Abstract
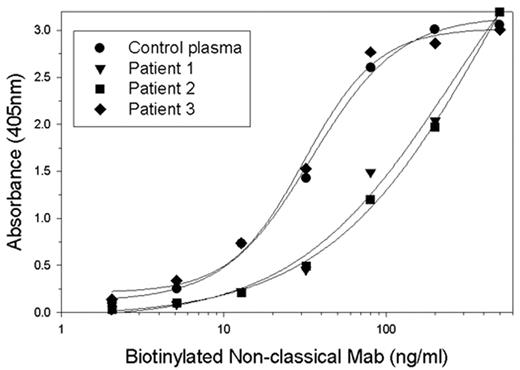
Approximately 30% of patients with severe hemophilia A will develop inhibitory antibodies to factor VIII (fVIII inhibitors). The immune response to fVIII currently is the most significant complication in the management of patients with hemophilia A. In addition, autoimmune antibodies to fVIII can develop in non-hemophiliacs, producing acquired hemophilia A, which frequently produces life- or limb-threatening bleeding. These inhibitors primarily are directed against the A2 or C2 domains of fVIII. The human response to the C2 domain of fVIII classically has been thought to inhibit fVIII activity by blocking its binding to phospholipid. We recently characterized the antibody response to the C2 domain of human fVIII in a murine hemophilia model and described 5 structural groups of antibodies. Groups A, AB, and B are classical anti-C2 antibodies. Groups BC and C consist of non-classical anti-C2 antibodies that inhibit the proteolytic activation of fVIII but do not block the binding of fVIII to phospholipid. Most non-classical antibodies have inhibitor titers greater than 10,000 Bethesda units/mg IgG. To determine if non-classical antibodies are present in fVIII inhibitor patients, patient plasmas were tested in an ELISA for their ability to block the binding of representative antibodies from the different anti-human fVIII C2 antibody groups. Classical and non-classical monoclonal antibodies (MAbs) were biotinylated and serially diluted into either fVIII deficient plasma or patient inhibitor plasma and then added to microtiter wells coated with fVIII. The ability of patient plasma to block the binding of the murine MAbs to fVIII was determined. A total of 16 patient plasmas were assessed: 4 from patients with a C2 predominant response, 2 with a non-C2 predominant response, and 10 with unknown specificities. Three of the 4 patients with C2 predominant responses had non-classical anti-C2 antibodies, while the 2 with non-C2 predominant responses did not. In the unknown plasmas, 6 of 10 had evidence of non-classical antibodies. Figure 1 shows representative results of the effect of 3 patient plasmas on the binding of a biotinylated non-classical MAb to fVIII. Patient plasmas 1 and 2 blocked MAb binding while patient plasma 3 did not. This study indicates that the majority of patients with fVIII inhibitors have non-classical anti-C2 antibodies in their response to fVIII.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal