Abstract
Growing interest in using endothelial cells for therapeutic purposes has led to exploring human embryonic stem cells as a potential source for endothelial progenitor cells. Embryonic stem cells are advantageous when compared with other endothelial cell origins, due to their high proliferation capability, pluripotency, and low immunogenity. However, there are many challenges and obstacles to overcome before the vision of using embryonic endothelial progenitor cells in the clinic can be realized. Among these obstacles is the development of a productive method of isolating endothelial cells from human embryonic stem cells and elucidating their differentiation pathway. This review will focus on the endothelial potential of human embryonic stem cells that is described in current studies, with respect to the differentiation of human embryonic stem cells to endothelial cells, their isolation, and their characterization.
Tissue vascularization and the clinical importance of endothelial progenitor cells
Progenitor endothelial cells (ECs) are promising key factors for many therapeutic applications. These applications include the following: cell transplantation for the repair of ischemic tissues, formation of blood vessels and heart valves, engineering of artificial vessels, repair of damaged vessels, and inducing the formation of blood vessel networks in engineered tissues.1-3 Vascularization of engineered tissue in vitro before transplantation is essential for building complex and thick tissues because it enhances cell viability during tissue growth, induces structural organization, and promotes integration following implantation.
Another area in which embryonic ECs can be beneficial is the study of human embryogenesis. In particular, ECs can serve as a model system for exploring central issues in human vasculogenesis and potentially elucidate vasculogenic and angiogenic mechanisms involved in the pathogenesis of vascular disease. Furthermore, it recently became evident that blood vessels do not just exchange metabolites between blood and tissue but play a more fundamental role in providing developmental cues to organs and differentiating cells. Early development of the pancreas depends on the presence of blood vessels, even in the absence of blood flow.4-6 A similar dependence on ECs for the development of the liver and kidney has also been reported.7,8 Angioblasts, or progenitor ECs, are associated with emerging buds of the embryonic lung and the nascent glandular portion of the stomach.7 Studies of neuronal stem cell proliferation and differentiation lend further support to the view that organs must develop in proximity to blood vessels.9 The potential overlap in signaling that occurs during neurogenesis and angiogenesis may even suggest that neurogenesis is regulated, in part, by an equilibrium between peripherally derived and centrally derived signaling molecules acting on both cell populations. Indeed, it has been shown that dividing cells in the mature hippocampus are immunoreactive for endothelial markers, thereby demonstrating the existence of neurogenesis within an angiogenic niche.10 All these findings raise the possibility that endothelial signaling in organogenesis is universal for development in vertebrates. Despite many attempts, the cellular origin of vascular endothelial progenitors remains vague.11-14 Understanding the complete role of endothelial signaling or the regulatory mechanisms of paracrine signaling in ECs, in the developing vasculature or in adult vessels, is still in its infancy. This highlights further the importance of studying basic vascular biology and the use of an endothelial model system. Formation of the first capillaries takes place mostly during the early stages of embryogenesis, when ECs are generated from precursor cells.15 Hence, isolated human embryonic ECs (hES-derived ECs) or progenitor cells can be useful for these previously mentioned investigations. Another advantage of hES-derived ECs is that their source can be kept pluripotent and can repopulate for hundreds of doublings.16
Human embryonic stem cells
Human embryonic stem cells (hESCs), derived from the inner cell mass of preimplanted blastocysts, have been shown to give rise to stable pluripotent cell lines that are capable of unlimited proliferation under specific culture conditions. Following their injection into immunodeficient mice, undifferentiated hESCs produce teratomas composed of multiple–tissue-type cells, thus demonstrating their pluripotent potential. In vitro aggregation of hESCs into clusters of cells or embryoid bodies (EBs) allows the spontaneous differentiation of hESCs into multiple tissue lineages that represent endoderm, ectoderm, and mesoderm origins.17-19 Since their first derivation by Thomson et al,18 various lineages have been derived from hESCs: neurons, cardiomyocytes, smooth muscle cells, hematopoietic cells, osteogenic cells, hepatocytes, insulin-producing cells, keratinocytes, and ECs.20-29 Furthermore, these cells appear to be weakly immunogenic, expressing only moderate amounts of major histocompatibility complex (MHC) class I and not any MHC class II proteins.16,30 Therefore, hESCs are a potential source for almost all cell types in the body and may serve to alleviate the shortage of organs needed for transplantation. Specifically, the isolation and use of hES-derived ECs has potential therapeutic implications that include cell transplantation for repair of ischemic tissues and tissue-engineered vascular grafts. Several recent studies have demonstrated the use of adult endothelial progenitor cells for such purposes.31,32 Therefore, it is of the utmost importance to study and compare the vasculogenic potential of adult ECs and ES-derived ECs. Due to burgeoning evidence of a common progenitor for both endothelial and hematopoietic cells, the study of endothelial progenitors may also shed light on the identification and development of hematopoietic progenitors. The derivation of engraftable hematopoietic stem cells from hESCs will have implications for human medicine that reach far beyond the treatment of hematologic malignancies because these cells may provide a powerful method for preventing immune rejection of other ESC-derived tissues.
Endothelial potential of hESCs
There is growing evidence for the various stages of differentiation of hESCs as they develop into endothelial cells. Several studies have explored the endothelial potential of hESCs, mainly demonstrating the spontaneous differentiation of EBs to vascular-like structures and isolating hES-derived ECs (Table 1).24,29,33,36,37,41 Both in vivo transplantation and in vitro assays have been used to characterize the vascular-endothelial differentiation capabilities of hESCs (Table 2). Multiple markers have been used for characterizing and identifying derived endothelial precursors. Expression of vascular endothelial cadherin (VE-cad), platelet endothelial cell adhesion molecule-1 (PECAM1), CD34, and Flk1 (human counterpart KDR, vascular endothelial growth factor receptor 2) and the ability to take up Dil-labeled acetylated low-density lipoprotein (Dil-Ac-LDL) have been used as markers for identifying endothelial precursors. Mature ECs were identified by selective staining for von Willebrand factor (VWF), endothelial nitric oxide synthase (eNOS), and E-selectin proteins. Two main approaches have been used for purifying progenitor ECs from hESCs: supplementing feeder layers or the medium with various growth factors, and selecting EBs for specific cell-surface molecules. These protocols differ considerably with respect to the source of the cells, the cell line, the use of undifferentiated cells versus differentiated EBs, the age of EBs, and the type of supplemented growth factors (Table 1).
Derivation of endothelial cells from hESCs
| Reference . | ES source . | EC derivation . |
|---|---|---|
| Kaufman24 | hES cells (H1, H1.1, and H9.2) | Undifferentiated hESCs were grown on mouse bone marrow cells (S17) or mouse yolk sac endothelial cells (c166) for 17 days. |
| Wang33 | Human EBs (H9, H1) days 9-10 | EBs were sorted by FACS for VE-cadherin/PECAM1+ cells and then the sorted cells were grown for 7 days in endothelial-supporting media containing pituitary extracts and VEGF. |
| Levenberg et al29 | Human EBs (H9) day 13 | FACS sorting for PECAM1+ cells. |
| Gerecht-Nir et al36 | hES cells (H9, H13, I9) | Undifferentiated hESCs were grown on IV collagen–coated dishes, filtered (40 μm mesh), and then recultured on IV collagen–coated dishes supplanted with VEGF. |
| Vodyanik41 | Undifferentiated hES cells (H1, H9) | Undifferentiated hES cells were grown on mouse bone marrow stromal cells (OP9, S17, and MS-5) for 10 days. |
| Zambidis34 | Human EBs (H1) days 7-9 | EBs were grown on methylcellulose and supplemented with 50 μg/mL ascorbic acid, 0.5% insulin/transferring/selenium. |
| Reference . | ES source . | EC derivation . |
|---|---|---|
| Kaufman24 | hES cells (H1, H1.1, and H9.2) | Undifferentiated hESCs were grown on mouse bone marrow cells (S17) or mouse yolk sac endothelial cells (c166) for 17 days. |
| Wang33 | Human EBs (H9, H1) days 9-10 | EBs were sorted by FACS for VE-cadherin/PECAM1+ cells and then the sorted cells were grown for 7 days in endothelial-supporting media containing pituitary extracts and VEGF. |
| Levenberg et al29 | Human EBs (H9) day 13 | FACS sorting for PECAM1+ cells. |
| Gerecht-Nir et al36 | hES cells (H9, H13, I9) | Undifferentiated hESCs were grown on IV collagen–coated dishes, filtered (40 μm mesh), and then recultured on IV collagen–coated dishes supplanted with VEGF. |
| Vodyanik41 | Undifferentiated hES cells (H1, H9) | Undifferentiated hES cells were grown on mouse bone marrow stromal cells (OP9, S17, and MS-5) for 10 days. |
| Zambidis34 | Human EBs (H1) days 7-9 | EBs were grown on methylcellulose and supplemented with 50 μg/mL ascorbic acid, 0.5% insulin/transferring/selenium. |
Summary of experiments in which hESCs were used to derive endothelial potential, indicating the source of the hESCs and the derivation procedure.
Summary of 3D in vitro and in vivo assays examining the vascularization potential of ESCs and ESC-derived endothelial cells
| In vitro . | In vivo . | Reference . |
|---|---|---|
| Vessellike formation was followed during EB differentiation for 2 weeks. | — | Levenberg et al29 |
| PECAM1+ cells were isolated from 13-day-old EBs and seeded on matrigel. Vessel formation was assessed. | — | Levenberg et al29 |
| PECAM1+ cells were isolated from 13-day-old EBs and seeded on PLLA/PLGA scaffolds. | Immediately after seeding, the seeded scaffolds were transplanted in the dorsal region of SCID mice. Vessel formation and function was evaluated. | Levenberg et al29 |
| Nine-day-old EB cells were seeded on matrigel or PLLA/PLGA scaffolds and cultured for 2 weeks in the presence of growth factor (RA, TGF-β, activin-a, or IGF-I). Vessel formation was examined. | Seeded PLLA/PLGA scaffolds were transplanted in the dorsal region of SCID mice. Vessel formation and function was evaluated. | Levenberg et al37 |
| PECAM1+ cells, isolated from 13-day-old EBs, were cocultured with myoblasts and embryonic fibroblasts on PLLA/PLGA scaffolds and cultured for 2 weeks from a vascularized muscle construct. | The seeded scaffolds were transplanted subcutaneously, intramuscularly, and replacing the interior abdominal muscle segment in SCID mice and rats. Vessel formation and function was evaluated. | Levenberg et al3 |
| Undifferentiated hESCs were seeded on alginate gels. After 30 days in culture, CD34+ cells forming lumens were observed. | — | Gerecht-Nir et al38 |
| hESCs were grown on IV collagen–coated dishes, filtered, and then seeded on type I collagen and matrigel. Vessel formation was assessed upon supplementing 50 μg VEGF and culturing for 7 to 12 days. | — | Gerecht-Nir et al36 |
| Rhesus ESCs were grown in the presence of growth factors (VEGF, bFGF, IGF-1, and EGF) for 29 days and then seeded on matrigel. | (1) A mixture of rhesus ESC–derived endothelial cells and matrigel were injected subcutaneously in SCID mice. Neovessel formation was detected. (2) A mixture of rhesus ESC–derived endothelial cells and matrigel were mounted on polyvinyl sponges and transplanted into SCID mice. Angiogenesis was detected. (3) oinjecting a mixture of tumor cells and rhesus ESC–derived endothelial cells subcutaneously in SCID mice increased tumor growth rate. | Kaufman et al39 |
| — | Vessel formation was studied during teratoma formation in SCID mice by injection of hESCs. Evaluation was performed 6 weeks after injection. | Gerecht-Nir et al40 |
| — | Vessel formation in 4-, 7-, and 8-week-old human embryos. | Gerecht-Nir et al40 |
| In vitro . | In vivo . | Reference . |
|---|---|---|
| Vessellike formation was followed during EB differentiation for 2 weeks. | — | Levenberg et al29 |
| PECAM1+ cells were isolated from 13-day-old EBs and seeded on matrigel. Vessel formation was assessed. | — | Levenberg et al29 |
| PECAM1+ cells were isolated from 13-day-old EBs and seeded on PLLA/PLGA scaffolds. | Immediately after seeding, the seeded scaffolds were transplanted in the dorsal region of SCID mice. Vessel formation and function was evaluated. | Levenberg et al29 |
| Nine-day-old EB cells were seeded on matrigel or PLLA/PLGA scaffolds and cultured for 2 weeks in the presence of growth factor (RA, TGF-β, activin-a, or IGF-I). Vessel formation was examined. | Seeded PLLA/PLGA scaffolds were transplanted in the dorsal region of SCID mice. Vessel formation and function was evaluated. | Levenberg et al37 |
| PECAM1+ cells, isolated from 13-day-old EBs, were cocultured with myoblasts and embryonic fibroblasts on PLLA/PLGA scaffolds and cultured for 2 weeks from a vascularized muscle construct. | The seeded scaffolds were transplanted subcutaneously, intramuscularly, and replacing the interior abdominal muscle segment in SCID mice and rats. Vessel formation and function was evaluated. | Levenberg et al3 |
| Undifferentiated hESCs were seeded on alginate gels. After 30 days in culture, CD34+ cells forming lumens were observed. | — | Gerecht-Nir et al38 |
| hESCs were grown on IV collagen–coated dishes, filtered, and then seeded on type I collagen and matrigel. Vessel formation was assessed upon supplementing 50 μg VEGF and culturing for 7 to 12 days. | — | Gerecht-Nir et al36 |
| Rhesus ESCs were grown in the presence of growth factors (VEGF, bFGF, IGF-1, and EGF) for 29 days and then seeded on matrigel. | (1) A mixture of rhesus ESC–derived endothelial cells and matrigel were injected subcutaneously in SCID mice. Neovessel formation was detected. (2) A mixture of rhesus ESC–derived endothelial cells and matrigel were mounted on polyvinyl sponges and transplanted into SCID mice. Angiogenesis was detected. (3) oinjecting a mixture of tumor cells and rhesus ESC–derived endothelial cells subcutaneously in SCID mice increased tumor growth rate. | Kaufman et al39 |
| — | Vessel formation was studied during teratoma formation in SCID mice by injection of hESCs. Evaluation was performed 6 weeks after injection. | Gerecht-Nir et al40 |
| — | Vessel formation in 4-, 7-, and 8-week-old human embryos. | Gerecht-Nir et al40 |
— indicates not available.
Endothelial cells isolated from hESCs
The first hESC-derived endothelial progenitors were isolated from 13-day-old EBs by fluorescence-activated cell sorting (FACS) of PECAM1+ cells. These cells expressed mature endothelial protein VWF in addition to expressing PECAM1, CD34, Flk1, and VE-cad and being capable of taking up Dil-Ac-LDL.29 Interestingly, endothelial progenitors derived from 9- to 10-day-old EBs, using an identical technique, also coexpressed the endothelial surface proteins PECAM1, CD34, Flk1, and VE-cad and possessed the ability to take up Dil-Ac-LDL. However, they did not express mature endothelial proteins, such as VWF and eNOS, or the common leukocyte marker CD45, an indicator of hematopoietic progenitor function.33 Therefore, these progenitors were called “primitive endothelial-like cells.”33 Zambidis et al34 grew mesodermal-hemato-endothelial colonies from cells isolated from 7- to 12-day-old human EBs (hEBs) seeded onto serum-free methylcellulose medium. These colonies showed Dil-Ac-LDL uptake and VE-cad expression. Other researchers have used undifferentiated hESCs grown on various feeder layers.24,36,41 hESCs grown on collagen IV–coated dishes for 6 days and then filtered through a 40-μm mesh strainer resulted in cells expressing specific endothelial progenitor markers, such as PECAM1, CD34, AC133, Tie2, and GATA3.36 Kaufman et al24 used mouse bone marrow stromal cells (S17 cell line) or mouse yolk-sac ECs (C166 cell line) as feeder layers for promoting hematopoietic differentiation of cultured hESCs. After 17 days, the cells had differentiated into an early hematopoietic subpopulation of CD34+CD3−CD45− cells. This subpopulation also contained ECs because about 50% of the CD34+ cells coexpressed PECAM1.24 Similar results were obtained when hESCs were grown on bone marrow stromal cells (OP9 cell line) for only 8 to 9 days.41 However, additional investigations of endothelial behavior or maturation were not reported in these studies.24,41
Most of the derived endothelial progenitors matured into competent endothelial cells, despite the various treatments and procedures. ECs isolated from hEBs after 13 to 15 days of differentiation displayed characteristics similar to vascular endothelium and expressed typical EC markers similar to those expressed in human umbilical vein endothelial cells (HUVECs), such as VE-cad, VWF, and Dil-Ac-LDL uptake (Figure 1). Furthermore, these cells displayed the proper organization of endothelial junctions. PECAM1 was distributed at the intercellular clefts, and the endothelial marker VWF was highly expressed in the cytoplasm. These cells were capable of forming tubelike structures in vitro and generating capillary structures when embedded in sponges. Their transplantation into immunodeficient mice resulted in the formation of microvessels containing blood cells and lined by cells positively stained for human PECAM1 and CD34.29
Isolated hESC-derived endothelial cells (PECAM1+ cells). The cells were grown in culture for up to 7 passages. Cells are stained with VE-cad (original magnification, × 1000; 100×/1.40 oil-immersion objective lens) and VWF (original magnification, × 400; 40×/1.3 objective lens) and show the uptake of Dil-labeled acetylated low-density lipoprotein (Dil-Ac-LDL; original magnification, × 200; 20×/0.5 NA air objective lens). Micrographs were taken at 25°C using a fluorescent motorized microscope (Axiovert 200; Carl Zeiss, Oberkochen, Germany) equipped with an Orca digital camera (Hamamatsu Photonics, Tokyo, Japan). OpenLab (ImproVision, Coventry, United Kingdom) and Fluoromount were used as imaging software and medium, respectively.
Isolated hESC-derived endothelial cells (PECAM1+ cells). The cells were grown in culture for up to 7 passages. Cells are stained with VE-cad (original magnification, × 1000; 100×/1.40 oil-immersion objective lens) and VWF (original magnification, × 400; 40×/1.3 objective lens) and show the uptake of Dil-labeled acetylated low-density lipoprotein (Dil-Ac-LDL; original magnification, × 200; 20×/0.5 NA air objective lens). Micrographs were taken at 25°C using a fluorescent motorized microscope (Axiovert 200; Carl Zeiss, Oberkochen, Germany) equipped with an Orca digital camera (Hamamatsu Photonics, Tokyo, Japan). OpenLab (ImproVision, Coventry, United Kingdom) and Fluoromount were used as imaging software and medium, respectively.
Primitive endothelial-like precursors isolated from hEBs after 9 to 10 days of differentiation were capable of developing into either endothelial or hematopoietic cells, depending upon in vitro culture conditions. Using selective conditions for endothelial cell culture containing pituitary extracts and vascular endothelial growth factor (VEGF) for 7 days, the cells became attached and spindle-shaped, strongly expressed PECAM1 and VE-cad, and were capable of Dil-Ac-LDL uptake. Although the primitive endothelial-like precursors failed to express mature endothelial proteins, under endothelial cell culturing conditions, VWF and eNOS were also expressed. Similar to the endothelial progenitors isolated from older EBs, these cells were also able to create an endothelial network in vitro, but their behavior in a 3-dimensional (3D) environment was not examined.33 The mesodermal-hemato-endothelial colonies, isolated from hEBs after 7 to 12 days of differentiation, developed into adhesive and nonadhesive cells after 2 to 4 weeks. Some adhesive cells were found to express PECAM1 (50%) and VE-cad (11%), but not CD45, and were capable of taking up Dil-Ac-LDL, indicative of endothelial maturation.34 The extent/degree of maturation of endothelial progenitors derived from undifferentiated hESCs was examined on collagen IV–coated dishes.36 VEGF supplementation conferred the ability to uptake Dil-Ac-LDL to these cells, which suggests endothelial cell behavior. However, only 20% were positively stained for PECAM1. Vascular smooth muscle cell (v-SMC) marker molecules, such as smooth muscle actin (SMA) and calponin, were also expressed following the addition of platelet-derived growth factor beta polypeptide (PDGFβ) to the culture medium. Seeding these cells on matrigel and supplementing with large amounts of VEGF (50 μg/mL) resulted in a typical tubelike arrangement of elongated ECs within the matrix. However, more CD34+ cells were observed in the vasculature arrangements than Flk1+ cells, which suggests that it is not completely predetermined that these structures will undergo endothelial differentiation.36
Because various manipulations were used to derive the endothelial progenitors, it is most likely that each study involved a different subset of cells. The most likely cause of these differences is that the progenitors were derived from cells at different stages of EB differentiation (Table 1). These differences are reflected in the expression of the various markers in progenitor cells isolated from 9- to 10-day-old EBs and 13-day-old EBs. Progenitors isolated from the younger EBs did not express mature endothelial cell markers but still expressed 1 or all of the endothelial markers PECAM1, VE-cad, and CD31, as well as being capable of Dil-Ac-LDL uptake. Comparison of the extent of expression of these markers in the different endothelial progenitors shows that progenitors isolated from 13- to 15-day-old EBs have the greatest expression (78%) (except for those isolated from 9- to 10-day-old EBs, where no quantification was reported).
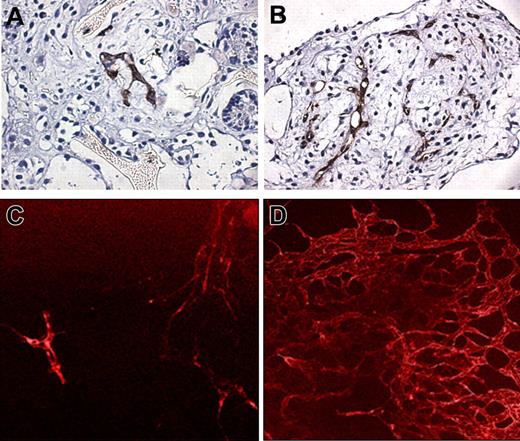
Several studies have explored 3D culture systems of hESCs on polymeric scaffolds or gels (Table 2).3,29,36-38,40,42 Using a 3D environment for cell culture brings the cells into close proximity, thereby enabling self-assembly and the formation of various components associated with the tissue microenvironment. The ability to culture cells in 3D is essential for understanding “real” in vivo cell interactions as well as the multifaceted mechanisms that control cell differentiation and the formation of complex tissue structures. Furthermore, this assay is a powerful tool for comparing the ability of the different progenitors to create a vascular network and to assess the extent of vascularization. Undifferentiated hESCs seeded on alginate scaffolds formed hEBs within 48 hours in culture. After 30 days in culture, tissuelike structures, such as epithelial sheets and tubes, connective tissue, and endothelial-like tubes, were observed.38 Human ESC-derived endothelial-like cells seeded on matrigel or collagen gels supplemented with 50 μg/mL VEGF exhibited a tubelike arrangement of elongated ECs after 7 to 12 days of culture.29,36 Coculturing hESC-derived endothelial progenitors isolated from 13-day-old EBs with myoblasts on a biodegradable polymer scaffold resulted in the formation of an endothelial vascular network in the skeletal muscle–engineered construct. While the myoblasts continued their differentiation to myotubes, the ECs organized themselves into tubular structures between the myoblasts, forming in vitro vessel networks in the engineered tissue (Figure 2A).3 Vascularized skeletal muscle–engineered constructs transplanted into immunodeficient severe combined immunodeficiency (SCID) mice became permeated with the host's blood vessels to the extent that 41% were functional, as assessed using a lectin assay (Figure 2B). This study shows that prevascularization of implants enhances vascularization of the implant and improves its survival following transplantation and also highlights the potential use of hESC-derived ECs as a cell source.3 These results are consistent with the outcome of another study, in which hESCs were cultured on 3D polymer scaffolds and supplemented with growth factors. This culturing resulted in the in vitro formation of a 3D vessellike network, which after transplantation intercalated with the host's blood vessels (Figure 2C,D).37 The possibility of inducing endothelial networks in other engineered tissues using various scaffolds is currently being investigated. A comprehensive in vivo study was performed using rhesus monkey ESC–derived ECs seeded on matrigel or matrigel-coated polyvinyl scaffolds.39 Upon transplantation into SCID mice, these seeded scaffolds exhibited intense vascularization and the formation of new vessels. The angiogenesis was attributed partly to the ability of the rhesus monkey ESC–derived ECs to secrete VEGF. Coinjection of rhesus ESC–derived ECs with tumor cells (C755 cell line) into SCID mice significantly increased tumor growth when compared with the injection of only tumor cells.39 However, the direct angiogenic effect of the rhesus ESCs was not evaluated. Gerecht-Nir et al40 studied teratoma formation following an injection of hESCs into SCID mice. Although small-diameter vessels of human origin could be detected in the core of the teratoma, the developing teratomas were mainly vascularized by invading blood vessels of the host rather than by the new vessels of human origin.40
Vessel formation of hESC-derived endothelial cells (ECs). (A) Vessellike structures formed in engineered skeletal muscle constructs in vitro (original magnification × 1000; 100 ×/1.40 oil-immersion objective lens). hESC-derived ECs (PECAM1+) were coseeded with skeletal myoblasts on PLGA-PLLA scaffold and cultured for 10 days. The construct was then fixed and immunostained for desmin (green), PECAM1 (red), and Dapi (blue). (B) The muscle constructs were implanted into murine muscle for 2 weeks, after which labeled lectin (red) was injected into the mice tail veins. Sections of the implant muscle were stained with human PECAM1 antibodies (green). The image shows functional (lectin perfused) human-derived endothelial vessels (original magnification × 1000; 100 ×/1.40 oil-immersion objective lens). Fluorescent micrographs were taken at 25°C using a fluorescent motorized microscope (Axiovert 200) equipped with an Orca digital camera (Hamamatsu). OpenLab and Fluoromount were used as imaging software and medium, respectively. (C,D) hEB differentiating cells were seeded on PLGA-PLLA scaffolds, grown for 2 weeks, and then transplanted in SCID mice. After 2 weeks, the constructs were retrieved and tissue was fixed and stained for antihuman PECAM1. Arrows indicate vessels lined by human endothelial cells. Original magnifications of panels C and D are × 1000 (100 ×/1.40 oil immersion objective lens) and × 400 (40 ×/0.6 NA air objective lens), respectively. Micrographs were taken at 25°C using an inverted microscope (Axiovert 200) equipped with an AxioCam colored camera (Carl Zeiss). AxioVision 3.1 (Carl Zeiss) was used as imaging software. DAB was used as fluorochrome.
Vessel formation of hESC-derived endothelial cells (ECs). (A) Vessellike structures formed in engineered skeletal muscle constructs in vitro (original magnification × 1000; 100 ×/1.40 oil-immersion objective lens). hESC-derived ECs (PECAM1+) were coseeded with skeletal myoblasts on PLGA-PLLA scaffold and cultured for 10 days. The construct was then fixed and immunostained for desmin (green), PECAM1 (red), and Dapi (blue). (B) The muscle constructs were implanted into murine muscle for 2 weeks, after which labeled lectin (red) was injected into the mice tail veins. Sections of the implant muscle were stained with human PECAM1 antibodies (green). The image shows functional (lectin perfused) human-derived endothelial vessels (original magnification × 1000; 100 ×/1.40 oil-immersion objective lens). Fluorescent micrographs were taken at 25°C using a fluorescent motorized microscope (Axiovert 200) equipped with an Orca digital camera (Hamamatsu). OpenLab and Fluoromount were used as imaging software and medium, respectively. (C,D) hEB differentiating cells were seeded on PLGA-PLLA scaffolds, grown for 2 weeks, and then transplanted in SCID mice. After 2 weeks, the constructs were retrieved and tissue was fixed and stained for antihuman PECAM1. Arrows indicate vessels lined by human endothelial cells. Original magnifications of panels C and D are × 1000 (100 ×/1.40 oil immersion objective lens) and × 400 (40 ×/0.6 NA air objective lens), respectively. Micrographs were taken at 25°C using an inverted microscope (Axiovert 200) equipped with an AxioCam colored camera (Carl Zeiss). AxioVision 3.1 (Carl Zeiss) was used as imaging software. DAB was used as fluorochrome.
Kinetics of expression of endothelial markers in hESCs
In an attempt to understand the process of ES differentiation, several studies have been undertaken that aimed at identifying the gene-expression profile of the endothelial cells and the kinetics of cell marker expression (Figure 3). The various stages of hESC differentiation as they developed into ECs forming vascular-like structures were followed in EBs. Within the EBs, PECAM1+ cells organized themselves into groups and formed specific channellike structures, which indicates that hESCs cultured to form EBs spontaneously differentiated to ECs and blood vessel–like structures. On the fourth day, a small number of PECAM1+ cells, which were localized in minute cell clusters, were detected in the EBs. From the sixth day, some sprouting of endothelial structures, which resembled capillaries, occurred. After 10 days, all the EBs contained extended areas of networklike capillary structures. The capillary area continued to increase over the following 3 days (Figure 4). To further characterize the vasculogenic potential, the expression of endothelial-specific genes was analyzed by using a reverse transcription–polymerase chain reaction (RT-PCR) during hESC differentiation on days 0 to 5, 8, 11, 13, 15, 18, and 20. In the undifferentiated cells, some genes, such as Flk1, AC133, and Tie2, were highly expressed, whereas low levels of expression occurred with other genes (GATA3, CD34). The expression of other genes (PECAM1, VE-cad, GATA2) only became noticeable after EB formation and differentiation. The gene expression of the EC adhesion molecules PECAM1, VE-cad, and CD34 increased progressively and reached their peaks between the 13th and 15th days. The 2 VEGF receptors Flk1 and Tie2 behaved differently in hESCs. Flk1 was expressed in undifferentiated cells and the expression was slightly increased upon differentiation, whereas Tie2 expression was unchanged during differentiation. Gene expression of the transcription factor GATA2 increased dramatically toward day 18. In addition, the expression in hESCs of AC133, a cell-surface marker of vascular hematopoietic stem and progenitor cells, remained constant over the period of differentiation. The time course of cell differentiation and the development of networklike capillary structures within the EBs correlated with the results of the RT-PCR analysis for RNA levels of the endothelial genes PECAM1, VE-cad, and CD34. The RNA levels were maximal between the 13th and 15th days.29
Summary of endothelial markers and the kinetics of their expression investigated during the differentiation of hESCs or EBS. Text colors within the figure correspond with the investigations listed at the bottom of the figure. D indicates down-regulated; U, up-regulated; +, expressed by undifferentiated hESCs (day 0 of EB differentiation); −, not expressed by hESCs. Where no data are given, expression was not reported.
Summary of endothelial markers and the kinetics of their expression investigated during the differentiation of hESCs or EBS. Text colors within the figure correspond with the investigations listed at the bottom of the figure. D indicates down-regulated; U, up-regulated; +, expressed by undifferentiated hESCs (day 0 of EB differentiation); −, not expressed by hESCs. Where no data are given, expression was not reported.
Vessellike network organization in 3D culture assay. (A,B) Dissociated EBs (8 days old) were seeded on PLGA-PLLA scaffolds and incubated for 2 weeks. The scaffold was then fixed in formalin and immunostained with anti-PECAM1 (original magnification, × 100; 10×/0.3 NA oil-immersion lens). Micrographs were taken at 25°C using an inverted microscope (Axiovert 200, Carl Zeiss) equipped with an AxioCam colored camera. AxioVision 3.1 was used as imaging software. DAB was used as fluorochrome. (C,D) A vessellike network was formed during differentiation of the EBs. hEBs obtained on day 6 (panel C) and day 13 (panel D) were fixed and stained for anti-PECAM1 to exhibit the advancement in the vascularization process and formation of complex vascular network (original magnification, × 100; 10×/0.3 NA oil-immersion lens). Micrographs were taken at 25°C using a fluorescent motorized microscope (Axiovert 200, Carl Zeiss) equipped with an Orca digital camera (Hamamatsu). OpenLab and Fluoromount were used as imaging software and medium, respectively.
Vessellike network organization in 3D culture assay. (A,B) Dissociated EBs (8 days old) were seeded on PLGA-PLLA scaffolds and incubated for 2 weeks. The scaffold was then fixed in formalin and immunostained with anti-PECAM1 (original magnification, × 100; 10×/0.3 NA oil-immersion lens). Micrographs were taken at 25°C using an inverted microscope (Axiovert 200, Carl Zeiss) equipped with an AxioCam colored camera. AxioVision 3.1 was used as imaging software. DAB was used as fluorochrome. (C,D) A vessellike network was formed during differentiation of the EBs. hEBs obtained on day 6 (panel C) and day 13 (panel D) were fixed and stained for anti-PECAM1 to exhibit the advancement in the vascularization process and formation of complex vascular network (original magnification, × 100; 10×/0.3 NA oil-immersion lens). Micrographs were taken at 25°C using a fluorescent motorized microscope (Axiovert 200, Carl Zeiss) equipped with an Orca digital camera (Hamamatsu). OpenLab and Fluoromount were used as imaging software and medium, respectively.
Wang et al33 investigated the hematopoietic commitment of hESCs in EBs (during days 3, 7, 10, 11, and 15). Using a colony-forming unit assay, they showed that CD45-expressing cells, an indicator for hematopoietic cells, emerged after 10 days of hEB development and functional hematopoietic cells could be observed on the 15th day. Expression of the endothelial cell adhesion molecules VE-cad and PECAM1 was not detected in hESCs. VE-cad–expressing cells were observed in discrete clusters in hEBs on the 7th and 10th days. PECAM1 expression was first observed on the third day and increased significantly between the 7th and 10th days. Ten-day-old hEBs containing PECAM1+ cells were devoid of committed hematopoietic (CD45) cells, whereas on the 11th day CD45+ cells appeared and were exclusively found adjacent to PECAM1+ cells. Most CD45+ cells costained with PECAM1 between the eleventh and fifteenth days of hEB development. This observation suggested that a subpopulation of VE-cad– and PECAM1-expressing cells within hEBs is associated with a subsequent hematopoietic commitment.
Zambidis et al34 identified the onset of hematopoietic progenitors and characterized the molecular kinetics of markers during 4 weeks of hEB differentiation. At the start of the experiment, undifferentiated hESCs expressed CD117, CD133, and Flk1, but there was low-to-undetectable expression of CD34 and PECAM1 RNA or surface protein. Between the twelfth and fifteenth day of hEB development, CD34 and PECAM1 expression peaked and these were coexpressed on the same hEB progenitors. CD45 was expressed on only 1% to 3% of hEB cells and not until about the fifteenth to thirtieth days of hEB differentiation. Similar results were also obtained when undifferentiated hESCs were supplemented with cytokines and bone morphogenic protein-4.17 Hematopoietic gene expression in hEBs has also been investigated using quantitative RT-PC, and a progressive pattern of expression of genes known (from murine studies) to initiate and regulate hematopoiesis was identified.34 The gene expression of key hematopoietic transcriptional regulators, including SCL, CDX4, GATA1, GATA2, EKLF, and PU.1, peaked between the sixth and tenth days of hEB differentiation. Increases in mRNA levels of these transcription factors coincided with increasing expression levels of PECAM1, CD34, and Flk1. These results suggest the existence of a coordinated developmental event in hemato-endothelial commitment of hEBs. The emergence of primitive hematopoiesis coincided with dramatic up-regulation of SCL, which was expressed abundantly in all hematopoietic colonies. On the ninth day of hEB development, the expression of CDX4 and SCL peaked. This event coincided with a peak in the number of primitive erythroid colony-forming cells and the subsequent emergence of definitive colony-forming cells.
In a subsequent study, Gerecht-Nir et al35 analyzed the changes in gene expression using DNA microarrays at weekly intervals over 21 days of hEB development. The resulting data confirmed the trends described in the above-mentioned research. Two main clusters of gene expression were identified: one consisting of down-regulated genes and the other consisting of up-regulated genes. The former contained genes involved in the undifferentiated state of hESCs, their pluripotency, and self-renewal capabilities, such as POU5 (OCT4), which is a transcription factor that is expressed in ESCs and down-regulated upon differentiation, and NANOG, which is a homeobox transcription factor that is involved in pluripotency and suppression of differentiation. The up-regulated group, as expected, expressed key markers of ECs and hematopoietic progenitors, such as PECAM1, vascular EC adhesion molecule-1 (VCAM1), SCL, as well as key cytokines, VEGF, Ang1, and TGFβ1, that are known to participate in the early stages of mammalian vascular development.
Despite the differences in growth conditions, the results of studies exploring the kinetics of molecular expression during hEB differentiation support trends similar to those that we described in our initial study (Figure 5). A review of the existing information (summarized in Figure 5) reveals that undifferentiated hESCs have low levels of expression of CD34 and GATA3 and high levels of expression of Flk1, Tie2, CD117, CD133, CDX4, EKLF, and PU.1. Between the second and third days of EB differentiation, the cells began to express PECAM1, SCL, GATA1, and GATA2. On the sixth day of EB differentiation, GATA3, SCL, PU.1, and EKLF reached their peak expression. In the same time scale, VE-cad–expressing cells appeared. Between the ninth and tenth days, expression of CDX4, GATA1, and GATA2 reached their peaks. This peak differs from our finding that the peak expression of GATA2 occurred on the eighteenth day. We suggest that this difference arose because of the use of a different medium for cell culture. This peak concurred with low expression of CD45 in some PECAM1 cells. The expressions of PECAM1, VE-cad, and CD34 reached their greatest levels between the twelfth and fifteenth days. Over the course of 20 days of differentiation of EBs, the expression of Tie2 and CD133 was constant, whereas the expression of Flk1 increased slightly. Accordingly, it is suggested that the formation of EBs and subsequent course of their vascular differentiation in a 3D environment closely resembles that of early in vivo vascular development.
Summary of endothelial markers and the kinetics of their expression during the differentiation of hEBs investigated/monitored over 20 days. Lines with arrows indicate the beginning and period of expression. Yellow triangles indicate the peak in expression (the time area of the peak is indicated by a thin line). Gene colors reflect the level of expression in undifferentiated hESCs: blue, low; red, high; and white, none.
Summary of endothelial markers and the kinetics of their expression during the differentiation of hEBs investigated/monitored over 20 days. Lines with arrows indicate the beginning and period of expression. Yellow triangles indicate the peak in expression (the time area of the peak is indicated by a thin line). Gene colors reflect the level of expression in undifferentiated hESCs: blue, low; red, high; and white, none.
Indeed, the endothelial progenitor markers, such as Flk1, SCL, and CD133, as well as other endothelial markers, such as CD31, CD34, and VWF, were expressed in a 4-week-old human embryo. The expression of VEGF, Ang1, Ang2, and Tie2 was also detected at this embryonic stage. However, the expression of sprouting and remodeling genes, such as VE-cad and VCAM1, was detectable only at the eighth week of embryonic development.40 It should be noted that in this study only 4-week-, 7-week-, and 8-week-old human embryos were examined. Interestingly, similar trends to those found during the differentiation of hEBs in a 3D environment were also observed in the course of differentiation of hESCs in a 2D environment in which OP9 mouse stromal cells were used to induce differentiation.41 The description of the kinetics of molecular expression emphasizes differences between mice and humans with respect to species. These differences pertain not merely to morphology, population doubling time, and growth factor requirements but also occur at the molecular level of differentiation of ESCs. Several genes important for regulating hematopoietic precursor development in mouse ESCs and embryos, such as LMO2, AML1, C-MYB, Flk1, FLT-1, and Tie2, are abundantly expressed at the mRNA level in both undifferentiated hESCs and in hEBs at all time points. These data suggest that there might be major differences between mouse and human embryonic regulation of differentiation. The expressions of Flk1 and Tie2 were increased only moderately during hEB differentiation but were relatively high at all times. This is distinct from the differentiation in murine EBs, in which these genes are not expressed in ESs or are expressed in very low levels and disappear on the first day of EB formation, to reappear only on or after the third day.29,34
It has been claimed that ESCs of nonhuman primates are more similar to hESCs than mouse ESCs in terms of their morphology, growth characteristics, and developmental potential.44 Unlike mouse ESCs, undifferentiated ESCs from cynomolgus monkeys already express Flk1, a feature similar to hESCs. However, Flk1 expression in these cells is not continuous and its re-expression on the eighth day of differentiation can be analogous to expression in 4-day-old differentiated murine ESCs.43 Rhesus monkey ESCs, grown in EGM2 medium containing VEGF, bFGF, EGF, and IGF for 5 to 10 days, exhibited endothelial-like morphology and UEA-1 binding and expressed integrin αvβ3, CD146, VWF, CD34, and Flk1. This picture is similar to HUVECs, but these cells did not express PECAM1 or VE-cad. Seeding these cells on matrigel and examining their in vivo behavior in SCID mice revealed the recruitment of new vessels into the matrigel and active vessel formation.39 The study of the biology of murine and primate ESCs contributes significantly to the research on hESCs. However, the differences that exist between these cell types need to be considered.
The collective results of the studies of Wang et al33 and Zambidis et al34 on the differentiation of EBs to endothelial-like cells clearly support the existence of a common origin for endothelial and hematopoietic cells. Investigating the hematopoietic ability of the progenitors isolated from 13-day-old EBs will lend further insight. Formation of blood islands in the extraembryonic yolk sac marks the onset of vasculogenesis and hematopoiesis in the developing mouse embryo.14,45 The concurrent development of blood vessels and ECs and their close proximity to the islands in the yolk sac blood has led to the hypothesis that they originate from a common precursor. This precursor is thought to be the hemangioblast and represents the first committed hematopoietic cell that develops from the uncommitted mesoderm.46 Indeed, endothelial and hematopoietic progenitors that share common markers are affected by common signals and influence each other.47
The endothelial progenitors isolated from hESCs have various phenotypes because of varying derivation protocols and the supplementation of growth factors and, more importantly, were isolated at different stages of development (Table 1). To date, no markers capable of distinguishing between these different endothelial subpopulations have been identified. Therefore, it is of the utmost importance that a universal assay to assess their vascularization potential be developed. Such an assay could be a 3D culture system and serve as a basic tool for quantifying and evaluating the differences between the diverse progenitors. This culture system could also reveal whether progenitors obtained at different developmental stages lead to variations in the extent of vascularization and shed light on vasculogenic and angiogenic mechanisms.
Endothelial progenitors were isolated from the bone marrow, spleen, cord blood, and circulating cells in peripheral blood of adult humans.11,48-51 Circulating endothelial progenitors constitutively express endothelial progenitor markers such as CD34 or Flk1. Upon the onset of endothelial differentiation, these cells express endothelial lineage–specific markers such as VE-cad or E-selectin.52 Isolated endothelial progenitors have been shown to home to the sites of new blood vessels and contribute to the functional vasculature. This has potential therapeutic application such as cell transplantation for repairing ischemic tissue and the tissue engineering of vascular grafts.49-51,53 However, ES-derived ECs have the advantage of being present in virtually unlimited amounts. Hence, it is very important to conduct further studies to examine and compare the vascular efficiency of embryonic endothelial progenitors with adult endothelial progenitors. Furthermore, recent evidence concerning the universality of endothelial signaling in organogenesis highlights the importance of studying the endothelial progenitors derived from different developmental stages in proximity to various internal tissue organs using a 3D culture assay. Deciphering endothelial cell signaling will undoubtedly lead to a better understanding of stem cell differentiation, which itself may require inducing factors from ECs. It may also provide clues to the mechanisms by which cancer progresses, given that vascular development is a major influence on tumor growth during tumorigenesis and may shed light on developmental issues in invertebrate biology.
Summary
This review emphasizes the advantages of hESCs as a cell source for ECs and their enormous therapeutic potential in cell therapy and tissue engineering. The collected evidence represents only the tip of the iceberg with respect to a complete understanding of the vascularization processes of hESCs. Hence, realization of the potential of hESC-derived ECs calls for further study in a number of directions. First, the molecular sequence of hESC differentiation toward endothelial lineage requires further elucidation. In addition, the collective knowledge on hematopoiesis should be used as a platform to address further basic questions regarding the differentiation routing of hESC-derived hematopoietic progenitor cells into ECs. These questions include the following. What is the kinetics of differentiation? Can this differentiation be controlled and induced in vitro? Can differentiated hESC-derived ECs be converted to hematopoietic cells? Further study might also contribute to the resolution of immunologic concerns that currently inhibit the clinical use of hESC-derived ECs. The vasculogenic potential of hESC-derived ECs should be investigated further with respect to their ability to vascularize various ischemic or engineered tissues both in vitro and in vivo. This information would be extremely important for the tissue engineering of complex internal organs and hence for alleviating various deficiencies in current procedures for transplanting such organs. Finally, understanding the global role of paracrine signaling of endothelial cells during embryonic development may open up new avenues for directing stem cell differentiation and tissue organization.
Authorship
Contribution: S.L., J.Z., Y.B., and R.L. wrote the review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shulamit Levenberg, Department of Biomedical Engineering, Technion–Israel Institute of Technology, Haifa, 32000, Israel; e-mail: shulamit@bm.technion.ac.il.
References
National Institutes of Health





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal