Abstract
Background: We sought to retrospectively analyze the clinical differences, at diagnosis and throughout the disease course, between patients with primary myeloifbrosis whom undergo blastic transformation (PMF-BP) and those whom expire from PMF complications without undergoing transformation.
Methods: An international collaborative database of patients with PMF which progressed to PMF BP, and a control group of individuals with PMF who expired secondary to PMF was created. Data regarding clinical course, bone marrow, karyotypic, quantitative JAK2V617F analysis (when able), laboratory values, and therapy at the diagnosis of PMF, PMF BP and up to 4 return visits (R1-4) in between these milestones was abstracted.
Results: COMPARISON AT DIAGNOSIS OF PMF: 136 cases of PMF who eventually underwent PMF-BP were included, with a control group of 42 PMF patients, were analyzed. Both groups of patients had similar demographic (median age at diagnosis 59 years (range 23–83)/ 59.1 (15–93); and disease parameters (median hemoglobin 10 g/dL (range 2–15.7) /9.8 (4.9–14.6); median platelet count 181 x 109/L (range 7–1400)/168 (16–1916)), and Lille PMF prognostic scores (p = n.s.) for PMF-BP vs. PMF control group respectively. Additionally, blasts (peripheral blood /marrow) were a median of 0% (range 0–18%)/ 2% (range 0–16%) for the study group versus 0.25% (range 0–5%)/ 3% (range 0–4%) (p = n.s. for both) for the control group, respectively. Both groups had similar rates of requiring therapy (86%; 93%) and survival from diagnosis (median 34 months (range 2–441); 29 months (1–236)) (p = n.s.). However, lactate dehydrogenase (LDH) (although high in both groups) was higher at diagnosis (median 802 IU/L (range 83–10353) vs. 416 IU/L (124–2197)) (p=0.04), as well as an abnormal marrow karyotype (85% vs. 25%; p<0.001) amongst the PMF BP group.
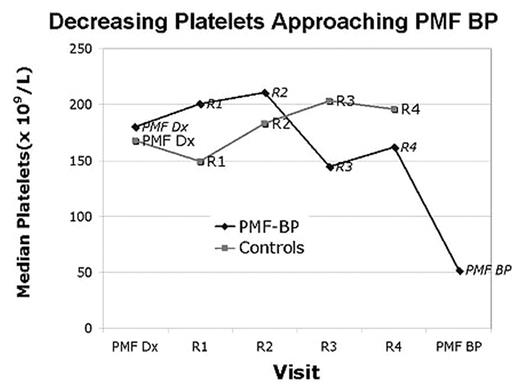
SUBSEQUENT CLINICAL COURSE: Analyzing the clinical evolution between groups demonstrated persistently higher LDH and worsening thrombocytopenia in the PMF-BP cohort (see figure).
Decreasing Platelets Approaching PMF BP
Peripheral blood blasts increased in both groups, but values above 10% were unique to the PMF BP group. Additionally marrow karyotype displayed clonal evolution developed in 56% of the PMF-BP patients as opposed to 14% of controls (p<0.001). Initial and serial quantitative JAK2V617F mutation analysis (available from 20 /19 patients from the PMF-BP and control group at diagnosis, with 11 / 12 serial samples) showed no difference between the groups, nor a pattern of mutation burden elevation with transformation to leukemia in the study group.
Conclusions: Increasing LDH, peripheral blood blast percentages >10%, and clonal evolution are features commonly seen in PMF patients whom evolve to blast phase and may be a harbinger of movement towards PMF BP.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal