Abstract
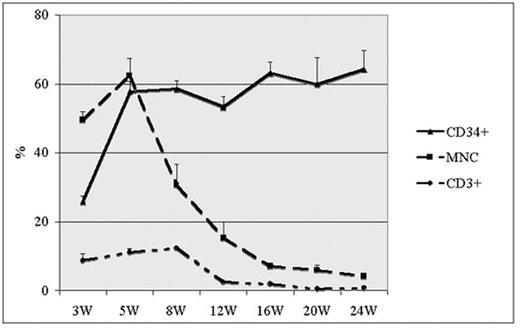
Despite sophisticated in vitro assays for primitive hematopoietic stem cells (HSC), transplantation assays remain the gold standard for HSC enumeration. Large animals better model human HSC activity, yet are cumbersome, costly, and outbred yielding high variability. Murine xenografting has been employed as a surrogate HSC assay, yet several remaining issues requiring further study include optimal conditioning, HSC source, cell dose, graft composition, and the lack of human erythroid output. NOD/LtSz-scid IL2Rgnull recipient mice were utilized in a series of transplantation studies to address these questions. We injected busulfan at a range of doses with or without low-dose irradiation, with 50mg/kg yielding consistent human HSC engraftment. To determine the contribution of HSCs vs mature elements to the human engraftment measured, we compared whole CB (n=6) to CB selected for CD34 (n=7) or CD3 (n=3). Human cells were detectable as early as 6 days after the transplant of whole CB or CD3 selected cells, were predominantly CD3+, peaked early, and declined after 4–8 weeks, whereas that from CD34 selected cells became detectable later, were predominantly CD20+ early and rose over the same period. The % human CD45+ cells in peripheral blood (PB) was similar between whole CB and CD34 selected graft recipients at 5 weeks (62.5±5.02% vs 57.8±2.89% P=0.46). Human CD14, CD16, CD20, CD3, CD56, IgM, IgG and CD41 positive cells were detectable from the PB, marrow and spleen of whole CB and CD34 selected graft recipients, but there were no detectable GPA positive red blood cells (RBCs). By 24 weeks, the % human CD45+ cells was significantly higher in CD34 selected graft recipients compared with the whole CB recipients (68.2±5.35% vs 4.27±0.67%, P<0.001). Human cells were also detectable in secondary transplant recipients performed after 6 months. Whole CB and CD34 selected CB grafts were then compared to CD34 selected mobilized PB grafts at equivalent doses. Human CD45+ cells in the CB group were significantly higher than in the PB group (58.5±2.51%,N=7 vs 18.4±1.92%,N=8,P<0.001). PB CD34+ dosing experiments, however, demonstrated dose dependent human engraftment (CD34+ 5x105 − 11.5±4.73% N=3; 2x106 − 18.4±1.92% N=8; 4x106 − 26.4 ±4.08% N=7). Despite the absence of human RBCs, human erythroid colonies confirmed by PCR were detectable up to 9 months, and human RBCs transfused into NOD/LtSz-scid IL2Rgnull mice were detectable up to 96 hours after infusion. We went on to determine whether human transferrin along with human cytokines (SCF, EPO, IL3, IL6, and GM-CSF) with or without anti-CD122 injected twice weekly could result in human RBC production. Though no RBCs were detectable in recipients without post-graft treatment, all mice treated with human transferrin demonstrated low-level human glycophorin A-positive cells in the circulation with no differences attributable to cytokine treatment. Our results demonstrate that busulfan conditioning produces dose dependent human HSC engraftment in the NOD/LtSz-scid IL2Rgnull mice. Further, CD34 selected CB grafts produce superior long-term engraftment rates. Finally, supplementation with human transferrin allows the detection human erythrocytes in this chimeric mouse assay, providing a potential model for the study of disorders affecting human red blood cells.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal