Abstract
Chronic lymphocytic leukemia (CLL) is commonly defined as a disease of failed apoptosis of B cells and remains an incurable disease. The mechanism of resistance to apoptosis in CLL is complex and influenced by numerous factors, including nuclear factor κB (NFκB)-mediated expression of antiapoptotic molecules. Recent evidence indicates that glycogen synthase kinase-3β (GSK-3β) positively regulates NFκB-mediated gene transcription and cell survival. Using malignant B cells collected from patients with CLL, we find that both GSK-3β and NFκB accumulate in the nucleus of CLL B cells, and pharmacologic inhibition of GSK-3 results in decreased expression of two NFκB target genes Bcl-2 and XIAP and a subsequent increase in CLL B-cell apoptosis ex vivo. Furthermore, we observed that inhibition of GSK-3 leads to a decrease in NFκB-mediated gene transcription but does not affect the nuclear accumulation of NFκB in CLL B cells. Last, using chromatin immunoprecipitation, we show that GSK-3 inhibition abrogates NFκB binding to its target gene promoters (XIAP, Bcl-2), in part through epigenetic modification of histones. Our results establish that inhibition of GSK-3 abrogates NFκB binding to its target gene promoters through an epigenetic mechanism, enhances apoptosis in CLL B cells ex vivo and identifies GSK-3 as a potential therapeutic target in the treatment of CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common human hematologic malignancy and, despite substantial scientific efforts, remains an incurable disease. Whereas some patients with CLL have a slow course of disease, most face inevitable progression and have fatal outcomes.1,2 CLL is characterized by the accumulation of largely nonproliferating leukemic B cells that are resistant to apoptosis.3 An increasing body of evidence suggests that the apoptotic block of CLL B cells is linked to constitutively activated signaling pathways, including an active NFκB pathway.4,5 In fact, CLL B cells exhibit high constitutive levels of NFκB activity compared with nonmalignant human B cells.5 Because NFκB regulates the expression of antiapoptotic molecules including Bcl-2 and XIAP, a sustained activation of NFκB pathway is critical for the survival of CLL B cells.6 Thus, identification of the altered pathways regulating NFκB activity in CLL B cells may lead to the discovery of novel therapeutic targets to antagonize NFκB activation and induce apoptosis in these leukemic B cells.
Glycogen synthase kinase (GSK)-3, a serine/threonine protein kinase, was first described as a component of the metabolic pathway for glycogen synthase regulation.7 Two homologous mammalian GSK-3 isoforms are encoded by different genes, GSK-3α and GSK-3β.8 It has been shown that, similar to the disruption of the NFκB p65 or IκB kinase β (IKKβ) genes, ablation of the murine GSK-3β gene is lethal to embryos as a result of TNFα-induced hepatocyte apoptosis and massive liver degeneration.9-11 These findings suggest a role for GSK-3β (but not GSK-3α) in the regulation of NFκB activation. The early steps leading to NFκB activation after tumor necrosis factor α (TNFα) treatment (degradation of IκBα and translocation of NFκB to the nucleus) were unaffected in GSK-3β–deficient mouse embryonic fibroblasts (MEFs), indicating that NFκB is regulated by GSK-3β at the level of the transcriptional complex.11 Consistent with this idea, we have recently shown that GSK-3β participates in NFκB-mediated pancreatic cancer cell survival and proliferation by regulating NFκB activity at a point downstream of the activation of the IKK complex.12 Taken together, these data rule out an effect of GSK-3β on the cascade of proteins that culminates in phosphorylation of IκBα and its degradation and suggest that GSK-3β may regulate the nuclear activity of NFκB p65/p50.
Although CLL B cells exhibit high constitutive levels of NFκB activity,5 the localization of GSK-3β in human CLL B cells and whether GSK-3β affects NFκB activity are unknown. In the present study, we find that GSK-3β accumulates in the nuclei of human CLL B cells. We demonstrate that pharmacologic inhibition of GSK-3 leads to depletion of its nuclear pool, suppression of NFκB transcriptional activity, decreased expression of antiapoptotic proteins (XIAP, Bcl-2), and enhanced apoptosis in CLL B cells. From a mechanistic perspective, we provide evidence that inhibition of GSK-3β affects histone modification at two NFκB target genes (XIAP, Bcl-2), resulting in its transcriptional repression and decreased survival of human CLL B cells.
Patients, materials, and methods
Patient selection and purification of lymphocytes
Blood was obtained from healthy donors or patients with CLL who had provided written informed consent. The Mayo Clinic Institutional Review Board, in accordance with the Declaration of Helsinki, approved the laboratory study. All patients with CLL had a confirmed diagnosis using the National Cancer Institute working group 1996 definition.13 Patients in this cohort were from all Rai stages and had not been treated for at least 6 weeks before blood processing for this study. Peripheral blood mononuclear cells (PBMC) were separated from heparinized venous blood by density gradient centrifugation. To remove adherent cells, PBMC were suspended in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and incubated in plastic dishes at 37°C for 1 hour before collection of nonadherent cells. To obtain at least 95% purity of CLL B cells, nonadherent cells were depleted of T cells by incubation with sheep erythrocytes. For some experiments, we purified CLL B cells from magnetic bead columns. In brief, highly purified CD19 + B cells (> 95%) were obtained from PBMC by standard negative selection using a cocktail of subset-specific antibodies conjugated with magnetic beads (Miltenyi Biotech, Auburn, CA). These purified CLL B cells were then either used immediately for the laboratory studies described below or cryopreserved in RPMI 1640 medium, 20% fetal calf serum (FCS), and 10% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen until use.
Reagents and cells
The GSK-3 inhibitor AR-A01441814 was obtained from Calbiochem (La Jolla, CA). Z-VAD-FMK (broad spectrum caspase inhibitor) was from BIOMOL Research Laboratories (Plymouth Meeting, PA). All other chemicals were obtained from Sigma (St Louis, MO). MEC1, a cell line developed from a patient with CLL15 was a kind gift from Dr. Frederico Caligaris-Cappio and maintained in RPMI 1640 medium supplemented with 10% FCS, l-glutamine, and penicillin/streptomycin. Primary CLL B cells and human splenic B cells were sorted by CD19+ (Miltenyi Biotec, Auburn, CA) and were then cultured in serum-free AIM-V (Gibco BRL) and RPMI (BIOMOL Research Laboratories) supplemented with 10% FCS, respectively. Cells were maintained at 37°C in an atmosphere containing 95% air/5% CO2 (v/v).
Immunoblot analysis and antibodies
For immunoblots, cells were lysed as described previously.12 Nuclear/cytosolic fractionation was done by the Dignam method.16 Protein sample concentration was quantified and equal amount (50 μg of whole, nuclear, or cytosolic protein extract) of protein was loaded in each well of SDS-polyacrylamide gel. Cell or tissue extracts were separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE), transferred to polyvinylidene diflouride membrane (PVDF), and probed as indicated in the figure legends. Antibodies for immunoblot analysis were obtained from the following suppliers: GSK-3β, Bcl-2, XIAP, phospho-p65 (Ser536), poly(ADP-ribose) polymerase (PARP), and ORC2 from BD Pharmingen (San Diego, CA); NFκB p65 from Santa Cruz Biotechnology (Santa Cruz, CA); Cu/Zn superoxide dismutase (SOD) from Stressgen (Victoria, BC, Canada); and β-actin from Novus (Littleton, CO). Bound antibodies were detected as described previously.12 Western blot band intensities were quantified using Image J software (http://rsb.info.nih.gov/ij/; National Institutes of Health, Bethesda, MD).
Immunocytochemical staining
CLL B cells and normal human B cells on glass coverslips were subjected to immunofluorescence staining17 to detect GSK-3β expression with the same primary antibody (diluted 1:1000) used for immunoblotting. After a wash in phosphate-buffered saline, cells were incubated with goat anti-mouse-tetramethylrhodamine isothiocyanate (TRITC) secondary antibody (Invitrogen, Carlsbad, CA). Nuclei were counterstained with Hoechst 33342. Images were collected using the Axio Vision 4.0 program on a Zeiss Axiovert 200 confocal microscope (Carl Zeiss Inc, Hallbergnoos, Germany) equipped with 100×/1.4 oil DiC objective. SlowFade mounting medium (Invitrogen) was used, and a Zeiss AxioCam HRC camera (Zeiss).
Apoptosis assay
Primary CLL B cells (1 × 106 cells) were treated with either vehicle (DMSO) or AR-A014418. After the treatment, cells were rinsed with 1 × PBS and analyzed for apoptosis/cell death levels by a fluorescein isothiocyanate (FITC)-labeled Annexin-V/propidium iodide assay. These cells were directly analyzed in a FACScan (BD Biosciences, San Jose, CA) with a sample size of at least 10 000 cells gated based on forward and side scatter. Storing and processing of data were accomplished using FACScan software as described previously.18
Reverse transcription-polymerase chain reaction
Expression of mRNA of XIAP and Bcl-2 was determined by reverse transcription-polymerase chain reaction (RT-PCR). cDNA was generated from 2 μg of total RNA by reverse transcription using a reverse transcription system kit (Promega, Madison, WI). To quantify the expression in each cDNA sample, the target was amplified by PCR in parallel with an internal control, β-2-microglobulin. Primer pairs that detect XIAP, Bcl-2, and β-2-microglobulin were used as described previously.12,19 Amplification was performed by using a thermal cycling program with various numbers of cycles for XIAP (25 cycles) and Bcl-2 (22 cycles) in which each cycle consisted of 94°C for 45 seconds, 52°C (XIAP) or 58°C (Bcl-2) for 45 seconds, and 72°C for 1 minute. Amplification for β-2-microglobulin (22 cycles) was performed following a cycling program, in which each cycle consisted of 94°C for 45 seconds, 55°C for 45 seconds, and 72°C for 1 minute. All PCR products were subjected to electrophoresis through a native 8% polyacrylamide gel and were visualized by staining with ethidium bromide.
Chromatin immunoprecipitation assay
MEC1 cells were treated with 25 μmol/L of AR-A014418 or control DMSO. The broad-spectrum caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (Z-VAD-FMK) was used in some experiments. At 12 hours after treatment, cells were cross-linked with formaldehyde for 15 minutes at 25°C, harvested in SDS lysis buffer (Upstate Biotechnology, Lake Placid, NY), and sheared to fragment DNA (500–1000 base pairs [bp]). Samples were then immunoprecipitated using an agarose-conjugated p65, dimethyl-histone H4 (Lys20), dimethyl-histone H3 (Lys9), trimethyl-histone H3 (Lys27) antibody, or mouse control IgG at 4°C overnight. Antibodies for chromatin immunoprecipitation (ChIP) analysis were obtained from the following suppliers: dimethyl-H3-K9, trimethyl-H3-K27, and dimethyl-H4-K20 from Upstate Biotechnology (Lake Placid, NY); NFκB p65 from Santa Cruz Biotechnology. After immunoprecipitation, samples were washed and eluted using the Chromatin Immunoprecipitation Kit (Upstate Biotechnology) according to the manufacturer's instructions. Cross-links were removed at 65°C for 6 hours, and immunoprecipitated DNA was purified using phenol/chloroform extraction and ethanol precipitation. Two hundred twenty base pairs of the Bcl-2 promoter and 250 bp of the XIAP promoter were detected in immunoprecipitated samples by PCR. PCR products were separated on a 2% agarose gel and visualized under UV light after staining with ethidium bromide.
Statistical analysis
Data were analyzed using Prism software (GraphPad Software, Inc., San Diego, CA). To evaluate the statistical significance of differences between group means, a one-way analysis of variance with a post-test was carried out, and P values less than .05 were considered statistically significant.
Results
GSK-3β was accumulated in the nucleus of CLL B cells
CLL B cell survival relies on different mechanisms to circumvent apoptosis, including the constitutive activation of NFκB.5,6 Consistent with the hypothesis that NFκB is constitutively active in CLL B cells, we found nuclear accumulation of p65 (RelA) in CLL B cells in 7 patients with CLL, whereas p65 was not detected in the nucleus of normal human B cells (Figure 1A-B). Because phosphorylation of the NFκB subunit p65 is associated with the transactivation potential of NFκB20 , we also assessed phosphorylation of p65 in CLL B cells (Figure 1B). We have shown that GSK-3β regulates NFκB activity at a point downstream of the activation of the IKK complex12 and aberrantly accumulates in nuclei of pancreatic cancer cells,21 implying a role for nuclear GSK-3β in the regulation of NFκB transcriptional activity in pancreatic cancer cells. In light of these results and knowing that NFκB is activated in CLL B cells,5,6 we sought to determine whether GSK-3β accumulates in the nuclei of CLL cells, where it might contribute to NFκB transcriptional activity. Using nuclear/cytosolic fractionation, we found nuclear localization of GSK-3β in malignant B cells obtained from 7 patients with CLL, but only traces of this protein were detected in the nuclear fraction of normal human B cells (Figure 1A-B). Using immunofluorescence staining, we detected nuclear accumulation of GSK-3β in CLL B cells, whereas only cytoplasmic expression of GSK-3β was observed in normal human B cells (Figure 1C). Thus, nuclear accumulation of NFκB p65 and GSK-3β seems to be a feature of CLL B cells.
GSK-3β accumulates in the nucleus of CLL B cells. (A) Equivalent amounts (50 μg) of nuclear and cytosolic proteins isolated from the indicated sample from patients with CLL and from normal human B cells were separated by SDS-PAGE, immunoblotted, and probed with antibodies to the indicated proteins. P = patient. Cu/Zn super oxide dismutase (Cu/Zn SOD) and ORC2 are used as markers for the purity of the cytoplasmic and nuclear proteins, respectively. (B) Nuclear/cytosolic fractions were prepared from the indicated samples from patients with CLL, and protein expression was analyzed as described in panel A. (C) Immunofluorescence staining of GSK-3β (probed with TRITC-labeled anti-mouse secondary antibody, red fluorescence) in CLL B cells (top) and normal human B cells (bottom). Nuclei were counterstained with Hoechst 33342 (blue fluorescence).
GSK-3β accumulates in the nucleus of CLL B cells. (A) Equivalent amounts (50 μg) of nuclear and cytosolic proteins isolated from the indicated sample from patients with CLL and from normal human B cells were separated by SDS-PAGE, immunoblotted, and probed with antibodies to the indicated proteins. P = patient. Cu/Zn super oxide dismutase (Cu/Zn SOD) and ORC2 are used as markers for the purity of the cytoplasmic and nuclear proteins, respectively. (B) Nuclear/cytosolic fractions were prepared from the indicated samples from patients with CLL, and protein expression was analyzed as described in panel A. (C) Immunofluorescence staining of GSK-3β (probed with TRITC-labeled anti-mouse secondary antibody, red fluorescence) in CLL B cells (top) and normal human B cells (bottom). Nuclei were counterstained with Hoechst 33342 (blue fluorescence).
Pharmacologic inhibition of GSK-3 induced apoptosis in CLL B cells
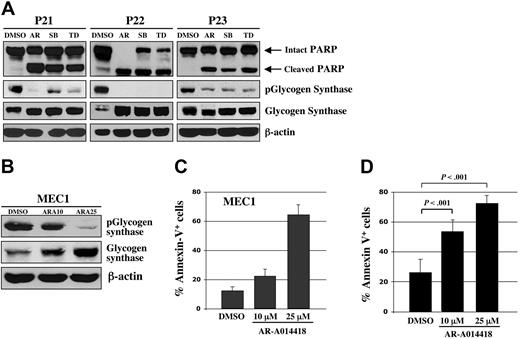
Using human malignant B cells from patients with CLL, we tested ex vivo the effect of 3 chemically distinct small-molecule inhibitors of GSK-3: AR-A014418 (ATP-competitive),14 SB216763 (ATP-competitive, arylindolemaleimide),22 and TDZD8 (non–ATP-competitive, thiadiazolidinone derivative).23 AR-A014418 inhibits GSK-3 kinase activity (IC50 = 104 nM) and does not significantly inhibit CDK2 or CDK5 (IC50 > 100 μmol/L) or 26 other kinases demonstrating the high specificity for GSK-3.14 SB216763 inhibits GSK-3 with an IC50 value of 100 nM with no significant inhibition of 24 other protein kinases.22 TDZD8, a potent inhibitor of GSK-3 (IC50 = 2 μmol/L), did not inhibit protein kinases A or C, CK-2, or CDK1/cyclin B kinases at > 100 μmol/L.23,24 Using Western immunoblotting, we estimated the level of GSK-3 inhibition by detection of the level of phosphorylated glycogen synthase, a primary GSK-3 substrate. We found that all 3 distinct GSK-3 inhibitors can decrease the level of phospho-glycogen synthase and induce apoptosis (as measured by PARP cleavage) in B cells obtained from patients with CLL (Figure 2A). Likewise, treatment of MEC1 CLL cells with different concentrations of AR-A014418 resulted in a dose-dependent inhibition of GSK-3 activity, as measured by the levels of phosphorylated glycogen synthase (Figure 2B).
Pharmacologic inhibition of GSK-3 induces apoptosis in CLL cells. (A) Malignant B cells from 3 patients with CLL were treated with 25 μmol/L concentrations of the 3 distinct GSK-3 inhibitors AR-A014418 (AR), SB216763 (SB), TDZD8 (TD), or diluent (DMSO); 24 hours after treatment, the cell pellet was collected and protein was obtained. Cell lysates were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted with the indicated antibodies. (B) MEC1 CLL cells were treated with DMSO or AR-A014418 at indicated concentrations for 24 hours and protein expression was analyzed as described in (A). ARA10 = 10 μmol/L AR-A014418; ARA25 = 25 μmol/L AR-A014418. (C) MEC1 cells were treated for 24 hours with DMSO or AR-A014418 at indicated concentrations, then assayed for apoptosis using Annexin-V-FITC staining as determined by flow cytometry. Columns, mean; bars, standard deviation (SD). (D) Malignant B cells from 10 patients with CLL were treated for 48 hours with diluent (DMSO) or AR-A014418 at indicated concentrations, then assayed for apoptosis using Annexin-V-FITC staining as determined by flow cytometry (mean ± SD; n = 10). Columns, mean; bars, SD.
Pharmacologic inhibition of GSK-3 induces apoptosis in CLL cells. (A) Malignant B cells from 3 patients with CLL were treated with 25 μmol/L concentrations of the 3 distinct GSK-3 inhibitors AR-A014418 (AR), SB216763 (SB), TDZD8 (TD), or diluent (DMSO); 24 hours after treatment, the cell pellet was collected and protein was obtained. Cell lysates were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted with the indicated antibodies. (B) MEC1 CLL cells were treated with DMSO or AR-A014418 at indicated concentrations for 24 hours and protein expression was analyzed as described in (A). ARA10 = 10 μmol/L AR-A014418; ARA25 = 25 μmol/L AR-A014418. (C) MEC1 cells were treated for 24 hours with DMSO or AR-A014418 at indicated concentrations, then assayed for apoptosis using Annexin-V-FITC staining as determined by flow cytometry. Columns, mean; bars, standard deviation (SD). (D) Malignant B cells from 10 patients with CLL were treated for 48 hours with diluent (DMSO) or AR-A014418 at indicated concentrations, then assayed for apoptosis using Annexin-V-FITC staining as determined by flow cytometry (mean ± SD; n = 10). Columns, mean; bars, SD.
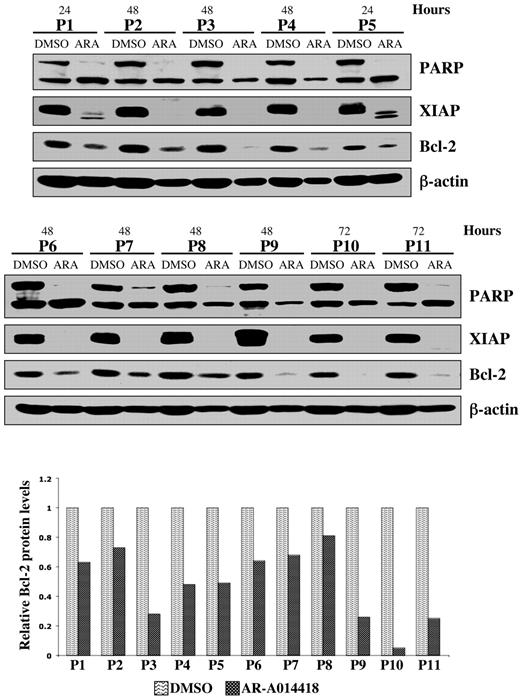
Using Annexin-V staining and subsequent flow cytometry, we detected apoptotic cells as an Annexin-V+ population within DMSO or AR-A014418–treated MEC1 CLL B-cell line and malignant B cells cultured ex vivo from each of 10 patients with CLL (Figure 2C-D). After completion of these experiments, we summarized the data for 10 patients with CLL and represented it as a mean value (Figure 2D). Although the mean number of apoptotic cells was 26 ± 9% in DMSO-treated malignant B cells, the apoptotic cell fraction in the AR-A014418-treated malignant B cells was significantly higher; the mean number of apoptotic cells reached 53 ± 7% (AR-A014418, 10 μmol/L) and 72 ± 5% (AR-A014418, 25 μmol/L) after 48 hours of exposure (Figure 2D; P < .001). We found that inhibition of GSK-3 in the MEC1 CLL B-cell line consistently resulted in a dose-dependent increase in the number of apoptotic cells (Figure 2C). Next, we treated malignant B cells from 11 patients with CLL with 25μmol/L AR-A014418 or diluent (DMSO control) for 24, 48, or 72 hours. Because malignant B cells are susceptible to apoptosis in culture, we detected some PARP cleavage in DMSO-treated CLL B cells (Figure 3). However, more pronounced or complete PARP cleavage was observed in AR-A014418–treated versus DMSO-treated malignant B cells in all 11 patients with CLL (Figure 3). Taken together, these results suggest that inhibition of GSK-3 induces apoptosis in CLL B cells.
Pharmacologic inhibition of GSK-3 decreases NFκB-mediated survival of CLL cells. Malignant B cells from 11 patients with CLL were treated with diluent (DMSO) or 25 μmol/L AR-A014418; at the indicated time after treatment, the cell pellet was collected and protein was obtained. Cell lysates were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted with the indicated antibodies. Western blot band intensities were quantified by using the Image J software (National Institutes of Health). The quantitative analysis of Bcl-2 protein levels (normalized to β-actin levels in each case) in DMSO versus AR-A014418–treated CLL B cells obtained from 11 patients with CLL are presented in the lower panel. ARA indicates AR-A014418; P, patient.
Pharmacologic inhibition of GSK-3 decreases NFκB-mediated survival of CLL cells. Malignant B cells from 11 patients with CLL were treated with diluent (DMSO) or 25 μmol/L AR-A014418; at the indicated time after treatment, the cell pellet was collected and protein was obtained. Cell lysates were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted with the indicated antibodies. Western blot band intensities were quantified by using the Image J software (National Institutes of Health). The quantitative analysis of Bcl-2 protein levels (normalized to β-actin levels in each case) in DMSO versus AR-A014418–treated CLL B cells obtained from 11 patients with CLL are presented in the lower panel. ARA indicates AR-A014418; P, patient.
Pharmacologic inhibition of GSK-3 decreased NFκB-mediated expression of antiapoptotic molecules in CLL B cells
We have shown that GSK-3β plays a critical role in NFκB-mediated survival of pancreatic cancer cells.12 Because CLL B-cell survival also relies on constitutively active NFκB, we have investigated whether inhibition of GSK-3 affects NFκB-mediated expression of antiapoptotic molecules in CLL B cells. Immunoblot analysis revealed a significant decrease in the expression of the antiapoptotic proteins XIAP and Bcl-2 in AR-A014418-treated CLL B cells from the 11 patients with CLL (Figure 3). Using RT-PCR, we found that inhibition of GSK-3 in malignant B cells from patients with CLL resulted in decreased expression of NFκB target genes XIAP and Bcl-2 (Figure 4A). To determine whether XIAP and Bcl-2 down-regulation was a cause or a consequence of caspase activation and apoptotic cell death, we treated CLL B cells with DMSO, AR-A014418, Z-VAD-FMK (irreversible, broad spectrum caspase inhibitor), or a combination of AR-A014418 and Z-VAD-FMK. We found that although Z-VAD-FMK could rescue the apoptotic effect of GSK-3 inhibition by AR-A014418 in CLL B cells, Z-VAD-FMK did not inhibit the decrease in XIAP and Bcl-2 mRNA or protein levels in AR-A014418–treated CLL B cells (Figure 4B-C). Thus, decreased expression of NFκB target genes XIAP and Bcl-2 in AR-A014418–treated CLL B cells occurs before the activation of caspases. Taken together, these results suggest that GSK-3 affects the expression of the NFκB target genes XIAP and Bcl-2 in CLL B cells, resulting in decreased cell survival.
Inhibition of GSK-3 decreased NFκB-mediated expression of antiapoptotic molecules in CLL cells. (A) CLL B cells from patients with CLL were treated with diluent (DMSO) or 25 μmol/L AR-A014418; 12 hours after treatment, the cell pellet was collected and RNA was obtained. RT-PCR analysis was performed as described in “Materials and methods.” P = patient; B2M = β-2-microglobulin. (B) MEC1 cells were treated with diluent (DMSO), 25 μmol/L AR-A014418 (ARA), 50 μmol/L Z-VAD-FMK (ZVAD), or AR-A014418 + Z-VAD-FMK; the cell pellet was collected and RNA (12 hours after treatment) and protein (24 hours after treatment) were obtained. Cell lysates were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted with the indicated antibodies (left panel). RT-PCR analysis was performed as described in “Materials and methods.” (C) CLL B cells from a patient with CLL were treated with diluent (DMSO) or 25 μmol/L AR-A014418 (ARA), 50 μmol/L Z-VAD-FMK (ZVAD), or AR-A014418 + Z-VAD-FMK; the cell pellet was collected and RNA (12 hours after treatment) and protein (24 hours after treatment) were obtained. mRNA and protein expression analysis was performed as described in panel B.
Inhibition of GSK-3 decreased NFκB-mediated expression of antiapoptotic molecules in CLL cells. (A) CLL B cells from patients with CLL were treated with diluent (DMSO) or 25 μmol/L AR-A014418; 12 hours after treatment, the cell pellet was collected and RNA was obtained. RT-PCR analysis was performed as described in “Materials and methods.” P = patient; B2M = β-2-microglobulin. (B) MEC1 cells were treated with diluent (DMSO), 25 μmol/L AR-A014418 (ARA), 50 μmol/L Z-VAD-FMK (ZVAD), or AR-A014418 + Z-VAD-FMK; the cell pellet was collected and RNA (12 hours after treatment) and protein (24 hours after treatment) were obtained. Cell lysates were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted with the indicated antibodies (left panel). RT-PCR analysis was performed as described in “Materials and methods.” (C) CLL B cells from a patient with CLL were treated with diluent (DMSO) or 25 μmol/L AR-A014418 (ARA), 50 μmol/L Z-VAD-FMK (ZVAD), or AR-A014418 + Z-VAD-FMK; the cell pellet was collected and RNA (12 hours after treatment) and protein (24 hours after treatment) were obtained. mRNA and protein expression analysis was performed as described in panel B.
GSK-3 regulated the binding of NFκB p65 to its target gene promoters in CLL cells
Although the role of nuclear GSK-3β is unclear, some reports have suggested that it is required to modulate the activity of several nuclear proteins, including transcription factors.25,26 We have found both GSK-3β and active NFκB p65 in the nuclei of malignant B cells obtained from 6 patients with CLL (Figure 1A). Our results, as shown in Figures 2,Figure 3–4, suggest that GSK-3 can regulate the expression of NFκB target genes, known to participate in CLL B-cell survival. These observations prompted us to further explore the relationship between aberrant GSK-3β nuclear accumulation and NFκB transcriptional activity in CLL B cells.
Treatment of CLL B cells with AR-A014418 for 12 hours resulted in a marked reduction in the expression of the NFκB target genes XIAP and Bcl-2 (Figure 4), suggesting a loss of NFκB transcriptional activity. To further investigate the role of GSK-3 in the regulation of NFκB activity, we determined whether NFκB p65 levels changed in the nuclei of MEC1 cells after inhibition of GSK-3. Consistent with the idea that GSK-3β positively modifies only NFκB transcriptional activity downstream to the IKK complex,12 we found that nuclear levels of p65 were not significantly changed in the nucleus of AR-A014418–treated MEC1 cells, but GSK-3β protein levels were diminished substantially (Figure 5A). These results indicate that the inhibition of GSK-3 does not affect the nuclear accumulation of p65 NFκB but might alter its ability to regulate target gene promoters in CLL B cells. To further evaluate this possibility, we analyzed whether p65 could bind to the promoters of its target genes after GSK-3 inhibition using ChIP in MEC1 cells and malignant B cells obtained from 5 patients with CLL. In fact, p65 was bound at both the Bcl-2 and XIAP promoters in DMSO-treated MEC1 cells (Figure 5B-C) and CLL B cells (Figure 5D) but was significantly diminished in cells treated with AR-A014418 (Figure 5B-D; compare DMSO with AR-A014418 [ARA]). It is noteworthy that Z-VAD-FMK treatment of MEC1 B cells did not affect the binding of p65 to its target gene promoters, and addition of Z-VAD-FMK to AR-A014418–treated MEC1 cells still resulted in an inability of p65 to bind to the promoters of XIAP and Bcl-2 (Figure 5C). Taken together, these data suggest that GSK-3 regulates the binding of p65 to the promoters of these 2 target genes in CLL B cells. Moreover, the inability of Z-VAD-FMK treatment to reverse the effects of AR-A014418 on p65 binding at the XIAP and Bcl-2 promoters further indicates that the effects of GSK-3 inhibition on NFκB-mediated gene transcription occur before the onset of apoptosis.
Inhibition of GSK-3 affects the binding of NFκB p65 to its target gene promoters in CLL cells. (A) MEC1 cells were treated with 25 μmol/L AR-A014418 for 0, 12, and 24 hours, as indicated. Nuclear/cytosolic fractions were prepared, and 50 μg of nuclear and cytosolic proteins were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted as indicated. (B-C) Binding of NFκB p65 to the promoters of its target genes XIAP and Bcl-2 was assayed with the use of chromatin immunoprecipitation (ChIP) in MEC1 CLL cells treated with 25 μmol/L AR-A014418 (ARA), 50 μmol/L Z-VAD-FMK, or AR-A014418 + Z-VAD-FMK for 12 hours. (D) Immunoprecipitated chromatin was analyzed by PCR for the binding of NFκB p65 to the promoters of its target genes XIAP and Bcl-2 in malignant B cells from 5 patients with CLL treated with 25 μmol/L AR-A014418 (ARA) for 12 hours.
Inhibition of GSK-3 affects the binding of NFκB p65 to its target gene promoters in CLL cells. (A) MEC1 cells were treated with 25 μmol/L AR-A014418 for 0, 12, and 24 hours, as indicated. Nuclear/cytosolic fractions were prepared, and 50 μg of nuclear and cytosolic proteins were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted as indicated. (B-C) Binding of NFκB p65 to the promoters of its target genes XIAP and Bcl-2 was assayed with the use of chromatin immunoprecipitation (ChIP) in MEC1 CLL cells treated with 25 μmol/L AR-A014418 (ARA), 50 μmol/L Z-VAD-FMK, or AR-A014418 + Z-VAD-FMK for 12 hours. (D) Immunoprecipitated chromatin was analyzed by PCR for the binding of NFκB p65 to the promoters of its target genes XIAP and Bcl-2 in malignant B cells from 5 patients with CLL treated with 25 μmol/L AR-A014418 (ARA) for 12 hours.
Inhibition of GSK-3 results in epigenetic silencing of the XIAP and Bcl-2 promoters in CLL B cells
Opening of chromatin to allow transcription factors to gain access to gene promoters and regulate gene transcription is one of the major functions of histone modifications (ie, phosphorylation, acetylation, and methylation).27 It has been suggested that GSK-3β does not affect histone acetylation, a hallmark of open chromatin structure and gene activation, at promoters of NFκB target genes.28 To evaluate the possibility that GSK-3 inhibition alters the ability of NFκB to regulate its target genes through transcriptional repression, we used ChIP to assess histone methylation at the NFκB target gene promoters in MEC1 cells and malignant B cells from 5 patients with CLL who were treated with diluent (DMSO) or 25 μmol/L AR-A014418 for 12 hours. Methylation of H3-K9 (histone H3, lysine 9), H3-K27 (histone H3, lysine 27), and H4-K20 (histone H4, lysine 20) are known features of repressive chromatin status and epigenetic gene silencing.29,30 We found a significant increase in methylation of H3-K9, H3-K27, and H4-K20 bound to the promoters of the NFκB target genes Bcl-2 and XIAP in AR-A014418–treated MEC1 cells and malignant B cells from patients with CLL (Figure 6A-B). We consistently found increased methylation of H3-K9, H3-K27, and H4-K20 at the promoters of the NFκB target genes Bcl-2 and XIAP in CLL B cells treated with a combination of AR-A014418 and caspase inhibitor Z-VAD-FMK (Figure 6C), indicating that the chromatin remodeling was taking place in the absence of caspase activation. These results suggest that GSK-3 contributes to the maintenance of active chromatin at NFκB target gene promoters, allowing p65 binding and transcriptional activation.
Inhibition of GSK-3 affects histone modification in CLL cells. (A-B) Twelve hours after treatment, genomic chromatin fragments from DMSO or 25 μmol/L AR-A014418 (ARA)-treated MEC1 cells (panel A) and malignant B cells from 5 patients with CLL (panel B) were immunoprecipitated with dimethyl-H3-K9, trimethyl-H3-K27, and dimethyl-H4-K20 antibodies. Immunoprecipitated chromatin was analyzed by PCR for the methylation of H3-K9, H3-K27, and H4-K20 at the XIAP and Bcl-2 promoters. PCR analysis on input chromatin (first 2 lanes) confirmed that equal chromatin amounts were used for ChIP. (C) Binding of NFκB p65 to the promoters of its target genes XIAP, and Bcl-2 was assayed in malignant B cells from a CLL patient treated with 25 μmol/L AR-A014418 (ARA) or 50 μmol/L Z-VAD-FMK (ZVAD) or AR-A014418 + Z-VAD-FMK for 12 hours by ChIP. Immunoprecipitated chromatin was also analyzed for the methylation of H3-K9, H3-K27, and H4-K20 at the XIAP and Bcl-2 promoters as described in (panels A and B).
Inhibition of GSK-3 affects histone modification in CLL cells. (A-B) Twelve hours after treatment, genomic chromatin fragments from DMSO or 25 μmol/L AR-A014418 (ARA)-treated MEC1 cells (panel A) and malignant B cells from 5 patients with CLL (panel B) were immunoprecipitated with dimethyl-H3-K9, trimethyl-H3-K27, and dimethyl-H4-K20 antibodies. Immunoprecipitated chromatin was analyzed by PCR for the methylation of H3-K9, H3-K27, and H4-K20 at the XIAP and Bcl-2 promoters. PCR analysis on input chromatin (first 2 lanes) confirmed that equal chromatin amounts were used for ChIP. (C) Binding of NFκB p65 to the promoters of its target genes XIAP, and Bcl-2 was assayed in malignant B cells from a CLL patient treated with 25 μmol/L AR-A014418 (ARA) or 50 μmol/L Z-VAD-FMK (ZVAD) or AR-A014418 + Z-VAD-FMK for 12 hours by ChIP. Immunoprecipitated chromatin was also analyzed for the methylation of H3-K9, H3-K27, and H4-K20 at the XIAP and Bcl-2 promoters as described in (panels A and B).
Discussion
Constitutively active NFκB is known as an important factor of cancer cell survival in human tumorigenesis.31 Human CLL B cells exhibit high constitutive levels of NFκB activity, and survival of CLL B cells mainly relies on NFκB-mediated expression of antiapoptotic molecules.5,6 It has been suggested that GSK-3β is an important regulator of NFκB transcriptional activity,11 and we have previously shown that inactivation of GSK-3 or genetic depletion of GSK-3β by RNA interference suppresses basal NFκB transcriptional activity, leading to decreased pancreatic cancer cell proliferation and survival.12 Recent studies have shown that inhibition of GSK-3 also decreases survival of colon and prostate cancer cells.32-34 Most recently, it has been demonstrated that GSK-3β positively regulates NFκB-mediated drug resistance in acute myeloid leukemia (AML), suggesting GSK-3β as a critical therapeutic target in resistant blasts of AML.35 Overall, these findings also suggest a more global role for GSK-3β in human malignancy.
However, we are unaware of any previous report that assessed the role of GSK-3 and the effect of its inhibition on NFκB-mediated cell survival in CLL. In the present study, we found aberrant nuclear accumulation of GSK-3β in malignant B cells obtained from patients with CLL. Further, we demonstrate that pharmacologic inhibition of GSK-3 leads to depletion of its nuclear pool, suppression of NFκB transcriptional activity, decreased expression of antiapoptotic proteins (XIAP, Bcl-2), and enhanced apoptosis in CLL cells. Moreover, we provide evidence that inhibition of GSK-3 affects histone modification, resulting in transcriptional repression of NFκB target genes (XIAP, Bcl-2) involved in CLL cell survival. Taken together, our results suggest GSK-3 as a novel potential therapeutic target in the treatment of CLL.
GSK-3β protein overexpression was recently reported in human pancreatic,21 ovarian,36 and colon32 carcinomas. From a physiological perspective, GSK-3β is expressed in the cytoplasm of normal human cells, including normal B cells. Although GSK-3β contains no identifiable nuclear localization or nuclear export signal sequences, it has been suggested to shuttle from the cytoplasm to the nucleus, where it is thought to participate in the regulation of gene transcription through the phosphorylation of transcription factors.25,26 We found nuclear accumulation of GSK-3β in pancreatic cancer cell lines and in most poorly differentiated human pancreatic adenocarcinomas.21 In the present study, we demonstrate aberrant nuclear accumulation of GSK-3β in human CLL B cells. Similar to our finding in pancreatic cancer,21 inhibition of GSK-3 leads to depletion of its nuclear pool in CLL B cells, whereas nuclear level of NFκB p65 remains unchanged. These results suggest that GSK-3β does not regulate nuclear accumulation of NFκB but might be required for NFκB transcriptional activity in human CLL B cells.
The exact mechanism by which GSK-3β affects NFκB transcriptional activity is unknown. It has been demonstrated that degradation of IκBα and translocation of NFκB to the nucleus were unaffected in TNFα-stimulated, GSK-3β–deficient MEFs, indicating that NFκB is regulated by GSK-3β at the level of the transcriptional complex.11 Consistent with this idea, we have recently shown that GSK-3β influences NFκB-mediated gene transcription in pancreatic cancer cells at a point distal to the Iκ kinase complex.12 These data suggest that GSK-3β must be regulating the nuclear activity of NFκB p65/p50. Consistent with this putative role of GSK-3β in regulating the nuclear activity of NFκB, a recent study demonstrated that loss of GSK-3β leads to decreased TNFα-induced binding of NFκB to the promoters of a subset of target genes, including antiapoptotic genes (eg, cIAP2), in GSK-3β–deficient MEFs.28 However, the underlying mechanism by which GSK-3 regulates p65 NFκB binding to target gene promoters was not defined.
Using CLL cell line MEC1 and malignant B cells obtained from patients with CLL, we have analyzed the effect of GSK-3 inhibition on the binding of p65 NFκB to the promoters of a subset of NFκB-dependent genes (XIAP, Bcl-2) involved in survival of CLL cells. Consistent with the data from the GSK-3β–deficient MEFS,28 we find that inhibition of GSK-3 leads to decreased binding of p65 to the promoters of XIAP and Bcl-2 in MEC1 cells and malignant B cells obtained directly from patients with CLL. Thus, GSK-3 may regulate the nuclear activity of NFκB in CLL B cells by affecting the binding of p65/p50 to the promoters of a subset of NFκB target genes. Although it is possible that GSK-3β controls NFκB nuclear activity through direct phosphorylation of NFκB p65, with p65 modification affecting DNA binding activity or dimerization,37 it is also possible that GSK-3β may affect chromatin structure, thereby facilitating accessibility of transcription factors such as NFκB to the promoter regions of target genes. Histone modifications play a critical role in the access of transcription factors to promoters and thereby regulate gene transcription.27 Methylation of H3-K9, H3-K27, and H4-20 are known marks of repressive chromatin status and epigenetic gene silencing.29,30 Significantly, we observed a substantial increase in methylation of H3-K9, H3-K27, and H4-K20 at NFκB target gene (Bcl-2, XIAP) promoters in AR-A014418–treated CLL cells. Our results suggest that GSK-3 plays an important role in regulating histone modification, and by this, GSK-3 may contribute to p65/p50 binding to the promoters and transcriptional activation of a subset of NFκB target genes in CLL cells.
In summary, our work identifies inhibition of GSK-3 as a new promising approach to CLL therapy, holding the potential to enhance apoptosis in human CLL cells. Our results suggest that GSK-3 plays an important role in regulating histone modification, and through this, GSK-3 may contribute to modulation of p65/p50 binding to the promoters and activation of its target genes in CLL B cells, thereby leading to increased CLL B-cell survival.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Mayo Foundation, by National Cancer Institute grants CA102701 (to D.D.B.) and CA95241 (to N.E.K.), by philanthropic support from Mr E. Spencer (to N.E.K.), and CA102701 and CA09724 (to M.E.F.-Z.).
Authorship
Contribution: A.V.O. designed and performed experiments, analyzed data, and wrote the manuscript; D.D.B. and N.E.K. designed research, analyzed data, and wrote the manuscript; and N.D.B. and M.E.F.-Z. performed experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel D. Billadeau, Department of Immunology and Division of Oncology Research, 200 First Street SW, Rochester, MN 55905; e-mail: billadeau.daniel@mayo.edu; or Neil E. Kay, Department of Hematology, Stabile 628, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; e-mail: kay.neil@mayo.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal