Abstract
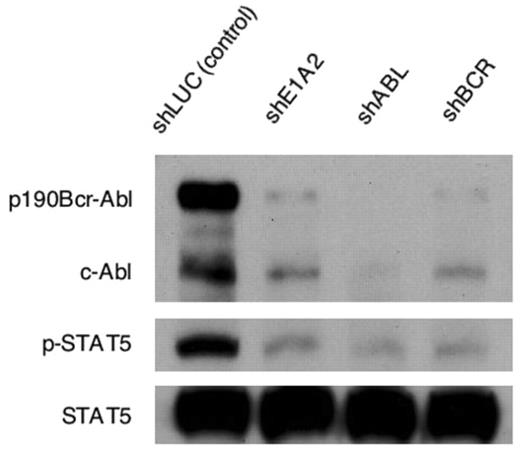
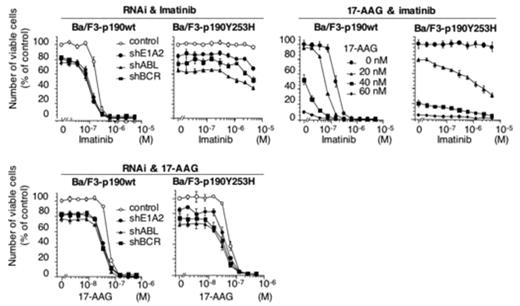
The 190kD (p190) and 210kD (p210) Bcr-Abl proteins are responsible for the development and progression of Philadelphia chromosome (Ph)-positive leukemia. We applied RNA interference (RNAi) technology to specific killing of p190+ cells. After screening a series of small hairpin RNA (shRNA) targeting p190, we determined the optimal sequences for gene silencing in the BCR (shBCR), junctional (shE1A2) and ABL (shABL) region of p190, respectively. Then, p190+ (KOPN30), p210+ (K562) and both negative (HL60) cell lines were infected with lentiviral vectors encoding these shRNAs, resulting in an efficient killing of KOPN30 cells. K562 cells were also sensitive to the two other than junction-specific shRNA and none of the anti-p190 shRNAs could affect survival of HL-60 cells, although shABL appeared to have some inhibitory effects on their growth, suggesting that Abl may be positively involved in proliferation of HL60 cells. Furthermore, colony formation derived from CFU-GEMM, BFU-E and CFU-GM was not influenced by shBCR. On the contrary, CFU-GEMM and BFU-E-derived colony formation was significantly suppressed by shABL, suggesting that Abl may play a certain role especially in normal erythropoiesis. Nonetheless, anti-p190 shRNAs may become therapeutic options in Ph-ALL by improvement of their delivery system such as CD19-targeted liposome formulation which we previously developed. In p190-transformed Ba/F3 cells, RNAi-mediated silencing of p190 specifically inhibited tyrosine phospohorylation of stat5 prior to their death, but did not affect phosphorylation of either Jak2, Akt, MEK1/2 or ERK1/2. These results offer another evidence for the critical role of Stat5 in BCR-ABL-induced transformation of hematopoietic cells. It is likely that p190 directly phosphorylates Stat5 and that Jak2 is remote from activation of Stat5 in this cell context, although the possible involvement of Src family kinases such as Hck and Lyn has not been excluded. In contrast, down-regulation of p190 by their treatment with 17-allyl-amino-geldanamycin (17-AAG) was associated with reduced phosphorylation of not only Stat5 but also Jak2 and Akt. shRNA targeting p190 also collaborated additively with imatinib and 17-AAG in growth inhibition of Ba/F3-p190 and BaF/3-p190Y253H cells. Collectively, RNAi-mediated silencing of p190 is a promising option both for therapeutic application and for delineating signal transduction in p190-expressing leukemia.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal