Abstract
Genetic modification of clinical-grade T cells is undertaken to augment function, including redirecting specificity for desired antigen. We and others have introduced a chimeric antigen receptor (CAR) to enable T cells to recognize lineage-specific tumor antigen, such as CD19, and early-phase human trials are currently assessing safety and feasibility. However, a significant barrier to next-generation clinical studies is developing a suitable CAR-expression vector capable of genetically modifying a broad population of T cells. Transduction of T cells is relatively efficient, but it requires specialized manufacture of expensive clinical-grade recombinant virus. Electro-transfer of naked DNA plasmid offers a cost-effective alternative approach, but the inefficiency of transgene integration mandates ex vivo selection under cytocidal concentrations of drug to enforce expression of selection genes to achieve clinically-meaningful numbers of CARneg T cells. We now report an improved approach to efficiently generating T cells from peripheral blood with redirected specificity. This was accomplished by introducing DNA plasmids from the Sleeping Beauty transposon/transposase system to directly express a CD19-specific CAR in both memory and effector T cells without co-expression of immunogenic drug-selection genes. The success of this approach was based upon the rationale design of
a next-generation codon-optimized CD19-specific CAR capable of coordinated signaling through chimeric CD28,
CD19+ artificial antigen-presenting cells (aAPC) derived from K562 and expressing desired co-stimulatory molecules, and
electro-transfer of two SB DNA plasmids expressing CAR transposon and an improved transposase.
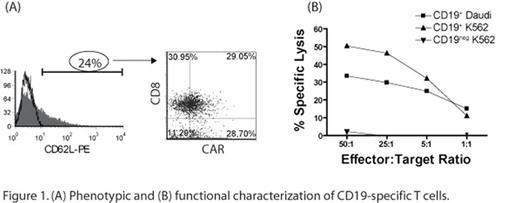
We report that introduction of a two-component SB system into primary human T cells results in efficient (∼60-fold improved expression compared with electro-transfer without transposase) and stable CAR gene transfer (60 fold as compared to single plasmid control) which can be numerically expanded to clinically-meaningful numbers within weeks on CD19+ aAPC, without the need for addition of drug-selection, and with the outgrowth of CD8+ and CD4+ CAR+ T-cell sub-populations. The improved CAR expression is due to SB transposon/transposase integration into chromosomal DNA compared with rare non-homologous end-joining process that mediates integration by electroporation alone. We demonstrate that the CAR+ T cells expressed memory cell markers (Figure 1A) as well as redirected-killing function of an effector-cell phenotype (Figure 1B). Our data have implications for improved in vivo therapeutic potential as memory T cells are associated with long-term persistence after adoptive transfer.
(A) Phenotypic and (B) funtional characterization of CD 19-specific T cells.
Author notes
Disclosure:Research Funding: Federal, private, institutional, and philanthropic funds were used in support of this research.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal