Abstract
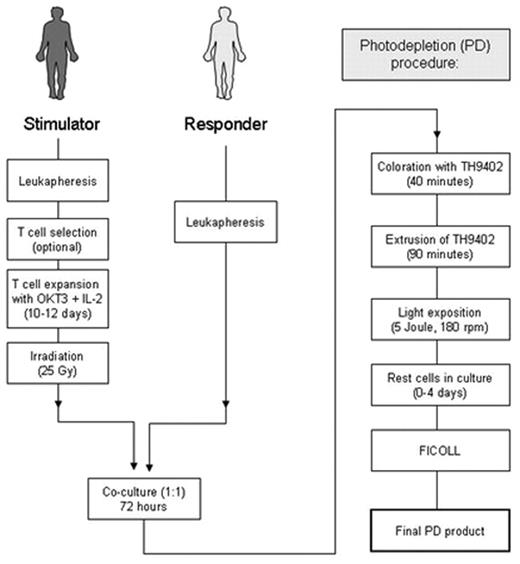
Selective allodepletion (SD) is a strategy to eliminate host-reactive donor T-cells from hematopoietic stem cell allografts to prevent graft versus host disease (GvHD) while conserving useful donor immune functions. To overcome fluctuations in activation-based surface marker expression and achieve a more consistent and effective allodepletion, we investigated a photodepletion (PD) process targeting activation-based changes in p-glycoprotein that result in an altered efflux of the rhodamide-based photosensitizer TH9402. Expanded lymphocytes, generated using anti-CD3 and IL-2, were co-cultured with responder cells from HLA-matched or mismatched donors. Optimal results were achieved when co-cultured cells were incubated with 7.5 μM TH9402, followed by dye-extrusion and exposure to 5 Joule/cm2 light energy at 5x106 cells/ml.
In six mismatched stimulator-responder pairs, the median reduction of allo-reactivity was 474-fold (range, 43–864) when compared to the unmanipulated responder. Third-party responses were maintained with a median 1.4-fold (range, 0.9–3.3) reduction. In three matched pairs alloreactive helper T-lymphocyte precursors (HTLps) were reduced below 1:100,000 while third party responses remained above 1:10,000. CMV-specific T cells and responses to SEB were preserved. The intracellular TH9402 amount after extrusion correlated significantly with the depletion efficacy obtained against the original stimulator (r2=0.76; p=0.0235) but not against the 3rd party (r2=0.05; p=0.68). This establishes a clinical scale process capable of highly efficient, reproducible, selective removal of alloreactive lymphocytes from lymphocyte transplant products performed under current good manufacturing practice (cGMP) being reliably capable of generating at least 107/kg SD T cells for transfusion using a single PD session. In establishing this SD approach, we set out to design a technique that could ultimately be adopted in many cell transplant processing centers. Recent review of our Investigational New Drug (IND) application by the Food and Drug Administration (FDA) and review of our clinical protocol by our Institutional Review Board have been successful, and a clinical trial is in progress where recipients with hematological malignancies are given a myeloablative preparative regimen followed by a transplant of selected CD34+ cells together with 5 x 106 SD T cells/kg from HLA-matched sibling donors.
Author notes
Disclosure:Employment: C.S. and A.V. are employed by Kiadis Pharma Inc. Ownership Interests: C.S. and A.V. are holding start-up stocks with Kiadis Pharma Inc (formerly Celmed). Research Funding: Part of this work was supported by a collaborative research and development agreement (CRADA) between Kiadis (formerly Celmed) Inc. and the NHLBI/NIH. SM received grant support from the Dr-Mildred-Scheel-Stiftung fuer Krebshilfe, Germany, a private welfare organization to support cancer research. The clinical trial that has been initiated based on the present work is being perfomed under a clinical trial agreement (CTA) between Kiadis Inc and the NIH/NHLBI. Membership Information: Not sure what that means: I am invited ASH reviewer for immunotherapy. Off Label Use: The Theralux photodynamic treatment system is being tested in a clinical trial at the NIH under an IND as stated in the abstract.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal