Abstract
Acute graft-versus-host disease (aGVHD) remains a serious complication following allogeneic stem-cell transplantation (SCT), and is mediated by infiltration of alloreactive donor T cells into recipient tissue. Chemokines and their receptors play a central role in controlling the recruitment of T cells into discrete tissue sites, and determine the clinical features of GVHD in murine models. In this study, we have analyzed the serum concentration of molecules that control leukocyte migration in serial samples from 34 patients following allogeneic SCT. The chemokine CXCL10 (IP-10) was significantly elevated (> 2-fold) in serum at the time of aGVHD. Because the ligand for CXCL10 is CXCR3, the number of CXCR3+ T cells was determined in peripheral blood, but was not increased during episodes of GVHD. To investigate the role of chemokines in the recruitment of T cells to the anatomic site of GVHD, skin biopsies were stained for CXCL10 and CXCR3 expression. CXCL10 expression was observed in the basal keratinocytes of the epidermis in patients with GVHD together with positive staining for CXCR3 on cells in dermal infiltrates. These findings indicate that CXCL10 plays a central role in the pathogenesis of skin aGVHD by the recruitment of CXCR3+ T cells to the sites of inflammation.
Introduction
Graft-versus-host disease (GVHD) remains a major complication following allogeneic stem-cell transplantation (SCT), and is associated with significant morbidity and mortality. The pathogenesis of GVHD is a multistep process,1 and is believed to be initiated by the conditioning regimen. This elicits a cytokine cascade involving IL-1 and TNF-α resulting in enhanced expression of major histocompatibility complex (MHC) and costimulatory molecules on tissue antigen-presenting cells (APCs). Donor T cells subsequently become activated through recognition of host alloantigens and differentiate into effector T cells that recruit other cell types and lead to local inflammation and target tissue destruction. The incidence of acute GVHD (aGVHD), occurring up to 100 days following SCT, can range from 10% to 80% depending on the degree of histoincompatibility between donor and recipient, the number of T cells in the graft, the patient's age, and the GVHD prophylatic regimen. aGVHD typically involves selective epithelial damage of the major target organs, which are skin, gut, and liver
There has been considerable interest in identifying the molecules involved in the pathogenesis of aGVHD, both for use as markers in monitoring disease outcome and as targets for future therapies in the management of GVHD. It is widely accepted that proinflammatory cytokines such as IFN-γ and TNF-α are important in the initiation of the disease. Recently, the role of chemokines and integrins in the migration of donor T cells to tissue sites of GVHD has been examined in mice following SCT.2 Gene expression profiling of GVHD target organs has identified elevated expression of the proinflammatory chemokines CCL2/MCP-1, CCL3/MIP-1α, CCL4/MIP-1β, CCL5/RANTES, CXCL9/MIG, and CXCL10/IP-10.3-5 However, only one study to date has looked at the recruitment of T cells to skin GVHD in humans.6 In this study, an increased percentage of CD4+CCR10+ T cells was found in the blood and in the dermal infiltrates of the skin from patients with GVHD. Furthermore, the infiltrate correlated with increased expression of the ligand CCL27/CTACK in the epidermis, thus indicating the potential importance of this receptor-ligand pair in the recruitment of T cells to skin in aGVHD. Here, we have used serial serum samples from 34 patients following SCT and assayed the level of molecules involved in leukocyte migration, which may have a role in the pathogenesis of GVHD. The chemokine CXCL10 was found to be increased more than 2-fold in aGVHD patients compared with control patients without GVHD. Skin biopsies from patients with aGVHD showed increased expression of CXCL10 in the epidermis together with CXCR3+ cells in dermal inflammatory foci. These findings indicate a role for the chemokine receptor pair CXCL10 and CXCR3 in the pathogenesis of skin GVHD in humans.
Patients, materials, and methods
Patients
A total of 34 patients (18 men and 16 women) who had undergone SCT were selected for study. Of these, 26 had received a graft from an HLA-matched sibling, with myeloablative conditioning used in 19 patients and nonmyeloablative conditioning used in 7 patients. A total of 8 patients had received HLA-matched unrelated donor (MUD) SCT (6 myeloablative and 2 nonmyeloablative). Patients were recruited following informed consent (approved by the South Birmingham Research Ethics Committee) from the University Hospitals Birmingham Foundation Trust or Heart of England Hospital Foundation Trust (Birmingham, United Kingdom), obtained in accordance with the Declaration of Helsinki. A total of 24 of the patients developed grade 1/II aGVHD according to Glucksberg criteria, of which 16 patients had skin GVHD, 5 had gut GVHD, 2 had mouth GVHD, and 1 had liver GVHD. The median age for patients with aGVHD was 41 years, compared with 41.5 years for patients who did not have aGVHD (Table 1).
Clinical features of patients in relation to the occurrence of aGVHD
| Clinical parameters . | Patients with aGVHD . | Patients without aGvHD . |
|---|---|---|
| No. patients | 24 | 10 |
| Median age, y (range) | 41 (22-52) | 41.5 (19-53) |
| Sex | ||
| Male | 12 | 6 |
| Female | 12 | 4 |
| GVHD grade | ||
| I | 23 | NA |
| II | 1 | NA |
| GVHD site | ||
| Mouth | 2 | NA |
| Skin | 16 | NA |
| Gut | 5 | NA |
| Liver | 1 | NA |
| Transplant donor | ||
| Sibling | 18 | 8 |
| MUD | 6 | 2 |
| Conditioning regimen | ||
| Myeloablative | 20 | 5 |
| Nonmyeloablative | 4 | 5 |
| Use of alemtuzumab | ||
| Yes | 9 | 6 |
| No | 18 | 4 |
| Clinical parameters . | Patients with aGVHD . | Patients without aGvHD . |
|---|---|---|
| No. patients | 24 | 10 |
| Median age, y (range) | 41 (22-52) | 41.5 (19-53) |
| Sex | ||
| Male | 12 | 6 |
| Female | 12 | 4 |
| GVHD grade | ||
| I | 23 | NA |
| II | 1 | NA |
| GVHD site | ||
| Mouth | 2 | NA |
| Skin | 16 | NA |
| Gut | 5 | NA |
| Liver | 1 | NA |
| Transplant donor | ||
| Sibling | 18 | 8 |
| MUD | 6 | 2 |
| Conditioning regimen | ||
| Myeloablative | 20 | 5 |
| Nonmyeloablative | 4 | 5 |
| Use of alemtuzumab | ||
| Yes | 9 | 6 |
| No | 18 | 4 |
Data are numbers of patients, except for median age.
NA indicates not applicable.
Conditioning
All patients received 1 of 2 forms of conditioning chemotherapy dependent on the type of stem cell transplant they received. Myeloablative conditioning was with cyclophosphamide (60 mg/kg over 2 days) together with total body irradiation (14.4 Gy in 8 fractions). Nonmyeloablative conditioning was with either BEAM (300 mg/m2 BCNU for 1 day, 200 mg/m2 etoposide a day for 4 days, 200 mg/m2 ara C a day for 4 days, and 140 mg/m2 melphelan a day for 1 day) or fludarabine (30 mg/m2/day for 5 days) and melphelan (140 mg/m2/day for 1 day). Patients also received GVHD prophylaxis with cyclosporin A and methotrexate at standard doses. In addition, MUDs and reduced-intensity allografts were T-cell depleted using 10 mg/day of alemtuzumab in vivo for 1 week following SCT.
PBMCs and serum
Peripheral blood was taken into sodium heparin tubes every 2 weeks after SCT until week 12 and then at monthly intervals. Peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation and frozen and stored in liquid nitrogen. Serum was prepared and stored at −80°C until use.
Serum cytokine and chemokine measurements
A panel of 9 cytokines and 6 chemokines (IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, GM-CSF, IFN-γ, TNF-α, CXCL8 [IL-8], CXCL10 [IP-10], CCL2 [MCP-1], CCL3 [MIP-1α], CCL5 [RANTES], and CCL11 [eotaxin]) were analyzed in serum using a multiplex fluorescent bead-based immunoassay (Upstate, Milton Keynes, United Kingdom) and run on a Luminex machine (Luminex, Austin, TX). The CXCL9 and CXCL11 chemokines were measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Abingdon, United Kingdom).
Flow cytometry
Up to 6-color flow cytometry was performed using the following antibodies: CCR7-FITC (R&D Systems), CLA-FITC, CXCR3-PE, β7PE, CD4–PE-CY7 (BD Pharmingen, Oxford, United Kingdom), CD45RO-FITC, CD45RO-PE, CD4-ECD, CD8-PC5, CD45RA-ECD, CD45RO-ECD, CD25-PC5 (Beckman Coulter, High Wycombe, United Kingdom), CD127-FITC (eBioscience/Insight, Wembley, United Kingdom), and CD8–Pacific blue (Caltag, Bucks, United Kingdom). Isotype control antibodies were used to determine background fluorescence. PBMCs were stained with antibodies for 20 minutes on ice, and in some cases were fixed in 2% paraformaldehyde (Fisher, Loughborough, United Kingdom) in phosphate-buffered saline (PBS) prior to analysis on an EPICS-XL flow cytometer (Beckman Coulter) or a Dako Cyan ADP (Dako, Ely, United Kingdom).
Immunohistochemistry
Skin biopsies were collected from patients suffering from histologically confirmed aGVHD and from healthy patients undergoing knee surgery to serve as controls. Paraffin-embedded sections measuring 5 μm thick were used for immunostaining. Sections were deparaffinized and antigen-retrieved using W/Cap (Surgipath Europe, Peterborough, United Kingdom) heated to 98°C for 30 minutes. To neutralize endogenous peroxidase activity, slides were pretreated with 0.3% hydrogen peroxide (Sigma, Dorset, United Kingdom) for 10 minutes. Primary antibodies were added at the following concentration: mouse anti-human CXCR3 (1:10 000; R&D Systems) and rabbit anti-human CXCL10 (1:500; Peprotech, London, United Kingdom) for 1 hour at room temperature in a humidified chamber. Immunoreactivity was detected using a chemate/envision kit (Dako) for 30 minutes and visualized using 3′3 diaminobenzidine tetrahydrochloride (Dako) as chromogen. Parallel slides were also stained using isotype controls to check for background staining. Slides were counterstained with Mayer hematoxylin (Surgipath Europe).
Images were viewed using a Nikon Eclipse E600 microscope equipped with a Nikon Plan Apo 40 ×/1.0 aperture objective (Nikon, Tokyo, Japan) and acquired using a Nikon DXM1200 digital camera and ACT software (Nikon UK, Kingston-upon-Thames, United Kingdom). Final images were obtained using Adobe Photoshop CS version 8.0 software (Adobe Systems, Uxbridge, United Kingdom).
Statistical analysis
All statistical analyses were performed using Prism (Graphpad, San Diego, CA). All comparisons were made using either parametric t test or the nonparametric Mann-Whitney U test for unpaired data. A P value of less than .05 was considered statistically significant.
Results
A wide range of clinical complications are seen within the first 100 days following SCT, with infections being particularly common. We wished to address the role of chemokines and cytokines solely in relation to aGVHD; therefore, patients who suffered significant infectious complications (viral, fungal, and bacterial infections), prior to or during aGVHD, were eliminated from the analysis. No infectious complications were observed in the control group who did not suffer from GVHD.
Effect of donor graft and conditioning on aGVHD outcome
The proportion of patients who developed aGVHD was similar whether they received a sibling or MUD graft (18 of 26 [69.2%] vs 6 of 8 [75%], respectively; Table 1). The incidence of aGVHD was reduced in patients who received a nonmyeloablative conditioning regimen (4 of 9 [44.4%]) compared with those who had myeloablative conditioning (20 of 25 [80%]). The use of alemtuzumab was associated with a predictable reduction in the numbers of individuals who developed GVHD (9 of 15 [60%]) versus no alemtuzumab (18 of 22 [81.8%]; Table 1).
Cytokine and chemokine serum levels following SCT in relation to the incidence of GVHD
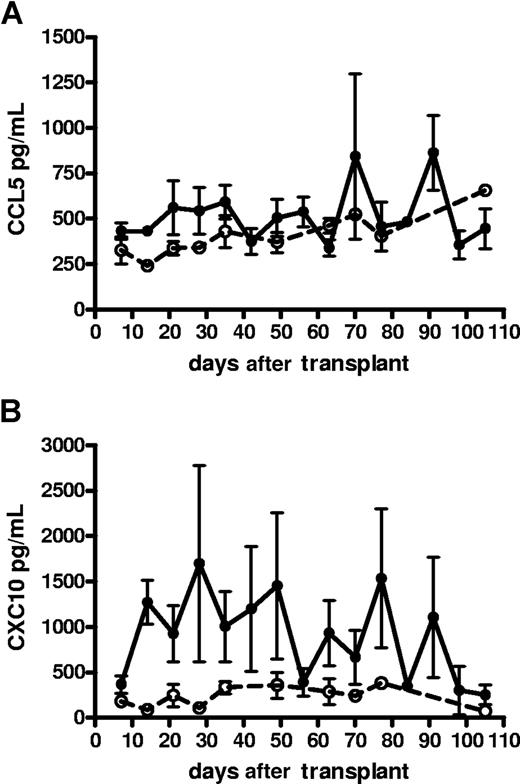
The median time for patients to develop aGVHD following SCT was 35 days, with a range from 14 to 100 days. A range of cytokines and chemokines were assayed within serum at the time of aGVHD (n = 24) and compared with matched samples from control patients (n = 10). At the time of aGVHD, most patients from both cohorts did not produce significant serum levels (higher than 90 pg/mL) of IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, TNF-α, IFN-γ, GM-CSF, CCL3, and CCL11 (Table 2). CXCL8-CXCL11, CCL2, and CCL5 were found at detectable levels in all patients (Table 2). CCL5 was significantly increased during the first 35 days after transplantation in patients with aGVHD (P < .05), but then returned to control values (Figure 1A). In contrast, CXCL10 was significantly elevated (> 2-fold) in the first 100 days in patients who suffered from episodes of aGVHD (P < .001; Figure 1B). CXCL10 serum levels resumed to normal levels (250 pg/mL) after 100 days (data not shown).
Cytokine and chemokine serum levels in patients at the time of aGVHD and in matched control group
| Cytokine/chemokine . | GVHD . | No GVHD . |
|---|---|---|
| IL-1β | 2 (2-6) | 2 (2-54) |
| IL-2 | 2 (2-4) | 2 (2) |
| IL-4 | 0.5 (0.5) | 0.5 (0.5) |
| IL-6 | 0.5 (0.5-78) | 3.5 (0.5-648) |
| IL-10 | 1.5 (1.5-10) | 1.5 (1.5-3) |
| IL-12 | 0.5 (0.5-8) | 0.5 (0.5-1) |
| TNF-α | 1.5 (1.5-14) | 1.5 (1.5-105) |
| IFN-γ | 0.5 (0.5-12) | 0.5 (0.5-5) |
| GM-CSF | 0.5 (0.5-4) | 0.5 (0.5) |
| CXCL8 | 71.5 (3-907) | 174 (0.5-434) |
| CXCL9 | 1625 (100-10 000) | 1133 (100-10 000) |
| CXCL10 | 582 (27-3728)* | 197 (41-775) |
| CXCL11 | 21 (1.5-252) | 12.6 (1.5-172) |
| CCL2 | 89 (7-638) | 129 (37-410) |
| CCL3 | 45 (45-2371) | 45 (45-7133) |
| CCL5 | 432 (227-2126) | 341 (262-517) |
| CCL11 | 9 (9-90) | 9 (9) |
| Cytokine/chemokine . | GVHD . | No GVHD . |
|---|---|---|
| IL-1β | 2 (2-6) | 2 (2-54) |
| IL-2 | 2 (2-4) | 2 (2) |
| IL-4 | 0.5 (0.5) | 0.5 (0.5) |
| IL-6 | 0.5 (0.5-78) | 3.5 (0.5-648) |
| IL-10 | 1.5 (1.5-10) | 1.5 (1.5-3) |
| IL-12 | 0.5 (0.5-8) | 0.5 (0.5-1) |
| TNF-α | 1.5 (1.5-14) | 1.5 (1.5-105) |
| IFN-γ | 0.5 (0.5-12) | 0.5 (0.5-5) |
| GM-CSF | 0.5 (0.5-4) | 0.5 (0.5) |
| CXCL8 | 71.5 (3-907) | 174 (0.5-434) |
| CXCL9 | 1625 (100-10 000) | 1133 (100-10 000) |
| CXCL10 | 582 (27-3728)* | 197 (41-775) |
| CXCL11 | 21 (1.5-252) | 12.6 (1.5-172) |
| CCL2 | 89 (7-638) | 129 (37-410) |
| CCL3 | 45 (45-2371) | 45 (45-7133) |
| CCL5 | 432 (227-2126) | 341 (262-517) |
| CCL11 | 9 (9-90) | 9 (9) |
Data are cytokine and chemokine median values, pg/mL (range).
P < .01, according to Mann-Whitney U test.
The kinetics of serum chemokine levels in SCT patients with and without GVHD. (A) CCL5 and (B) CXCL10 levels in patients with (—) or without (- - -) aGVHD. Data presented as means plus SE bars.
The kinetics of serum chemokine levels in SCT patients with and without GVHD. (A) CCL5 and (B) CXCL10 levels in patients with (—) or without (- - -) aGVHD. Data presented as means plus SE bars.
The influence of the transplant conditioning regimen on cytokine and chemokine levels following SCT
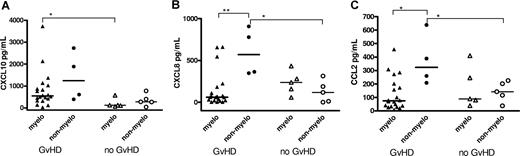
Serum cytokine and chemokine levels were examined in relation to whether patients received a myeloablative (n = 24) or nonmyeloablative conditioning regimen (n = 9). CXCL10 levels were higher in patients with aGVHD in both groups (P < .001), although this result was only significant for the myeloablative subset, most likely due to the relatively low number of patients in the nonmyeloablative group (Figure 2A). Interestingly, CXCL8 and CCL2 serum levels were significantly elevated in patients with aGVHD from the nonmyeloablative group compared with controls, but this was not observed in the myeloablative subgroup (Figure 2B,C).
Comparison of cytokine and chemokine levels between myeloablative and nonmyeloablative SCT patients with and without GVHD. Patients with and without GVHD were subdivided into those who underwent myeloablative transplantation and those who underwent nonmyeloablative transplantation. Solid lines represent median markers. P values were calculated using the Mann-Whitney U test (*P < .05; **P < .01).
Comparison of cytokine and chemokine levels between myeloablative and nonmyeloablative SCT patients with and without GVHD. Patients with and without GVHD were subdivided into those who underwent myeloablative transplantation and those who underwent nonmyeloablative transplantation. Solid lines represent median markers. P values were calculated using the Mann-Whitney U test (*P < .05; **P < .01).
Patients were also subdivided into those that received T-cell depletion with alemtuzumab and those who had an unmanipulated stem cell graft. Again, CXCL10 was significantly elevated (P < .05) in both patient groups who experienced aGVHD compared with controls (data not shown). The only difference between the groups was in CXCL8 serum levels that were reduced in patients with aGVHD compared with controls who received a T-cell–replete graft (P < .05; data not shown).
Chemokine receptor expression is not increased on T cells in peripheral blood
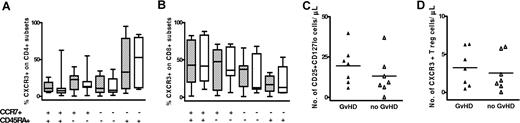
The finding that CXCL10 serum levels were significantly raised in GVHD patients after SCT suggested that expression of its ligand, CXCR3, might be elevated on T cells within peripheral blood. CXCR3 expression was therefore determined on naive, central memory, effector memory, and effector memory revertant CD4+ and CD8+ T cells using CCR7 and CD45RA as markers (Figure 3A-B, respectively). T regulatory (reg) cells also express CXCR3. T reg numbers did not differ between aGVHD patients and controls (Figure 3C), nor did their expression of CXCR3 (Figure 3D). Thus, despite higher CXCL10 levels in serum from patients with aGVHD, CXCR3 expression on different T-cell subsets in the periphery was not altered compared with control SCT patients.
CXCR3 expression on naive, memory, and regulatory T-cell subsets from SCT patients with and without GVHD. CXCR3 expression on naive (CCR7+CD45RA+), central memory (CCR7+CD45RA−), effector memory (CCR7−CD45RA−), and effector memory revertant (CCR7−CD45RA+) CD4+ (A) and CD8+ (B) T cells from SCT patients with GVHD (▨) and without GVHD (□). The y-axis represents the proportion of T cells positive for CXCR3. Bars represent median and range. (C) Regulatory T-cell numbers in PBMCs. PBMCs were gated on CD4+CD45RO+ T cells and T reg frequency was determined by their positive expression for CD25 and low expression for CD127. (D) CXCR3 expression on T reg cells. ▴ indicates SCT patients with GVHD; ▵ indicates control SCT patients without GVHD. The y-axis represents the number of cells positive for marker(s) per microliter of peripheral blood. Solid line represents the mean.
CXCR3 expression on naive, memory, and regulatory T-cell subsets from SCT patients with and without GVHD. CXCR3 expression on naive (CCR7+CD45RA+), central memory (CCR7+CD45RA−), effector memory (CCR7−CD45RA−), and effector memory revertant (CCR7−CD45RA+) CD4+ (A) and CD8+ (B) T cells from SCT patients with GVHD (▨) and without GVHD (□). The y-axis represents the proportion of T cells positive for CXCR3. Bars represent median and range. (C) Regulatory T-cell numbers in PBMCs. PBMCs were gated on CD4+CD45RO+ T cells and T reg frequency was determined by their positive expression for CD25 and low expression for CD127. (D) CXCR3 expression on T reg cells. ▴ indicates SCT patients with GVHD; ▵ indicates control SCT patients without GVHD. The y-axis represents the number of cells positive for marker(s) per microliter of peripheral blood. Solid line represents the mean.
The expression of CXCR3 on T cells within peripheral blood is unlikely to be an accurate reflection of expression on lymphocytes at the site of tissue damage. The clinical manifestations of aGVHD are seen predominantly in the skin, gut, and liver. The expression of chemokine receptor molecules that define the tissue-specific migration of lymphocytes was therefore determined on cells within peripheral blood. CLA was used to define T cells with skin homing properties (Figures S1C,D, available on the Blood website; see the Supplemental Materials link at the top of the online article) and β7 integrin for identifying cells with gut-homing potential (Figures S1E,F). No differences in the percentage or absolute count of T cells expressing these markers were observed between patients with aGVHD and control patients after SCT.
Chemokine expression in aGVHD skin biopsies
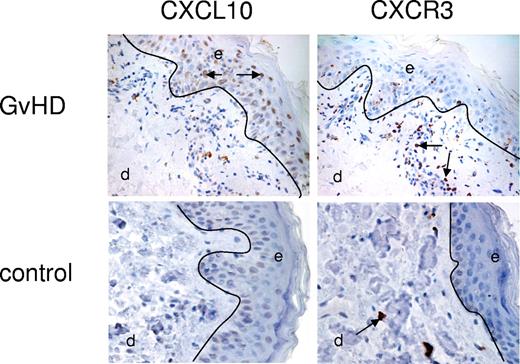
In order to ascertain if CXCL10 or CXCR3 expression was increased at the site of GVHD, skin biopsies were obtained from patients at time of aGVHD and compared with skin samples from healthy controls. CXCL10 and CXCR3 expression was determined by immunohistochemistry. CXCL10 expression was expressed by basal keratinocytes at the epidermal layer of the skin in all 11 biopsies taken from patients with GVHD (Figure 4). In contrast, CXCL10 expression was seen at a low level in only 1 biopsy from 5 healthy controls. In addition, the dermis in patients with GVHD was characterized by both interstitial and perivascular infiltrates, which in some cases merged with the epidermal layer. These infiltrates contained considerable numbers of CXCR3+ cells (Figure 4). Although scattered CXCR3+ T cells were identified in the dermal layers of control patients (Figure 4), no significant interstitial infiltrates were observed.
Expression of CXCL10 and CXCR3 in skin biopsies from patients with aGVHD and control patients. A representative example from 11 patients with GVHD and 5 control patients. Single enzymatic staining for CXCL10 (ligand; brown stain) showed significant expression of CXCL10 in basal keratinocytes of the epidermal (e) layer of aGVHD skin versus no expression on control tissue. CXCR3 expression (brown stain) was located to the dermal (d) infiltrates located in close proximity to the epidermis in aGVHD skin. Control tissue lacked these infiltrates but showed some CXCR3 expression on scattered T cells within the dermis. Arrows indicate positive staining. See “Immunohistochemistry” for complete image acquisition information.
Expression of CXCL10 and CXCR3 in skin biopsies from patients with aGVHD and control patients. A representative example from 11 patients with GVHD and 5 control patients. Single enzymatic staining for CXCL10 (ligand; brown stain) showed significant expression of CXCL10 in basal keratinocytes of the epidermal (e) layer of aGVHD skin versus no expression on control tissue. CXCR3 expression (brown stain) was located to the dermal (d) infiltrates located in close proximity to the epidermis in aGVHD skin. Control tissue lacked these infiltrates but showed some CXCR3 expression on scattered T cells within the dermis. Arrows indicate positive staining. See “Immunohistochemistry” for complete image acquisition information.
Discussion
aGVHD is a significant cause of morbidity and mortality in patients following allogeneic SCT. GVHD is initiated by presentation of recipient alloantigen to donor T cells by host antigen-presenting cells. Recent murine studies have indicated that after transplantation, donor T cells migrate initially to lymphoid tissue, where they undergo expansion for up to 3 days prior to dissemination to target organs.2 In murine models, the systemic release of inflammatory cytokines such as TNFα and IFNγ by T cells activated within lymphoid tissue leads to the release of the chemokines CXCL9, CXCL10, and CXCL11 at tissue sites.2 These recruit CXCR3+ T cells, which are believed to mediate the tissue damage characteristic of GVHD. A second wave of T cells that produce CCR5 ligands, such as CCL5, is then induced and may play a role in limiting tissue damage.7
The importance of the cytokine cascade in the development of GVHD has been studied extensively, but a consensus view has not yet emerged. Release of proinflammatory cytokines such as IL-6, IL-8 (CXCL8), TNF-α, and IFN-γ has been correlated positively with aGVHD.8-14 Although IL-6, IL-8, TNF-α, and IFN-γ were studied in our cohort, they did not serve as significant markers, except for in the nonmyeloablative transplantation group, where IL-8 (CXCL8) levels were significantly elevated in the aGVHD group. This could be attributed to different treatment regimens used by different transplantation centers.
Chemokines and their receptors play a critical role in leukocyte chemoattraction,15 and murine models have demonstrated their importance in the pathogenesis of aGVHD.2 As such, they may prove to be a more accurate and sensitive marker for the development of GVHD, but very little has been reported on their potential role in human disease. Faaj et al6 studied CD4+ T-cell chemokine expression in 15 pediatric SCT patients with skin GVHD and observed recruitment of CD4+CCR10+ T cells to inflammatory dermal infiltrates, together with enhanced epidermal expression of CCL27. Another study showed a correlation between CCL5 and other reported aGVHD markers, IL-6, TNF-α, and soluble IL-2R, in serum after allogeneic SCT.16
In this study, we looked at the serum level of 9 cytokines and 8 chemokines in serial samples from 35 SCT patients with and without aGVHD. Patients had received myeloablative or nonmyeloablative conditioning regimens, and those with severe infection were excluded in order to remove potential confounding factors for chemokine production. We found that the IFN-γ–inducible chemokine, CXCL10, was significantly elevated in patients with aGVHD. Serum CXCL10 levels were increased in all patients at the time of aGVHD and fell to basal levels following resolution of disease; however, the severity of aGVHD did not correlate with increased CXCL10 expression (data not shown). The average increase in serum levels was 2-fold, and this was maintained during the 100-day aGVHD window. Murine GVHD models have also demonstrated an increase in serum CXCL10 at the time of GVHD,17 and reinforce the view that inflammatory T helper 1 (TH1) immune responses are critical in initiating tissue damage. In our study, the increase in CXCL10 was seen after both myeloablative and nonmyeloablative SCT. Mapara et al17 have recently studied the influence of murine SCT conditioning regimens on the induction of chemokine expression and demonstrated that myeloablative conditioning led to greater CXCL10 expression.
The ligand for CXCL10 is CXCR3, and the increased serum level of CXCL10 suggests that CXCR3+ T cells may play a critical role in the development of GVHD. Indeed, levels of CXCL10 are also increased in murine GVHD,17 and in another study the transfer of CXCR3−/− T cells has been associated with decreased GVHD mortality in contrast with wild-type splenocytes.17,18 In our study, no differences in the number of CXCR3+ T cells or CXCR3 expression on naive, memory, or regulatory T-cell subsets within peripheral blood were observed between patients with aGVHD and control patients, although this may reflect homing of such populations to peripheral tissue. Skin was the predominant target organ for aGVHD in our cohort, and biopsies were taken to determine the immunohistochemical expression of CXCR3 and CXCL10 in skin GVHD. Significant expression of CXCL10 was observed in basal keratinocytes of the epidermis in patients with GVHD, similar to what has been described in atopic dermatitis19 and lichen planus.20,21 The pathology of cutaneous aGVHD is characterized by interstitial dermal T-cell infiltrates, and in our cohort, dermal CXCR3+ T cells were identified in close proximity to epidermal CXCL10 expression, supporting a direct pathogenic role for these T cells. In contrast, skin from control biopsies showed only scattered CXCR3+ T cells in the dermal layer and blood vessels, with no inflammatory foci. Since T reg cells can express chemokine receptors such as CXCR3, FoxP3 expression was also examined in 4 GVHD skin biopsies. FoxP3+ cells were identified in only 1 patient and here represented 2% of the inflammatory infiltrate (data not shown), suggesting that the CXCR3+ cells were predominantly effector cells. Most inflammatory conditions such as psoriasis and eczema express significant levels of FoxP3+ T cells in skin lesions,22,23 although a paucity of these cells has been observed for some conditions, such as cutaneous lupus erythematosus,22 leishmaniasis,23 and leukemic Sézary syndrome.24 Interestingly, mucosal biopsies from patients with acute and chronic GVHD are numerically deficient for FoxP3 in contrast with those patients who suffer infectious complications after SCT.25 Several studies have examined the frequency and function of T reg cells in GVHD, but these have focused on measurements in peripheral blood. The lack of T regs in mucosal25 and now skin biopsies thus provides strong evidence for a potential pathophysiologic mechanism for the uncontrolled inflammation that occurs in GVHD.
Interestingly, CXCL9 and CXCL11, which share the same receptor (CXCR3) as CXCL10, were not elevated in the serum from patients with aGVHD. This indicates that it is the specific interaction of CXCL10 with CXCR3 that is critical for the recruitment of T cells to the skin in patients with aGVHD. The precise physiologic roles of CXCL9, CXCL10, and CXCL11 remain uncertain, and increased understanding in this regard will provide more clues as to the pathogenesis of skin aGVHD.
Serum CCL5 levels were also increased in patients with aGVHD in the first month after SCT, which is intriguing given the proposed role of this chemokine in the late stages of murine GVHD and the protective effect of CCR5 blockade in hepatic GVHD.26 In our study, there was no temporal dissociation in the increase in serum CXCL10 or CCL5 levels, which may partially reflect biological differences between human and experimental murine disease. The serum level of CCL2, CCL3, or CCL11 was not influenced by the development of GVHD.
We propose that serum CXCL10 is an accurate marker for the development of aGVHD, and this may prove of value in clinical decision-making. In addition, it is noteworthy that current therapeutic approaches for GVHD are not optimal. Although corticosteroids are usually effective in controlling aGVHD, they are associated with general immunosuppression, and the outlook for patients with steroid-unresponsive disease remains very poor. Understanding of the specific molecules that are critical in the pathogenesis of aGVHD may help to facilitate earlier diagnosis of disease and direct the development of more specific therapies. Our data suggest that the CXCL10-CXCR3 interaction is an important therapeutic target to modify the development of aGVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank Paul Murray, John Gregory, and Gary Reynolds for help with immunohistology, and Andrew Filer for skin biopsies from healthy knee surgery controls.
This work was supported by the Leukemia Research Fund, grant 9904.
Authorship
Contribution: K.P.P. designed and performed experiments, analyzed and interpreted data, performed statistical analysis, and drafted the paper. C.H. performed the experiments. S.J.C. performed experiments and analyzed the data. J.A. consented and collected clinical material and patient information. S.N. performed some experiments. P.M. and C.C. consented and provided clinical information on patients. P.A.H.M. drafted the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karen P. Piper, CRUK Institute for Cancer Studies, University of Birmingham, Vincent Drive, Birmingham, B15 2TT United Kingdom; e-mail: k.p.piper@bham.ac.uk.