Abstract
The WT1 gene on chromosome 11p13 encodes a zinc-finger protein regulating gene transcription. A recent study conducted on 70 CN-AML patients (pts) found that 7 (10%) harbored WT1 mutations (WT1mut) and suggested that WT1mut are associated with failure to achieve complete remission (CR) (
Summers et al., Leukemia 2007;21:550
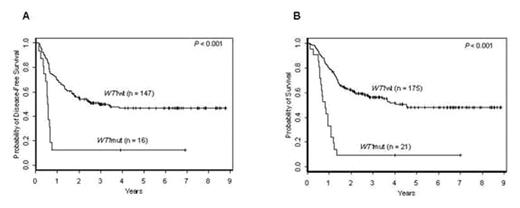
). However, the prognostic impact of WT1mut remains to be elucidated in a larger set of homogeneously treated adults with de novo CN-AML and in the context of established prognostic molecular markers. We studied the prevalence and prognostic impact of WT1mut in 196 younger [<60 years (yrs)] adults with de novo CN-AML who were intensively treated on frontline CALGB protocols incorporating autologous stem cell transplantation for consolidation, i.e. 9621 or 19808. The median follow-up for pts alive was 4.2 yrs (range, 1.2–8.9 yrs). Diagnostic bone marrow and/or blood specimens were studied for WT1mut by denaturing high-performance liquid chromatography and DNA direct sequencing. The samples were also analyzed for FLT3-ITD, FLT3-TKD, MLL-PTD, NPM1 and CEBPA mutations, and ERG and BAALC expression levels. Twenty-one (11%) of the 196 pts had WT1mut [15 had WT1mut in exon 7 (WT1mut7), 5 WT1mut in exon 9 (WT1mut9) and 1 WT1mut in both exons]. WT1mut7 were frameshift or nonsense mutations predicted to result in a truncated WT1 protein; WT1mut9 were missense mutations leading to single amino-acid substitutions. At diagnosis, WT1mut pts had higher white blood cell counts (WBC; P=0.01), more frequently harbored FLT3-ITD (P=0.06) and were more often high ERG (P=0.01) and high BAALC (P=0.006) expressers than unmutated WT1 (WT1wt) pts. While CR rates were not significantly different between WT1mut and WT1wt pts (76% vs 84%, P=0.36), WT1mut pts had worse disease-free survival [(DFS); P<0.001; 3-yr DFS rates, 13% vs 50%; Figure A] and worse overall survival [(OS); P<0.001; 3-yr OS rates, 10% vs 56%; Figure B] than WT1wt pts. In a multivariable analysis, WT1mut independently predicted worse DFS (P=0.009) when controlling for CEBPA (CEBPAmut vs CEBPAwt, P=0.005) and FLT3-ITD/NPM1 risk status (FLT3-ITD-negative and NPM1mut vs all other combinations of FLT3-ITD and NPM1 mutation status, P<0.001). The risk of relapse was 2.9 times higher for WT1mut pts than for WT1wt pts (95% CI: 1.3–6.3). WT1mut also independently predicted worse OS (P<0.001), and conferred a 3.6 times higher risk of death compared with WT1wt pts (95% CI: 1.8–7.2), when controlling for CEBPA (P=0.02), FLT3-ITD/NPM1 risk status (P<0.001), and WBC (P=0.01). In conclusion, we show here for the first time, in a relatively large set of intensively treated younger CN-AML pts, that WT1mut independently predicts very poor outcome. We propose that in future trials, WT1mut analysis should be considered for molecularly-based risk assessment and risk-adapted treatment stratification of CN-AML pts.Author notes
Disclosure: No relevant conflicts of interest to declare.
2007, The American Society of Hematology
2007

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal