Abstract
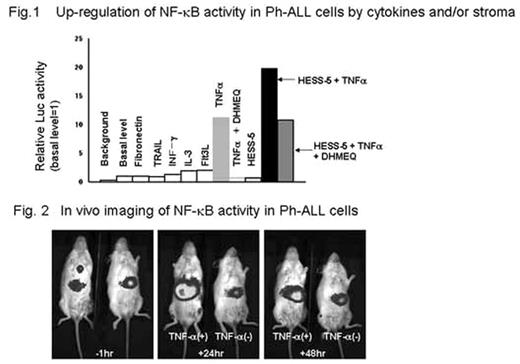
Constitutive activation of NF-kB via Bcr-Abl has been demonstrated in primary blast cells and cell lines derived from Philadelphia chromosome (Ph) -positive acute lymphoblastic leukemia (Ph-ALL). However, the microenvironmental (cytokine and/or stroma cell) regulation of NF-kB activity in Ph-ALL has not been clarified. To gain insight into these unsolved issues, we lentivirally transduced IMS-PhL1 cells with NF-kB/luciferase (kB/Luc) reporter construct and established a bioluminescence imaging model of Ph-ALL for in vitro and in vivo analysis. Unstimulated PhL1-kB/Luc cells revealed a weak but significant Luc activity over the background, verifying constitutive activation of NF-kB. Among a panel of cytokines, only TNFa potently up-regulated Luc activity in PhL1-kB/Luc cells about 10-fold over the basal level. DHMEQ, a specific inhibitor of nuclear translocation of p65, eradicated constitutive and TNFa-inducible NF-kB activity of PhL1 cells and induced their substantial apoptosis dose-dependently. A series of Ph-ALL cell lines were similarly sensitive to treatment with DHMEQ, suggesting the critical role of constitutive NF-kB activity in survival of Ph-ALL cells. When PhL1-kB/Luc cells were seeded onto a layer of murine HESS-5 stroma cells, Luc activity was not changed. Intriguingly, TNFa stimulation of PhL1-kB/Luc cells in the presence of HESS-5 cells caused synergistic enhancement of Luc activity up to 20 fold over the basal level. This up-regulation was canceled by blocking cell to cell contact with a transwell membrane, suggesting that the direct cell contact may be essential for such a synergistic effect. In HESS-5 cells, NF-kB activity was markedly augmented in response to TNFa, but this up-regulation was not sensitive to DHMEQ. Furthermore, the inhibitory effects of DHMEQ on Luc activity as well as viability of TNFa-treated PhL1-kB/Luc cells were significantly alleviated in the presence of HESS-5 cells. (Fig 1) Taken together, TNFa-triggered HESS-5 cells are likely to up-regulate NF-kB activity of PhL1 cells through DHMEQ-insensitive alternate pathway. Finally, PhL1-kB/Luc cells were transplanted into NOD-SCID mice and subjected to periodic monitoring with a CCD camera. (Fig 2) We successfully detected constitutive and TNFa-inducible bioluminescent signals of leukemia cells. Unexpectedly, by far the strongest constitutive signal was captured in the liver, although massive leukemic infiltration was observed in bone marrow and spleen, implying that hepatic microenvironment may offer proper stimuli to activate NF-kB and constitute leukemic niche. In conclusion, p65-dependent and independent pathways are involved in microenvironmental up-regulation of NF-kB activity, which contribute to survival, expansion and presumably drug-resistance of Ph-ALL cells. The present bioimaging model helps us to dissect the regulatory mechanism of NF-kB signal by cytokines and cellular interactions.
Up-regulation of NF-κB activity in Ph-ALL cells by cytokines and/or stroma.
Fig. 2 In vivo imaging of NF-κB activity in Ph-ALL cells
Up-regulation of NF-κB activity in Ph-ALL cells by cytokines and/or stroma.
Fig. 2 In vivo imaging of NF-κB activity in Ph-ALL cells
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal