Abstract
The phosphoinositide 3-kinase (PI3K/Akt) pathway is activated in acute myelogenous leukemia (AML) and is promising for targeted inhibition. Ninety-two patients with primary AML were analyzed for PI3K/Akt constitutive activation. Fifty percent of the patients presented with constitutive PI3K activation (PI3K +). No difference was observed between PI3K + and PI3K − groups concerning age, sex, white blood cell count, lactate dehydrogenase (LDH) level, bone marrow blast cells, French-American-British (FAB) classification, cytogenetics, RAS or nucleophosmin (NPM) mutations. Slightly more FLT3-ITD was detected in the PI3K − group (P = .048). The complete remission rate was similar between the 2 groups. With a median follow-up of 26 months, we observed for PI3K + and PI3K − patients, respectively, 56% and 33% overall survival (P = .001) and 72% and 41% relapse-free survival (P = .001). Constitutive PI3K/Akt activity is a favorable prognosis factor in AML, even after adjustment for FLT3-ITD, and may confer a particular sensitivity to chemotherapy. A better understanding of the downstream effectors of the PI3K/Akt pathway is needed before targeting in AML.
Introduction
Several recent articles have highlighted that the class IA phosphoinositide 3-kinase/serine-threonine kinase (PI3K)/Akt pathway is activated in acute myelogenous leukemia (AML), both in blast cells and in the immature CD34 +, CD38Low, and CD123 + population.1-4 Most PI3K activity in AML is caused by the p110δ isoform of PI3K, the activity of which can be specifically inhibited by IC87114.5,6 The mechanisms leading to PI3K activation are unclear and no activating mutation in the PI3KCD gene coding this isoform has been identified.7
Two studies have suggested that the prognosis of AML patients with an upregulated PI3K/Akt pathway is worst, suggesting that this pathway could constitute a therapeutic target.2,8 To ascertain this hypothesis, we prospectively studied constitutive PI3K/Akt/FOXO3A activation in 92 de novo AML patients.
Patients, materials, and methods
Patients
Ninety-two patients with de novo AML treated in the AML-2001 trial from the Groupe Ouest-Est des Leucémies et Autres Maladies du Sang (GOELAMS) were included, after approval by the GOELAMS Institutional Review Board and receipt of signed informed consent in accordance with the Declaration of Helsinki. The patients received cytosine-arabinoside (200 mg/m2, days 1-7) and daunorubicin (60 mg/m2, days 1-3) or idarubicin (8 mg/m2, days 1-5), and a second course (daunorubicin 35 mg/m2, days 17-18 or idarubicin 8 mg/m2, days 17-18, and cytosine-arabinoside 1 g/m2 every 12 hours days 17-20) if bone marrow blasts were more than 5% at day 15. All patients in complete remission received low-dose consolidation with daunorubicin (60 mg/m2, days 1-2) or idarubicin (12 mg/m2, days 1-2), and cytosine-arabinoside (100 mg/m2, days 1-7). Patients with HLA-identical siblings underwent allogenic stem cell transplant (up front until 50 years old; after a consolidation and with a reduced intensity conditioning after 51 years old). Other patients received one consolidation (idarubicin 12 mg/m2 or daunorubicin 60 mg/m2, days 1-2 and cytosine-arabinoside 3g/m2 every 12 hours days 1-4), followed by autologous stem cell transplantation or high-dose chemotherapy in favorable-risk karyotypes.
Cell processing and PI3K/Akt activity detection
Fresh blast cells from the bone marrow were studied at diagnosis for PI3K/Akt activation and FOXO3A (a Forkhead transcription factor) phosphorylation. Highly infiltrated samples (75 [81%] of 92) were studied by Western blot, and others (17 [19%] of 92) by flow cytometry. We previously reported that both methods gave identical results when tested on the same samples.4 Blast cells were starved for 4 hours in cytokine and serum-free medium before testing for constitutive or FLT3-ligand (a growth factor for hematopoietic progenitors)–stimulated Akt and FOXO3A phosphorylation by Western blot analysis.
Antibodies used were anti-p-Akt (Ser 473 and Thr 308, Cell Signaling Technologies, Danvers, MA), anti-pFOXO3A (Thr 32; Upstate Biotechnology, Charlottesville, VA), anti-beta-actin (Sigma, St Louis, MO), and anti-Akt1/2 (Cell Signaling Technologies).
Dual color flow cytometry of Akt Ser 473 phosphorylation in the CD45low leukemic population was performed as described.4
FLT3, N-RAS, K-RAS, and NPM gene mutational status
Statistical analysis
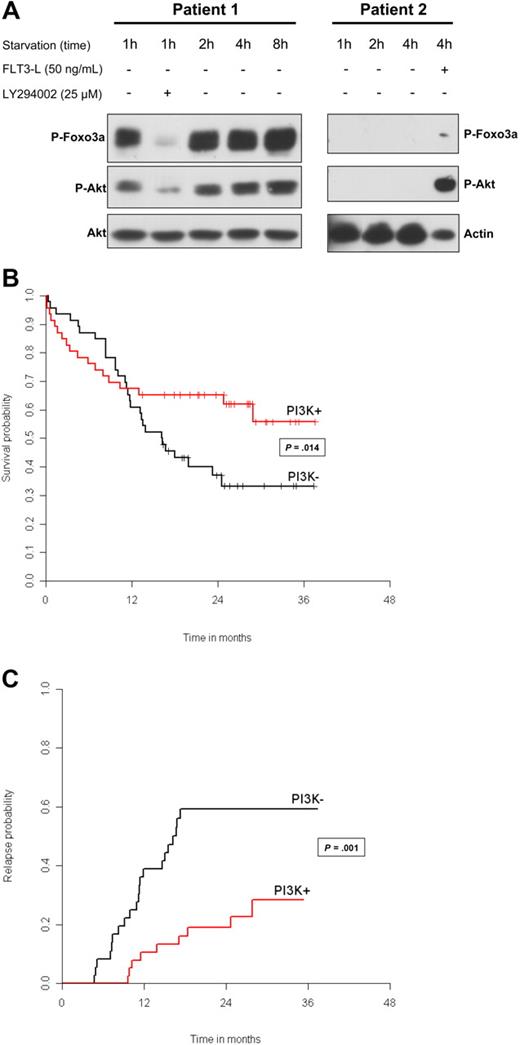
Patients with AML can be separated into 2 groups: with constitutive activation of PI3K (PI3K + group) and without PI3K activation (PI3K − group). (A) Blast cells from patients 1 and 2 were analyzed for PI3K/Akt activation at the indicated times, after starvation in serum- and cytokine-free medium, to determine constitutive and sustained activation of this signaling pathway. Proteins from 106 cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blot using antibodies directed against p-Akt (Ser 473) and pFOXO3A (Thr 32). To ensure equal loading, blots were reprobed with anti-actin or anti-Akt antibodies. LY294002 was used at 25 μM for 30 minutes to inhibit PI3K/Akt. The functionality of the PI3K/Akt pathway was tested in patient 2, after 15-minute stimulation with FLT3 ligand at 50 ng/mL. Vertical lines were drawn to indicate cuts made to put together nonadjacent regions from the same gel. (B) Kaplan-Meier curves for overall survival according to the PI3K status. Classical log-rank test was used to compare survival distribution between subgroups. We tested the proportional hazard hypothesis using a test proposed by Grambsch et al.14 To investigate for a mixed effect (early and late effect) of PI3K/Akt activation, we tested the null hypothesis of no short-term and no long-term effect, using the model proposed by Broët et al.15 This latter test allows for testing 3 null hypotheses: (1) no long-term and short-term effect; (2) no short-term effect; and (3) no long-term effect (whatever a short-term effect is). Here, the long-term effect represents the effect on the proportion of patients alive after 30 months. (C) Cumulative incidence curves for relapse according to the PI3K status. Relapse probabilities were obtained from crude cumulative incidence estimates, treating death before relapse and relapse as competing risk events. Crude cumulative incidence curves for relapse were compared between subgroups using the Gray test.16
Patients with AML can be separated into 2 groups: with constitutive activation of PI3K (PI3K + group) and without PI3K activation (PI3K − group). (A) Blast cells from patients 1 and 2 were analyzed for PI3K/Akt activation at the indicated times, after starvation in serum- and cytokine-free medium, to determine constitutive and sustained activation of this signaling pathway. Proteins from 106 cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blot using antibodies directed against p-Akt (Ser 473) and pFOXO3A (Thr 32). To ensure equal loading, blots were reprobed with anti-actin or anti-Akt antibodies. LY294002 was used at 25 μM for 30 minutes to inhibit PI3K/Akt. The functionality of the PI3K/Akt pathway was tested in patient 2, after 15-minute stimulation with FLT3 ligand at 50 ng/mL. Vertical lines were drawn to indicate cuts made to put together nonadjacent regions from the same gel. (B) Kaplan-Meier curves for overall survival according to the PI3K status. Classical log-rank test was used to compare survival distribution between subgroups. We tested the proportional hazard hypothesis using a test proposed by Grambsch et al.14 To investigate for a mixed effect (early and late effect) of PI3K/Akt activation, we tested the null hypothesis of no short-term and no long-term effect, using the model proposed by Broët et al.15 This latter test allows for testing 3 null hypotheses: (1) no long-term and short-term effect; (2) no short-term effect; and (3) no long-term effect (whatever a short-term effect is). Here, the long-term effect represents the effect on the proportion of patients alive after 30 months. (C) Cumulative incidence curves for relapse according to the PI3K status. Relapse probabilities were obtained from crude cumulative incidence estimates, treating death before relapse and relapse as competing risk events. Crude cumulative incidence curves for relapse were compared between subgroups using the Gray test.16
Results and discussion
Constitutive activation of PI3K is detected in 50% of patients
We first determined that a 4-hour starvation clearly divided AML patients into 2 groups: one with PI3K constitutive activation (PI3K +) and the other without PI3K activation (PI3K −; Figure 1A). In patient 1 (PI3K + ), Akt (Ser 473) and FOXO3A (Thr 32) phosphorylations were detected with the same intensity during 8 hours of starvation. LY294002 inhibited both phosphorylations. Patient 2 is representative of the PI3K − group and had no detectable Akt or FOXO3a phosphorylation. The functionality of the PI3K/Akt pathway was detected by stimulating the cells with FLT3-ligand as shown in patient 2. All PI3K − samples presented an activation of the pathway after cytokine stimulation. In 15 samples tested, a perfect correlation between Akt phosphorylation on Ser 473 and on Thr 308 residues was found (data not shown). Foxo3A phosphorylation was only detected in PI3K + patients. The PI3K + group represented 46 (50%) of 92 patients.
Association between PI3K status and clinical and biologic data
Patient characteristics according to their PI3K status are reported in Table 1. There was no difference between the 2 groups for age, sex, FAB subtypes, cytogenetic subgroups, myelodysplastic syndrome (MDS) features, white blood cell counts, LDH levels, bone marrow blast percentage, or tumor burden. The rates of N-RAS/K-RAS and NPM mutations were similar between PI3K + and PI3K − patients. FLT3-ITD were slightly more frequent in the PI3K − group (P = .048; Table 1).
Characteristics of the 92 patients according to their PI3K/Akt status
| . | TotalN=92 . | PI3K + N=46 (50%) . | PI3K − N=46 (50%) . | P . |
|---|---|---|---|---|
| Mean age, y (SD) | 44 (12) | 45 (12) | 44 (12) | 0.82 |
| Male, N (%) | 41 (45) | 19 (42) | 22 (48) | 0.59 |
| Median WBC count, × 109/L (range) | 12 (0.4-252) | 8.6 (0.7-140) | 16.9 (0.4-252) | 0.12 |
| LDH over 400 UI/L, N (%) | 58 (81) | 31 (82) | 27 (79) | 0.82 |
| Tumor burden, N/total (%) | 31/77 (40) | 16/39 (41) | 15/38 (40) | 0.89 |
| % BM blasts, median (range) | 51 (0-100) | 70 (22-98) | 58 (0-100) | 0.13 |
| FAB, N (%) | ||||
| M0 | 9 (10) | 4 (9) | 5 (11) | — |
| M1 | 20 (22) | 9 (20) | 11 (24) | — |
| M2 | 32 (35) | 17 (37) | 15 (33) | — |
| M4 | 19 (21) | 8 (17) | 11 (25) | — |
| M5 | 11 (12) | 8 (17) | 3 (7) | 0.55 |
| Cytogenetic subgroup, N (%)* | ||||
| Unfavorable | 20 (23) | 7 (16) | 13 (30) | — |
| Intermediate | 54 (61) | 29 (66) | 25 (57) | — |
| Favorable | 14 (16) | 8 (18) | 6 (13) | 0.30 |
| MDS features, N (%) | 17 (19) | 9 (20) | 8 (17) | 0.79 |
| Mutations, N (%) | ||||
| FLT3-ITDpos | 17/79 (22) | 5/40 (13) | 12/39 (31) | 0.048 |
| RAS (N-RAS and K-RAS) | 9/60 (15) | 6/32 (19) | 3/28 (11) | 0.48 |
| NPM1-mut | 17/70 (24) | 8/33 (24) | 9/37 (24) | 0.99 |
| NPM1-wt/FLT3-ITDneg | 45/69 (64) | 23/33 (70) | 21/36 (58) | — |
| NPM1-mut/FLT3-ITDneg | 10/69 (13) | 5/33 (15) | 4/36 (11) | — |
| NPM1-wt/FLT3-ITDpos | 8/69 (12) | 2/33 (6) | 6/36 (17) | — |
| NPM1-mut/FLT3-ITDpos | 8/69 (12) | 3/33 (9) | 5/36 (14) | 0.51 |
| Outcome: CR, N (%) | 74 (80) | 38 (83) | 36 (78) | 0.59 |
| . | TotalN=92 . | PI3K + N=46 (50%) . | PI3K − N=46 (50%) . | P . |
|---|---|---|---|---|
| Mean age, y (SD) | 44 (12) | 45 (12) | 44 (12) | 0.82 |
| Male, N (%) | 41 (45) | 19 (42) | 22 (48) | 0.59 |
| Median WBC count, × 109/L (range) | 12 (0.4-252) | 8.6 (0.7-140) | 16.9 (0.4-252) | 0.12 |
| LDH over 400 UI/L, N (%) | 58 (81) | 31 (82) | 27 (79) | 0.82 |
| Tumor burden, N/total (%) | 31/77 (40) | 16/39 (41) | 15/38 (40) | 0.89 |
| % BM blasts, median (range) | 51 (0-100) | 70 (22-98) | 58 (0-100) | 0.13 |
| FAB, N (%) | ||||
| M0 | 9 (10) | 4 (9) | 5 (11) | — |
| M1 | 20 (22) | 9 (20) | 11 (24) | — |
| M2 | 32 (35) | 17 (37) | 15 (33) | — |
| M4 | 19 (21) | 8 (17) | 11 (25) | — |
| M5 | 11 (12) | 8 (17) | 3 (7) | 0.55 |
| Cytogenetic subgroup, N (%)* | ||||
| Unfavorable | 20 (23) | 7 (16) | 13 (30) | — |
| Intermediate | 54 (61) | 29 (66) | 25 (57) | — |
| Favorable | 14 (16) | 8 (18) | 6 (13) | 0.30 |
| MDS features, N (%) | 17 (19) | 9 (20) | 8 (17) | 0.79 |
| Mutations, N (%) | ||||
| FLT3-ITDpos | 17/79 (22) | 5/40 (13) | 12/39 (31) | 0.048 |
| RAS (N-RAS and K-RAS) | 9/60 (15) | 6/32 (19) | 3/28 (11) | 0.48 |
| NPM1-mut | 17/70 (24) | 8/33 (24) | 9/37 (24) | 0.99 |
| NPM1-wt/FLT3-ITDneg | 45/69 (64) | 23/33 (70) | 21/36 (58) | — |
| NPM1-mut/FLT3-ITDneg | 10/69 (13) | 5/33 (15) | 4/36 (11) | — |
| NPM1-wt/FLT3-ITDpos | 8/69 (12) | 2/33 (6) | 6/36 (17) | — |
| NPM1-mut/FLT3-ITDpos | 8/69 (12) | 3/33 (9) | 5/36 (14) | 0.51 |
| Outcome: CR, N (%) | 74 (80) | 38 (83) | 36 (78) | 0.59 |
Differences between groups were tested for categorical variables using the χ2 test or the Fisher exact test, when appropriate. Differences between groups for continuous variables were tested using the Student test or the Wilcoxon rank-sum test, when appropriate.
CR indicates complete remission; FAB, French-American-British classification; FLT3-ITD, Internal Tandem Duplication of the FLT3 receptor; WBC, white blood cell; SD, standard deviation; and —, not applicable.
Cytogenetic subgroups: karyotype risk groups were defined according to the SWOG criteria.
Overall survival and relapse-free survival are better in patients with constitutive PI3K
Complete remission was obtained in 74 (80%) of 92 patients with no difference between the 2 groups (P = .60). With a median follow-up of 26 months (range, 3 days to 37 months), 48 patients died. Overall survival was 45% (95% confidence interval [CI], 34%–58%) for the entire population. Figure 1B shows a clear nonproportional effect between the 2 groups, confirmed by the test by Grambsch et al14 (P = .002). A 2-phase effect is present (Figure 1B), with 2 hazard rates crossing each other at approximately 1 year. When testing for short-term and long-term effects, the test is highly significant (P = .014), with PI3K activation being associated with a better long-term survival. For an actually unknown reason, slightly more patients in the PI3K + group died from infection before 12 months without AML relapse (short-term effect, P = .027). Nevertheless, the overall survival rate at 30 months was 56% (95% CI, 41%-76%) in the PI3K + group and 33% (95% CI, 21%-52%) in the PI3K − group.
Thirty patients relapsed. Crude cumulative incidence curves for relapse are presented in Figure 1C. Relapse free survival was 72% (95% CI, 54%-89%) in the PI3K + group and 41% (95% CI, 24%-58%) in the PI3K − group (P = .001).
Because there was more FLT3-ITD in the PI3K − patients, and a nonsignificant imbalance in term of unfavorable cytogenetics (PI3K + 16% and PI3K − 30%), explorative multivariate models were performed. The PI3K activation still played a significant prognostic role on overall survival and relapse-free survival after adjustment for FLT3-ITD (P = .01 and P = .007, respectively) and cytogenetics (P = .014 and .008, respectively).
Two studies have correlated Akt phosphorylation to an adverse overall survival in AML.2,8 In their study, Kornblau et al8 did not starve blast cells before testing for Akt phosphorylation, and they did not test for constitutive PI3 activation. Accordingly, they observed that 91% of blast samples presented Akt phosphorylation, whereas we observed constitutive activation in 50% of AML samples only. Moreover, the populations studied were not similar, because all patients in our study had de novo AML, whereas 32% in Kornblau's study had AML secondary to MDS or chemotherapy.8 In their study, Min et al2 observed that Akt was undetectable in pAkt-negative patients. Clearly, their samples are different from those tested in our study in which pAkt could be induced in all PI3K − samples stimulated by FLT3-ligand.
One hypothesis for the lower relapse rate of PI3K + patients may be that PI3K drives immature leukemic populations in the S phase, increasing their susceptibility to cell cycle-dependant chemotherapies. Overall, these results suggest that precise identification of Akt targets is needed before targeting this pathway. As recently reported in cancer models, inhibiting the Akt pathway may lead to unexpected dark side effects.12,13
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all participating investigators from the GOELAMS (Groupe Ouest-Est d'Etude des Leucémies et Autres Maladies du Sang).
This work was supported by l'Association Laurette Fugain and by grants from the Association pour la Recherche contre le Cancer (ARC).
Authorship
Contribution: J.T., V.B., and N.C. performed research. C.E. and P.B. performed the statistical analysis. S.P., B.L., N.I., F.W., and F.D. provided patient samples. P.C.L., V.H., and O.B. performed molecular analysis. P.M., C.L., and D.B. designed research and wrote the paper.
J.T. and C.E. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Didier Bouscary, Département d'Hématologie, Institut Cochin, 27 rue du Faubourg Saint-Jacques, F-75014 Paris, France; e-mail: bouscary@cochin.inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal