Abstract
Nonrandom and somatically acquired chromosomal translocations can be identified in nearly 50% of human acute myeloid leukemias. One common chromosomal translocation in this disease is the 8q22;21q22 translocation. It involves the AML1 (RUNX1) gene on chromosome 21 and the ETO (MTG8, RUNX1T1) gene on chromosome 8 generating the AML1-ETO fusion proteins. In this review, we survey recent advances made involving secondary mutational events and alternative t(8;21) transcripts in relation to understanding AML1-ETO leukemogenesis.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous disease that is classified based on the presence of specific cytogenetic abnormalities as well as the French-American-British (FAB) classification of the leukemic cells and immunophenotype. One of the common translocations identified in leukemia is between chromosome 8q22 and chromosome 21q22 (Figure 1a).1 It is associated with nearly 40% of cases of FAB-M2 AML and 8% to 20% of all cases of AML depending on the genetic background and geographic location of the population. The (8;21) translocation is also observed in approximately 6% of AML M1 and, more rarely, in AML M0, M4, M5, and other myeloproliferative syndromes.2,3 The involved genes are, on chromosome 8, MTG8 or ETO, meaning myeloid translocation gene or eight twenty-one, respectively,4,5 and AML1 (acute myeloid leukemia factor 1) on chromosome 21.4 The commonly used name for the t(8;21) fusion protein is AML1-MTG8 or AML1-ETO, and we refer to it as AML1-ETO in this review. AML1 was also discovered from other studies that are not related to t(8;21) and has several different names.6 Its HUGO (Nomenclature Committee of the Human Genome Organization) name is RUNX1. In correlation, MTG8/ETO is named RUNX1T1 for RUNX1 translocation 1.
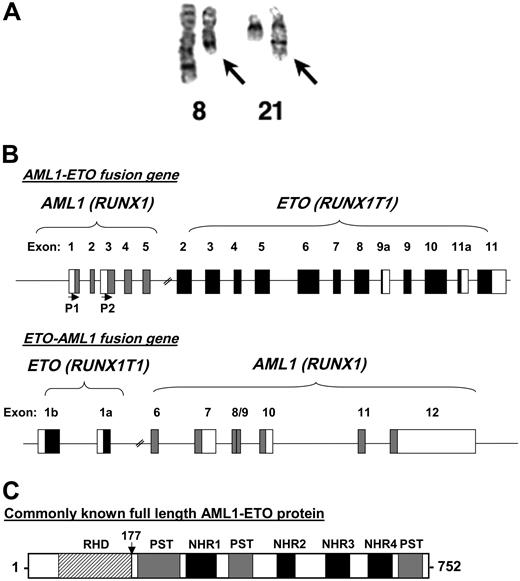
The 8;21 chromosomal translocation. (A) A chromosome preparation of a t(8;21) patient depicting normal chromosome 8, a shorter chromosome 8 with the translocated chromosome 21 (left arrow), normal chromosome 21, and a longer chromosome 21 fused to the portion of chromosome 8 (right arrow). The image was kindly provided by Dr Janet Rowley. (B) Genomic structure of t(8;21). Depicted are the exons of both AML1 and ETO organized following the reciprocal translocation. The first 5 exons of AML1 are fused to exons 2 through 11 of ETO. P1 and P2 with the horizontal arrows denote the start sites of transcription based on the usage of the distal (P1) or proximal (P2) promoters of AML1. The reciprocal ETO-AML1 fusion shows exons 1b and 1a of ETO fused to exons 6 through 12 of AML1. Filled boxes and blank boxes indicate translated and untranslated exon sequences, respectively. (C) Protein structure of AML1-ETO. As explained in the text, the commonly known full-length AML1-ETO protein is a 752-amino acid protein. The N-terminal portion of AML1 up to its runt homology domain (RHD) is fused to most of the ETO protein. Shown are the regions of homology to the Drosophila Nervy protein (NHR1 to NLH4) and 3 proline-serine-threonine-rich (PST) regions. The vertical arrow points to the fusion junction between AML1 and ETO.
The 8;21 chromosomal translocation. (A) A chromosome preparation of a t(8;21) patient depicting normal chromosome 8, a shorter chromosome 8 with the translocated chromosome 21 (left arrow), normal chromosome 21, and a longer chromosome 21 fused to the portion of chromosome 8 (right arrow). The image was kindly provided by Dr Janet Rowley. (B) Genomic structure of t(8;21). Depicted are the exons of both AML1 and ETO organized following the reciprocal translocation. The first 5 exons of AML1 are fused to exons 2 through 11 of ETO. P1 and P2 with the horizontal arrows denote the start sites of transcription based on the usage of the distal (P1) or proximal (P2) promoters of AML1. The reciprocal ETO-AML1 fusion shows exons 1b and 1a of ETO fused to exons 6 through 12 of AML1. Filled boxes and blank boxes indicate translated and untranslated exon sequences, respectively. (C) Protein structure of AML1-ETO. As explained in the text, the commonly known full-length AML1-ETO protein is a 752-amino acid protein. The N-terminal portion of AML1 up to its runt homology domain (RHD) is fused to most of the ETO protein. Shown are the regions of homology to the Drosophila Nervy protein (NHR1 to NLH4) and 3 proline-serine-threonine-rich (PST) regions. The vertical arrow points to the fusion junction between AML1 and ETO.
The t(8;21) generates the 2 fusion genes AML1-ETO and ETO-AML1 (Figure 1B). AML1-ETO mRNA is easily detectable using polymerase chain reaction (PCR) primers on 2 sides of the fusion point. However, ETO-AML1 mRNA was not identified using a similar approach (E. Kanbe, D.-E.Z., unpublished data, February 2003). This result indicates that the ETO-AML1 transcript is not expressed, is expressed at an extremely low level, or is highly unstable due to degradation. All of the studies on t(8;21) have therefore focused on AML1-ETO.
Most of the coding region of the ETO gene is fused to the AML1 amino terminus containing the DNA-binding runt homology domain (RHD) to generate an AML1-ETO fusion protein (Figure 1C).4,5,7 The ETO gene has 14 exons. The original cloned AML1-ETO cDNA contained ETO exons 2 through 11; the fusion transcript produces an AML1-ETO protein of 752 amino acids (Figure 1C).8 The ETO portion of the full-length AML1-ETO protein contains 3 proline-serine-threonine (PST)-rich regions and 4 Nervy homology regions (NHR1-4) (Figure 1C).9 The PST-rich regions have multiple potential kinase phosphorylation sites (SP [Serine-Proline] and TP [Threonine-Proline]). Phosphorylation of ETO has been reported although no kinase involved in its phosphorylation has been identified.10 NHR1, also called the TAF (TATA box binding protein associated factor) homology domain, shares a sequence similarity with TAF110 and other related TAFs. NHR2 has a hydrophobic amino acid heptad repeat, which is critical for ETO oligomerization.11 NHR3 contains a predicted coiled-coil structure. NHR4 is a myeloid-Nervy-DEAF1 (MYND) homology domain with 2 predicted zinc finger motifs.
Expression of the AML1-ETO fusion gene is under the control of the AML1 promoter. The AML1 gene has 2 promoters, the P1 (distal) and the P2 (proximal) promoters,12 whose arrangement is conserved in human, mouse, and zebrafish.13 The protein encoded by the transcript from the P1 promoter is 27 amino acids longer at the N terminus than the protein encoded by the P2 transcript. Studies of alternate AML1 promoter usage have been limited, but it is known that transcripts originating from P1 are present in day 7, 11, 15, and 17 of mouse embryos, but transcripts originating from P2 are only detectable at day 7.14 Differences are also observed when embryonic stem (ES) cells undergo hematopoietic differentiation in vitro.14 The P1 promoter was shown to be the major one used in hematopoietic stem cells and T cells.15 The published AML1-ETO cDNA originated from the AML1 P2 promoter.8 AML1-ETO transcripts originating from the P1 promoter transcripts have not yet been identified, and it still remains to be determined if such a transcript has complete functional similarity to the P2-derived transcript.
The consequences of AML1-ETO expression on cell biologic processes in both cell line and primary models have been extensively reviewed.9,16-18 Overall, its role in blocking cell cycle and promoting apoptosis contradicts its function in promoting leukemogenesis. However, AML1-ETO blocks myeloid, lymphoid, and erythroid differentiation in many of these models. Furthermore, a role of AML1-ETO in positively influencing stem cell renewal in primary human and murine hematopoietic stem cells and cell line models has been uncovered that relies on direct or indirect gene regulation of factors involved in stem cell maintenance. A recent review by Elagib and Goldfarb17 surveyed AML1-ETO's molecular role in regulating stem renewal and blocking hematopoietic differentiation, including interactions with various lineage-specific transcription factors, histone deacetylases, and methylases. Therefore, this review addresses AML1-ETO mouse models, the requirement and identification of additional point mutations, and the identified splice variant(s) of AML1-ETO.
Additional mutations and mouse models of t(8;21) leukemogenesis
In t(8;21) AML patient cells, 1 allele of AML1 and 1 allele of ETO are still normal. Furthermore, the AML1-ETO fusion gene is under the control of AML1 regulatory elements. However, all heterozygous AML1-ETO knock-in mice die around 12.5 days of embryogenesis and fail to establish definitive hematopoiesis.19,20 These major phenotypes are identical to those reported in homozygous AML1-deficient mice,21,22 indicating that AML1-ETO may dominantly block AML1 function during early embryo development.
To circumvent early embryo lethality, several other AML1-ETO transgenic mouse models have been established by different procedures, including tetracycline regulatable,23 myeloid lineage-specific MRP8 promoter directed,24 hematopoietic stem cell Sca-1 locus,25 and Cre recombinase-mediated conditional AML1-ETO expression transgenic mice.26,27 Except for mice with the Sca-1 locus-directed AML1-ETO expression, all of these transgenic mice remained healthy with normal hematopoiesis during their life span. Mice with AML1-ETO expression by the Sca-1 locus developed a myeloproliferative disorder with a latency of 6 months and a penetrance of 82% at 14 months following skin lesion epidermal hyperplasia as early as 4 weeks of age with hyperkeratosis and ulceration at older age.25 Interestingly, similar skin lesions around the eyes were observed in chimeras of AML1-ETO knock-in mice (L. Liu, D-E.Z., unpublished data, March 1997). Furthermore, virally transduced AML1-ETO expression and bone marrow transplantation approaches were also not able to induce leukemia development in recipient mice.28,29 However, abnormal expansion of hematopoietic early progenitors and extension of the life span of hematopoietic cells without leukemogenesis were noticed in these last models. Thus, the Sca-1 model and the retroviral approaches demonstrate that targeting of the right progenitor and/or stem cells is important for establishing a role of AML1-ETO in self-renewal biology. Furthermore, these models suggest that AML1-ETO may alter the epigenetic environment of stem cells, without causing acute leukemia, dependent on its ability to interact with histone deacetylases and methylases. However, the treatment of MRP8-AML1-ETO transgenic or conditional AML1-ETO knock-in mice with the DNA alkylation mutagen N-ethyl-N-nitrosourea (ENU) produced AML in more than 50% of the MRP8-AML1-ETO mice,24 while 31% of the conditional AML1-ETO mice developed granulocytic sarcoma that is commonly observed in t(8;21) patients.27 Thus, these results provided direct evidence that AML1-ETO is critical for causing myeloid leukemia, requiring one or more additional mutations for leukemogenesis.
The nature of additional mutations in patients associated with AML1-ETO-mediated leukemogenesis is evidenced by additional cytogenetic abnormalities such as the loss of one of the sex chromosomes.30 As shown in Table 1, 35% of female t(8;21) AML patients are missing one X chromosome and 56% of male t(8;21) AML patients lack the Y chromosome. Although loss of the sex chromosomes is a natural phenomenon associated with aging, the loss of sex chromosomes tends to occur at a significantly younger age and at a much higher frequency in patients with t(8;21) AML than in the general population.50 Furthermore, sex chromosome loss in other types of AML occurs in less than 5% of patients.31,50 These statistics suggest that the loss of certain sex chromosome genes may cooperate with t(8;21) in AML development. Furthermore, trisomy 8, trisomy 4, and chromosome 9 deletion are also associated with t(8;21) AML (Table 1). In addition to cytogenetic abnormalities, mutations of growth factor receptors, protooncogenes, and transcription factors are also identified in t(8;21) AML, which include stem cell factor receptor (c-KIT), FMS-related tyrosine kinase 3 (FLT3), N-RAS, PU.1, and AML1 (Table 1). Other chromosome translocations related to the development of myeloid proliferation diseases, such as t(5;12) (encoding the TEL-PDGFRβ fusion protein) and t(9;22) (encoding the BCR-ABL fusion protein), are also reported in a few t(8;21) AML patients.51,52 The finding of activating mutations in kinases involved in leukemia associated with t(8;21), such as c-KIT and FLT3-ITD, impacts future cytogenic and mutagenic data collection from patients by molecular methods at presentation, thereby facilitating individual patient-directed chemotherapeutic treatments. This is exemplified by the possible use of imatinib in the case of the c-KIT N822K mutation46 or, in the case of c-KIT D816 mutations, the SRC/ABL inhibitor dasatinib53 or nilotinib,54,55 an ABL tyrosine kinase inhibitor. In the case of FLT3 mutants possibly combining treatments with FLT3-specific inhibitors such as PKC41256-58 or sunitinib.59,60
Cytogenetic abnormalities and gene mutations associated with t(8; 21) leukemia
| Mutations . | Frequency, no./total (%) . | References . |
|---|---|---|
| Cytogenetic abnormalities | ||
| −X in female patients | 115/331 (35) | 31,,,,,-37 |
| −Y in male patients | 235/419 (56) | 31,,,,,-37 |
| Del (9q) | 80/454 (18) | 32,36,37 |
| Trisomy 4 | 8/75 (11) | 3 |
| Trisomy 8 | 28/454 (6) | 32,36,37 |
| Others | 77/454 (16) | 32,36,37 |
| Molecular genetic mutations | ||
| FLT3-length mutation: FLT3/ITD | 27/387 (6.9) | 36,38,,-41 |
| FLT3 D853 activating mutations | 5/184 (2.7) | 36,38,41 |
| c-KIT D816 (D>Y, D>V, D>H, D>I) | 43/351 (12.3) | 36,38,42,,-45 |
| c-KIT N822K | 10/54 (19) | 46 |
| NRAS: codons 12,13, 61 | 40/469 (8.5) | 36,38,47,-49 |
| PU.1 | 1/19 (5.3) | 36 |
| AML1 | 1/26 (3.8) | 36 |
| Mutations . | Frequency, no./total (%) . | References . |
|---|---|---|
| Cytogenetic abnormalities | ||
| −X in female patients | 115/331 (35) | 31,,,,,-37 |
| −Y in male patients | 235/419 (56) | 31,,,,,-37 |
| Del (9q) | 80/454 (18) | 32,36,37 |
| Trisomy 4 | 8/75 (11) | 3 |
| Trisomy 8 | 28/454 (6) | 32,36,37 |
| Others | 77/454 (16) | 32,36,37 |
| Molecular genetic mutations | ||
| FLT3-length mutation: FLT3/ITD | 27/387 (6.9) | 36,38,,-41 |
| FLT3 D853 activating mutations | 5/184 (2.7) | 36,38,41 |
| c-KIT D816 (D>Y, D>V, D>H, D>I) | 43/351 (12.3) | 36,38,42,,-45 |
| c-KIT N822K | 10/54 (19) | 46 |
| NRAS: codons 12,13, 61 | 40/469 (8.5) | 36,38,47,-49 |
| PU.1 | 1/19 (5.3) | 36 |
| AML1 | 1/26 (3.8) | 36 |
Using a mouse retroviral-mediated transduction bone marrow transplantation model, it has been shown that AML1-ETO cooperates with the oncogenic factors TEL-PDGFRβ or the FLT3 internal duplication (FLT3/ITD).38,61 Two other recently reported mouse leukemia models with AML1-ETO include the expression of AML1-ETO in blood cells without IRF8 and the coexpression of AML1-ETO with Wilms tumor gene WT1.29,62 Both of these genes have been implicated in AML by loss and gain of expression, respectively. An increase of the cell cycle inhibitor p21WAF1 has been reported in AML1-ETO-expressing cells,63 suggesting a role in t(8;21)-related leukemogenesis. Interestingly, AML1-ETO induced leukemia in p21WAF1-deficient mouse bone marrow cells,64 suggesting that the bypass of pathways induced by the fusion protein itself may be another mechanism of AML1-ETO leukemogenesis.
Taken together, studies with AML1-ETO mouse models and the observed cytogenic and mutagenic events in patients fully support the notion that AML1-ETO is a critical factor but is not sufficient for leukemogenesis. In addition, the observation of the t(8;21) aberration still being detected after remission in a patient that contained both t(8;21) and an activating mutant of c-KIT suggests that the chromosomal translocation is the first event.46 Furthermore, AML1-ETO is often detected in remission patients, suggestive of minimal residual disease. Further evidence that the translocation occurred in stem cells comes from AML1-ETO being detected in B cells.65,66 In summary, several approaches that mimic additional gene mutations in human t(8;21) AML or that counteract AML1-ETO-induced inhibition of cell cycle progression have been useful in generating AML1-ETO leukemic mouse models. More challenging will be to identify the molecular mechanisms of additional cytogenetic defects, such as loss or gain of specific chromosomes, associated with t(8;21) AML, because these losses and gains suggest the presence of possibly one or more tumor suppressors or amplified oncogenes.
Transcribed isoforms of t(8;21) and their impact on leukemogenesis
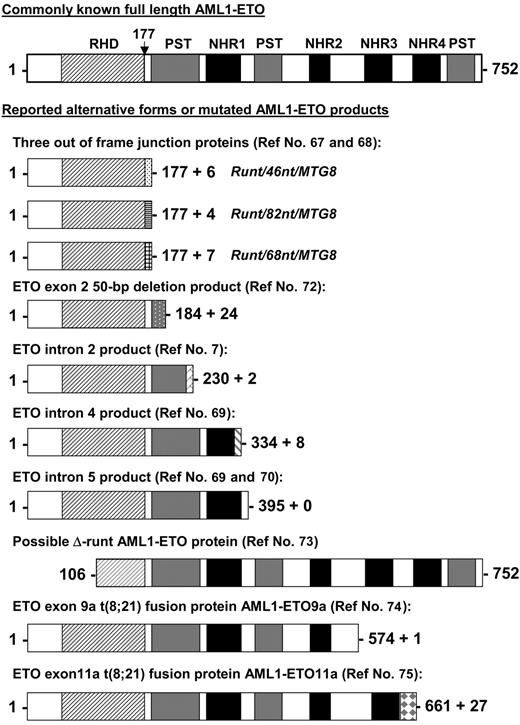
Besides the full-length AML1-ETO transcript, alternative AML1-ETO transcripts have been reported in t(8;21) patient samples and/or in leukemia cell lines with t(8;21). The predicted proteins of these alternative transcripts are shorter versions of the full-length AML1-ETO (Figure 2). Three AML1-ETO transcripts (runt/46nt/MTG8, runt/82nt/MTG8, and runt/68nt/MTG8) with insertions between exon 5 of AML1 and exon 2 of ETO have been detected in t(8;21) patient samples.67,68 These insertions cause AML1 to be out of frame with ETO and result in an in-frame stop codon before the ETO sequence. AML1-ETO transcripts containing intron 2, 4, or 5 of ETO were also reported by several groups.7,69,70 Reverse transcriptase (RT)-PCR studies showed that the ETO intron 4- and intron 5-containing transcripts coexisted with the full-length AML1-ETO in all t(8;21) patient samples tested.69,71 An AML1-ETO transcript with a 50-nucleotide deletion in exon 2 of ETO was reported as the only fusion transcript in one patient sample.72 This transcript is capable of generating an AML1-ETO protein that contains amino acids 1 to 184 of the full-length AML1-ETO and 24 additional amino acids (Figure 2). Alternate splicing of AML1 amino-terminal exons that exclude exons 2 and 3 has been reported.73 This could conceivably produce an AML1-ETO transcript with a substantial portion of the DNA-binding RHD deleted (Figure 2). However, AML1-ETO transcripts with this structure have not yet been detected. Furthermore, the alternatively spliced isoforms of AML1-ETO, AML1-ETO9a,74 and AML1-ETO11a,75 are coexpressed with full-length AML1-ETO in t(8;21) patient samples. Both of these transcripts generate C-terminal truncated AML1-ETO proteins (Figure 2). The detection of these shorter AML1-ETO transcripts in t(8;21) AML patients raises the question as to whether AML1 truncation per se or its fusion to ETO is crucial to leukemia development and whether fusion proteins derived from incompletely processed alternative transcripts or alternative spliced products contribute to leukemogenesis.
Other possible AML1-ETO fusion proteins from reported alternate transcripts. Shown are other known alternative AML1-ETO predicted gene products identified in cell lines and patient samples aligned to the full-length fusion protein. The vertical arrow points to the fusion junction between AML1 and ETO. RHD indicates runt homology domain; NHR1 to NLH4, Drosophila Nervy protein homology regions; and PST, proline-serine-threonine-rich regions. References identifying these alternative products are indicated.
Other possible AML1-ETO fusion proteins from reported alternate transcripts. Shown are other known alternative AML1-ETO predicted gene products identified in cell lines and patient samples aligned to the full-length fusion protein. The vertical arrow points to the fusion junction between AML1 and ETO. RHD indicates runt homology domain; NHR1 to NLH4, Drosophila Nervy protein homology regions; and PST, proline-serine-threonine-rich regions. References identifying these alternative products are indicated.
In summary, these alternative AML1-ETO transcripts are capable of producing fusion proteins lacking either entire or various portions of the C-terminal ETO protein. In addition to the recent discovery of the AML1-ETO9a splice variant, which is addressed next, the question is raised as to whether various of these shorter AML1-ETO fusion proteins contribute to the development of leukemia in human t(8;21) patients in a cooperative manner.
A C-terminal truncated form of AML1-ETO (AML1-ETOtr) was found to cause leukemia.63 AML1-ETOtr was generated in vivo by a single-nucleotide insertion in the AML1-ETO DNA sequence, leading to the early termination of protein translation thereby lacking the NHR3 and NHR4 domains. This finding triggered the identification of the alternatively spliced AML1-ETO9a transcript in t(8;21) AML described in the paragraph above, which also leads to rapid leukemia development in mice.74 These results suggest that the C-terminal portion of ETO inhibits the leukemogenic potential of AML1-ETO. It is likely that one type of mutation that can promote AML1-ETO-induced leukemogenesis in mice is to directly or indirectly block the molecular events associated with the C terminus of AML1-ETO. Unlike their full-length counterpart, AML1-ETOtr and AML1-ETO9a did not have an inhibitory effect on myeloid cell cycle progression although they retained the ability to block myeloid cell differentiation.63,74 Moreover, coexpression of AML1-ETO and AML1-ETO9a results in an earlier onset of leukemia development, blocking myeloid cell differentiation at an earlier stage of hematopoiesis compared with the expression of AML1-ETO9a alone.74 This suggests that fusion proteins from various alternatively spliced transcripts of the 8;21 translocation may cooperate in promoting leukemogenesis.
It will be, however, interesting to solidify the contribution of the AML1-ETO9a splice variant to a prognostic value in patients, as observed with the prognosis value associated with c-KIT mutations in t(8;21).44,45 —specifically, to see how the level of transcript determines the overall survival. As has been observed in t(12;21) childhood ALL cases, high expression of the reciprocal AML1-TEL fusion gene is an independent poor prognostic factor.76 Furthermore, expression of the splice variants of survivin, a member of the inhibitor of apoptosis protein family, survivin-2B and survivin-DeltaEx3, is valuable in prognosis in adult and childhood AML following treatment, respectively.77 In addition, it will be of interest to see if the AML1-ETO9a transcript is present during remission compared with full-length AML1-ETO. One scenario could be envisioned in which the loss of the AML1-ETO9a splice variant at remission and the continued presence of the full-length AML1-ETO transcript would suggest that the alternative splicing of the t(8;21) transcript is itself a secondary event involving posttranscriptional regulation. Furthermore, with the discovery of additional mutations in t(8;21) patients, the question remains if AML1-ETO9a is also present and to what level. These studies will help identify if AML1-ETO9a is truly a secondary deregulated posttranscriptional event that will promote leukemia in the absence of any other secondary mutagenic event or if it is one of the pieces of the puzzle in the complex regulation of AML1-ETO leukemogenesis. In addition, the question persists as to the contribution of the C-terminal region that is known to interact with the NCoR repressor protein.78,79 Specifically, does NCoR actually function as a “proteonomic” tumor suppressor in t(8;21)? This is interesting because NCoR is located on chromosome 17p11.2, which is often lost in various malignant tumors80 including AML/MDS patients.81 However, from the literature it is evident that the loss of chromosome 17 is not a common event in t(8;21) leukemia. Thus, the loss of the “proteonomic” pathway of NCoR may be a way forward in t(8;21)-associated leukemia with the loss of the NHR4 zinc finger domain in both AML1-ETO9a and AML1-ETO11a splice variants. Although NCoR should associate as well with the N-terminal region of both AML1-ETO9a and AML1-ETO11a, the loss of a protein complex involving the NHR4/NCoR and/or other interacting proteins like HDACs suggest that this NHR4/NCoR interaction is crucial for blocking the leukemogenic potential of AML1-ETO. Furthermore, recently it was shown that the NHR4 domain is also important in blocking p300 acetylation of GATA-1, inhibiting erythroid differentiation.82
Interestingly, a recent report describes how AML1-ETO9a and the mutant AML1-ETOtr are able to break through the mitotic arrest induced by various mitosis poisons causing aneuploidy.83 Next to the observation of AML1-ETO deregulating DNA repair genes84 that are important in genome stability, these observations demonstrate that AML1-ETO splice variant(s) may affect the normal mitotic checkpoint.83 It does this specifically by negatively targeting securin involved in regulating mitosis by preventing anaphase through its association with the separase protease, which is responsible for loss of sister chromatid cohesion through cleavage of the Scc1/hRad21 cohesin subunit.83 These observations support a potential model in which AML1-ETO and its splice variant(s) are the first event that is able to promote secondary mutagenic events promoting full-blown leukemia development and the associated gain and loss of chromosomal material.
These new developments suggest a new model of t(8;21) leukemogenesis in which stem cell renewal and loss of DNA repair ability caused by AML1-ETO drive the ideal condition for leukemia development, promoting secondary mutagenic events. Alternatively, deregulated splicing of AML1-ETO may lead to an alternate model in which the requirement of secondary mutations in other genes is not directly required. Thus, AML1-ETO9a may drive leukemia development in cooperation with AML1-ETO, in addition to gain and loss of genomic material (Figure 3). The discovery of AML1-ETO9a, which promotes leukemia development on its own or in cooperation with AML1-ETO, points to the importance of not just understanding full-length AML1-ETO but to the need to also better understand its splice variant(s). The cooperative leukemogenesis with the combined AML1-ETO and AML1-ETO9a expression highlights that t(8;21) may follow a mechanism of leukemogenesis in the footsteps of some other known oncogenes, such as HER2,85 cyclin D1,86,87 and TRKA,88 that display increased transformation activity observed with the splice isoforms ΔHER2, cyclin D1b, and TRKA-III, respectively. Although it is known that full-length ETO protein is expressed in hematopoietic cells, it still has to be confirmed that splicing of exon 9a occurs in these cells and, more specifically, in which differentiated cells it happens. This as the expression of ETO in human myeloid cells is very low compared with its family members MTG16 and MTGR1,89 in agreement with AML1-ETO blocking granulocytic differentiation by sequestering NCoR from MTG16.90 The ability of ETO9a generation by normal splicing events also opens the can of worms as to whether the AML1-ETO9a spliced form is expressed before or after leukemia development. This is because varying levels of AML1-ETO9a are observed in t(8;21) patients74 in addition to patients in remission still containing AML1-ETO-positive cells.65,67 Thus, the possibility of deregulated alternative processing of AML1-ETO mRNA being required for transformation is brought forth, as is seen in the case of the receptor tyrosine phosphatase PTPN6, which undergoes RNA editing and alternative splicing leading to the production of an inactive protein unable to block receptor tyrosine kinases like c-KIT, providing ideal conditions for leukemia development.91 Moreover, the phenomenon of deregulated splicing events as a mechanism of cancer development is evident by observations of altered RNA processing of tumor suppressors and oncogenes (reviewed by Kalnina et al92 and Scholzova et al93 ). The presence of alternative splice forms of fusion genes is not uncommon, because the MLL-AF4 fusion gene expresses various splice variants due to the presence of cryptic splice sites94 ; however, direct evidence for a role of these alternative transcripts in leukemogenesis has not been presented as yet. There is further evidence of BCR-ABL signaling deregulating the splicing of the Ikaros gene in t(9;22) pre-B-cell leukemia.95 In addition, that BCR-ABLp210 is able to induce the expression of various splicing factors in primary human CD34+ hematopoietic progenitor cells deregulating splicing of the PYK2 gene.96 Thus, the identification of alternative splicing of AML1-ETO in patient samples and the cooperative mouse model of AML1-ETO/AML1-ETO9a leukemogenesis show that not all has been learned in mechanisms of transformation by the 8;21 translocation.
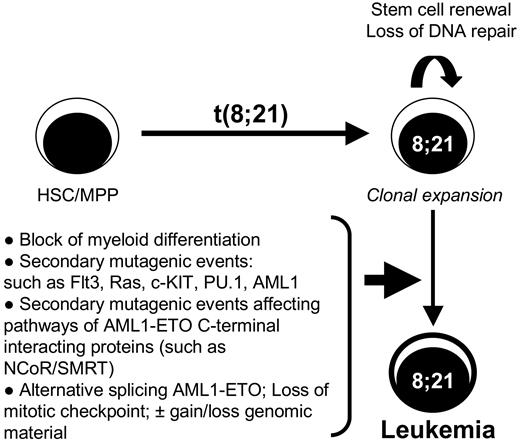
Diagram of the multistep leukemogenesis process associated with t(8;21). This model shows that after the initial event of t(8;21) within the hematopoietic stem cells (HSC) or multipotential progenitor (MPP), the cells will initially expand by the stem cell renewal program induced by the AML1-ETO fusion proteins associated with a block in myeloid differentiation. The loss of DNA repair mechanisms elicited by AML1-ETO creates an ideal environment for the acquisition of additional transforming mutagenic events such as FLT3 and Ras or deregulation of the NCoR/SMRT pathway. However, the appearance of alternative splice variants such as AML1-ETO9a may contribute to the speed of transformation or may even cooperate with AML1-ETO, being itself a secondary event by deregulated alternative splicing. The expression of AML1-ETO isoforms may also further aid in the gain and/or loss of genomic material often observed in t(8;21) leukemia through the deregulation of the mitotic checkpoint.
Diagram of the multistep leukemogenesis process associated with t(8;21). This model shows that after the initial event of t(8;21) within the hematopoietic stem cells (HSC) or multipotential progenitor (MPP), the cells will initially expand by the stem cell renewal program induced by the AML1-ETO fusion proteins associated with a block in myeloid differentiation. The loss of DNA repair mechanisms elicited by AML1-ETO creates an ideal environment for the acquisition of additional transforming mutagenic events such as FLT3 and Ras or deregulation of the NCoR/SMRT pathway. However, the appearance of alternative splice variants such as AML1-ETO9a may contribute to the speed of transformation or may even cooperate with AML1-ETO, being itself a secondary event by deregulated alternative splicing. The expression of AML1-ETO isoforms may also further aid in the gain and/or loss of genomic material often observed in t(8;21) leukemia through the deregulation of the mitotic checkpoint.
Thus, AML1-ETO alternative splicing leading to the loss of the interaction of C-terminal portion of AML1-ETO with other proteins is one possible route of leukemogenesis by t(8;21). Alternatively, loss of function or binding of proteins interacting with C-terminal portion of AML1-ETO or their further downstream pathways is another possible mechanism of leukemogenesis by t(8;21). Next to interacting with NCoR, the NHR4 domain of AML1-ETO or ETO also interacts with the silencing mediator of retinoic acid and thyroid hormone receptor (SMRT).78,79 Further, there is tangible evidence of SMRT functional tumor suppressive role in non-Hodgkin lymphoma in a haploinsufficient manner.97 Specifically, the SMRT gene located at chromosome 12q24 is often abrogated by chromosomal rearrangements, decreasing its transcript and protein levels in cell lines and primary patient lymphoma samples. This chromosome 12q24 region is also implicated in AML.98-100 Further down-regulation of SMRT by antisense strategy conferred transformation of fibroblasts in cooperation with overexpressed hTert (telomerase) and SV40-LargeT in soft-agar assays. In addition, reconstitution in lymphoma cell lines with exogenous SMRT expression induced apoptosis.97 Furthermore, the presence of the FLT3-internal tandem duplication mutant in AML is able to block SMRT-specific regulation of PLZF growth inhibitory action.101 Thus, bypass of the tumor suppressive roles of either NCoR or SMRT pathways associated with the C terminus of AML1-ETO by mutagenic events is a putative mechanism of leukemogenesis involving t(8;21) and requires further investigation.
Future directions
Although AML1-ETO9a is a potent inducer of leukemia in mice, AML1-ETO9a mutants that are unable to bind to DNA are not leukemogenic (M.Y., D.-E.Z., unpublished data, February 2005). This result in addition to previous studies suggests that the DNA-binding domain of t(8;21) fusion proteins is important in directing the expression of certain important genes that control cell proliferation, differentiation, and survival. Thus, further studies to elucidate the complex mechanism of t(8;21) leukemogenesis should address issues such as (1) the clinical significance of the expression levels of alternatively spliced AML1-ETO isoforms, (2) the function of the AML1-ETO/alternatively spliced AML1-ETO heterodimers/homodimers, (3) the mechanism of regulated AML1-ETO splicing, (4) localization, (5) deregulated gene expression in the presence or absence of the splice variants and the analysis of newly identified potential targets on their specific roles in leukemia development, and (6) and altered or enhanced protein-protein interactions due to the presence of splice variants.
Future development of therapeutic regimens to effectively target the AML1-ETO fusion protein(s), such as decreasing its RNA or protein stability, preventing its binding to DNA and cofactors, and altering its cellular localization, should be valuable approaches against t(8;21) leukemogenesis. The generation of various mouse models described above further form ideal primary models, as exemplified by the use of AML1-ETOtr in testing a new drug and combinations of drugs102,103 and testing the new generations ofchemotherapeutics and/or alternative molecular biologic approaches for treating t(8;21) leukemia.
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Cancer Institute (CA96735 and CA104509). We apologize to all researchers whose relevant work could not be cited due to space limitations.
National Institutes of Health
Authorship
Contribution: All authors contributed to writing this review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dong-Er Zhang, MEM-L51, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037; e-mail: dzhang@scripps.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal