Abstract
Previous studies have shown that granulocyte colony stimulating factor (G-CSF) mobilization skews T-cells toward a type 2 cytokine profile, potentially impacting GVHD and other immune mediated events that occur after allogeneic hematopoietic stem cell transplantation (HCT). AMD3100, a selective antagonist of CXCR4, rapidly mobilizes hematopoietic progenitor cells into the circulation, has a synergistic effect on CD34+ cell mobilization when combined with G-CSF, and is currently being evaluated as a single agent to mobilize allografts. Apheresis collections mobilized with a single injection of AMD3100 contain a similar number of T-cells as those collected following 5 daily doses of G-CSF. We investigated whether T-cells mobilized with AMD3100 undergo changes in cytokine polarization status as described to occur with G-CSF mobilization. Using real time PCR, we investigated the expression of 84 genes associated with TH1, TH2, and TH3 T-cell pathways at baseline and following mobilization with a single injection of AMD3100 (dosed at 240 or 320 mcg/kg; n=12 subjects) or following 5 daily doses of G-CSF(n=5 subjects). RNA was extracted from CD3+ T-cells isolated using immunomagnetic beads (>95% purity) from PBMCs collected immediately before mobilization and 6 hours after AMD3100 administration or 5 days after G-CSF mobilization. The RT2 Profiler ™ PCR Array was used which contains pathway specific cytokine genes associated with TH1, TH2, and TH3 cells. Expression levels of 16 genes changed significantly (false discovery rate=0.10) from baseline following G-CSF mobilization; 9 genes were up-regulated and 7 genes were down-regulated from baseline. Five up-regulated and 4 down-regulated genes had greater than a 2-fold change in expression (Figure). In contrast, none of the 84 genes examined, including the 16 altered with G-CSF, changed significantly following AMD3100 administration. Our results are concordant with current literature that shows the expression of several genes effecting T-cell cytokine polarization are altered in G-CSF mobilized T-cells. It has been suggested that the TH2 polarization in G-CSF mobilized products contributes to the comparable incidence of acute GVHD and the higher incidence of chronic GVHD compared to bone marrow allografts. In contrast, T-cells mobilized with AMD3100 appear similar to non-mobilized T-cells, and do not undergo a change in TH1- and TH2-related gene expression. Whether the differences in cytokine polarization of T-lymphocytes mobilized with AMD3100 compared to G-CSF will impact immune reconstitution or other immune sequela (i.e. GVHD, graft-vs.-tumor) associated with HCT is currently being assessed in a pilot allogeneic transplantation trial in humans using AMD3100 to mobilize donors.
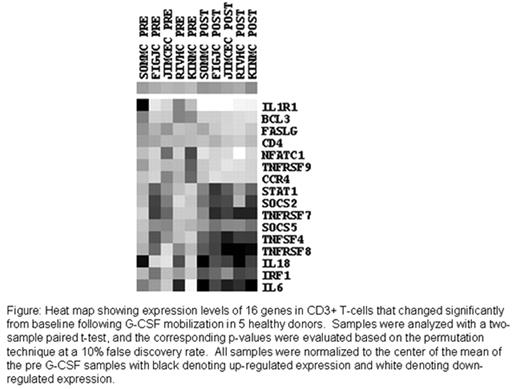
Heat map showing expression levels of 16 genes in CD3+ T-cells that changed significantly from baseline following G-CSF mobilization in 5 healthy donors. Samples were analyzed with a two-sample paired t-test, and the corresponding p-values were evaluated based on the permutation technique at a 10% false discovery rate. All samples were normalized to the center of the mean of the pre G-CSF samples with black denoting up-regulated expression and white denoting down-regulated expression.
Heat map showing expression levels of 16 genes in CD3+ T-cells that changed significantly from baseline following G-CSF mobilization in 5 healthy donors. Samples were analyzed with a two-sample paired t-test, and the corresponding p-values were evaluated based on the permutation technique at a 10% false discovery rate. All samples were normalized to the center of the mean of the pre G-CSF samples with black denoting up-regulated expression and white denoting down-regulated expression.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal