Abstract
Tyrosine and serine phosphorylation of the common β chain (βc) of the granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and IL-5 receptors is widely viewed as a general mechanism that provides positive inputs by coupling the receptor to signaling pathways that stimulate several cellular functions. We show here that despite the known action of Tyr577 in βc to recruit Shc–PI-3 kinase (PI3K) pathway members, Tyr577 plays, surprisingly, a negative regulatory role in cell function, and that this is mediated, at least in part, through the uncoupling of SH2-containing inositol 5′-phosphatase (SHIP) from βc. Fetal liver cells from βc/βIL-3−/− mice expressing human GM-CSF receptor α chain and βc Tyr577Phe mutant showed enhanced colony formation and expansion of progenitor cells in response to GM-CSF. Dissection of these activities revealed that basal survival was increased, as well as cytokine-stimulated proliferation. As expected, the recruitment and activation of Shc was abolished, but interestingly, Gab-2 and Akt phosphorylation increased. Significantly, the activation of PI3K was enhanced and prolonged, accompanied by loss of SHIP activity. These results reveal a previously unrecognized negative signaling role for Tyr577 in βc and demonstrate that uncoupling Shc from cytokine receptors enhances PI3K signaling as well as survival and proliferation.
Introduction
Granulocyte-macrophage colony-stimulating factor (GM-CSF) exerts its biological activities through the binding and activation of cell-surface receptors that consist of a ligand-specific α subunit (GMRα) and a common β subunit (βc).1,2 Ligand binding results in the assembly of oligomeric α and βc receptor complexes and the initiation of intracellular signaling cascades through the activation of associated tyrosine kinases.3 For example, JAK2 is critical for tyrosine phosphorylation of the cytoplasmic domain of the βc subunit and the subsequent recruitment of SH2- and PTB-domain proteins.2 These events are important for the activation of multiple downstream signaling pathways, including the PI-3 kinase (PI3K) pathway and the Ras/mitogen-activated protein kinase (MAPK) pathway. In some cases, the recruitment of signaling proteins occurs via the direct binding of SH2 or PTB domains to the cytoplasmic tail of βc, such as for SHP2 (also named PTP1D or SH-PTP2), which binds tyrosine 612 via its SH2 domain,4 and Shc, which binds tyrosine 577 via its PTB domain.5,6 The tyrosine phosphorylation of these and other proteins recruited to βc allows the further recruitment of additional SH2- and PTB-domain–signaling proteins and promotes the assembly of a network of signaling complexes on the receptor cytoplasmic tail
GM-CSF (and interleukin-3 [IL-3]) have been shown to regulate cell survival predominantly via the PI3K pathway.7 Two possible mechanisms for the recruitment of PI3K to βc have been reported. Our previous studies have shown that the phosphorylation of Ser585 of βc in response to cytokine is required for the binding of the phospho-serine–binding 14–3-3 family of proteins, and subsequent recruitment and activation of PI3K.8 We have shown that this Ser585-signaling pathway regulates cell survival alone and is not required for cell proliferation.8,9 In addition, others have shown that Tyr577 of βc binds the PTB domain of Shc and is important for subsequent recruitment of a Grb2/Gab2/PI3K complex, although its role in mediating cell survival or proliferation has remained unclear.10 Other studies have shown that Tyr577 together with Tyr612 plays a role in PI3K signaling in response to GM-CSF.11 While these studies12 have been important in identifying receptor tyrosines important for initiating positive signaling, it is now clear that receptor tyrosine residues can also promote negative regulation of intracellular signaling.13 For example, the suppressors of cytokine signaling (SOCS) family of proteins are important negative regulators of signaling and directly bind phosphotyrosine residues in cytokine receptors via their SH2 domains.12,14 Another example is the tyrosine phosphatase, SHP2, which is also recruited to cell-surface receptors via its SH2 domain and has negative effects on cytokine signaling.15 In these instances, negative regulation is important in signal termination and is critical in preventing sustained or prolonged receptor activation, which leads to deregulated cellular responses. Although a number of the proteins involved in the negative regulation of cytokine function have been recognized,13,16 identifying specific receptor tyrosine residues involved in initiating such functions has proved more difficult. In fact, understanding the receptor-proximal events in the negative regulation of cytokine function has lagged considerably behind our understanding of how receptors positively activate specific pathways.
One important mechanism by which growth factor–induced PI3K signaling is down-regulated is via lipid phosphatases. These include the hematopoietic-restricted SH2-containing inositol 5′-phosphatase, SHIP1 (originally called SHIP), and the more widely expressed SHIP2. SHIP1 and SHIP2 dephosphorylate PIP3 in the 5′ position to give PI(3,4)P2, thereby removing docking sites for PH-domain proteins and terminating downstream PI3K signaling.17 SHIP1 and SHIP2 exert tight control over PI3K signaling, ensuring the production of PIP3 is both rapid and transient in response to growth factors and cytokines.17 Such rapid and transient PI3K activation has been observed following GM-CSF (and IL-3) stimulation and would suggest an important role for the hematopoietic SHIP1 in attenuating cytokine-mediated PI3K signaling.8,18 In fact, SHIP1−/− mice demonstrate deregulated PI3K signaling in the hematopoietic compartment with expansion of granulocyte-macrophage progenitors in both the bone marrow and spleen,19,20 which exhibit enhanced sensitivity to a number of cytokines, including GM-CSF.19,21 Although these studies demonstrate an important role for SHIP1 in negatively regulating GM-CSF–mediated PI3K signaling, the mechanisms by which this occurs are not known.
We have now identified an important molecular mechanism by which the βc subunit of the GM-CSF/IL-3/IL-5 receptor negatively regulates the PI3K pathway and modulates specific GM-CSF biologic activities. We have developed a model system that allows both the functional and biochemical analysis of GM-CSF activities in primary mouse hematopoietic cells. This system uses fetal liver (FL) cells derived from βc−/−/βIL-3−/− (double knockout) mice22 that are transduced with wild-type (wt) or mutant βc. Such a system allows us to dissect the biological roles and signaling pathways regulated by specific βc residues in a βc-null background. Using this approach, we show here that the substitution of Tyr577 of βc, which is the major binding site of Shc, unexpectedly resulted in increased survival, proliferation, and colony formation in response to GM-CSF. Examining receptor-proximal and downstream components of the JAK/STAT, Ras/MAPK, and PI3K pathways suggested that the uncoupling of Shc is accompanied, paradoxically, with enhanced Gab2 phosphorylation and prolonged activation of PI3K. In addition, the Tyr577 mutant also showed a concomitant loss of activity of the SH2-containing phosphatase SHIP, but with enhanced phosphorylation of the prosurvival residue Ser585 on βc.
Materials and methods
Transduction of FL and murine BM cells
FL or bone marrow (BM) cells from mice devoid of both endogenous GM-CSF receptor beta subunits (β common and IL-3–specific β chain [βc−/−/βIL-3−/−]22,23 were transduced with bicistronic retroviral constructs to express both α and βc subunits of the human GM-CSF receptor. The constructs were based on pRUF,24,25 with an internal ribosome entry site (IRES) from the encephalomyocarditis virus (ECMV) to drive expression of the α chain. Expression of βc was from the U3 long terminal repeat (LTR). Retroviral constructs were transfected into ψ2 cells, and cells surface expressing both α and β subunits were selected using fluorescence-activated cell sorting (FACS).26 Transductions were as described,27 with stem cell factor (SCF; 100 ng/mL) as cytokine support. Transduced cells were analyzed by immunofluorescence (IF) using a monoclonal antibody (mAb) to the GM-CSF receptor α chain (4H1)28 to quantitate the percentage of transduced cells and level of expression of the receptors.
Antibodies
Polyclonal antibodies (pAbs) that specifically recognize the phospho-Tyr577 of βc were generated by immunizing New Zealand white rabbits with a phospho-peptide (Mimotopes, Clayton, Australia; CDFNGPYLGPPH, where Y is phosphorylated) conjugated to keyhole limpet hemocyanin (KLH). pAbs raised against 14–3-3 (EB1) and phospho-Ser585 of βc were previously described.9 Monoclonal antibodies (mAbs) to human GM-CSF receptor βc (1C1) and α chain (4H1) were previously described.28 Anti-p85, anti-Shc, and 4G10 antiphosphotyrosine mAbs were from Upstate Biotechnology (Lake Placid, NY); anti–phospho-SHIP, anti–phospho-Akt Thr308, and anti–phospho-Akt Ser473 were from Cell Signaling (Beverly, MA); antiactive extracellular signaling–related kinase (Erk) and anti–phospho-Erk pAbs were from Promega (Madison, WI); antiphosphorylated signal transducer and activator of transcription 5 (STAT5) mAb was from Zymed (San Francisco, CA); and anti–phospho–Janus kinase 2 (JAK2) pAb was from Affinity Bioreagents (Golden, CO). Antibodies to Gab2 and SHP2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All antibodies were used according to the manufacturers' instructions.
Biological assays
Colony assays were performed as described previously.29 In all assays, control groups with no cytokines (diluent control of PBS) or a cocktail of IL-6 (50 ng/mL), SCF (100 ng/mL), and erythropoietin (Epo; 4 U/mL) were included. For delta assays, FL cells were transduced and set up in liquid cultures with either GM-CSF at 100 ng/mL or a cocktail of SCF (50 ng/mL), IL-6 (100 ng/mL), and G-CSF (10 ng/mL) for up to 10 days. After this time, cells were harvested, counted, and plated in colony assays. Cell survival was determined by annexin V–FLUOS (Roche, Indianapolis, IN) staining as previously described.9,29 Transduced FL cells were plated at 1.5 × 105/mL in IMDM containing either 10% heat-inactivated (HI)–FCS or 0.5% HI-FCS. Each serum concentration had groups containing no GM-CSF, 50 ng/mL human (h) GM-CSF, or cocktail (of SCF, IL-6 and G-CSF). After 48 hours, cells were harvested and stained with 4H1 and anti-mouse IgG-PE antibody, and with annexin V–FLUOS. The proportion of cells which were viable (annexin V–FLUOS−) from all cells expressing the GM-CSF receptor was determined by flow cytometry. Proliferation assays were conducted as previously described.30
Immunoprecipitations and immunoblot analysis
Transduced FL or stable CTL-EN cells were factor deprived for 12 hours, stimulated with 50 ng/mL GM-CSF, and lysed in either RIPA buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1.0% NP-40, 0.5% deoxycholate, and 0.1% SDS) for Western blotting with phosphospecific antibodies, or NP-40 lysis buffer8 for immunoprecipitations, and processed as previously described.8 Signals were developed using enhanced chemiluminescence (ECL; Amersham Pharmacia, Piscataway, NJ; or West Dura; Pierce, Rockford, IL).
PI3K assay
CTL-EN cells expressing GM-CSF receptors were factor deprived and stimulated with GM-CSF at 50 ng/mL for up to 60 minutes. Cells were lysed in NP-40 lysis buffer, and tyrosine-phosphorylated proteins were immunoprecipitated using 4G10 antibody. PI3K assays were performed as previously described.8
PtdIns(3,4,5)P3 5-phosphatase assay
Results
Transduction of FL, murine BM, and CTL-EN cells with GM-CSF receptors
We generated retroviral IRES constructs that allow coexpression of both α and β subunits of the GM-CSF receptor (GMR) in primary cells or cell lines. FACS-sorted ψ2 cells were used, in coculture, to transduce BM or FL cells harvested from mice at 13.5 days after coitum (dpc). We transduced cells from βc−/−/βIL-3 mice to avoid potential cross-talk between the introduced receptor mutants and the endogenous murine (m) βc receptor.32 Importantly, primary cells allow multiple biological readouts to be examined, including cell survival, proliferation, and colony formation.29 wtβc and Tyr577Phe mutant receptors were expressed at equivalent levels. Transduction efficiency for wtβc on FL cells ranged from 10.2% to 53.9% (29.1% ± 4.1%, mean ± SEM) and for Tyr577Phe, ranged from 8.6% to 54.2% (27.7% ± 4.3%). The mean fluorescence intensity (MFI) of the GMRα wtβc in FL cells for 12 experiments ranged from 0.49 to 4.6, and on GMRα Tyr577Phe ranged from 0.50 to 3.6 (averages ± SE were 1.74 ± 0.42 for wtβc and 1.53 ± 0.30 for Tyr577Phe). Similarly, we observed equivalent levels of GMRα expression in BM cells (data not shown). In addition, we also used the previously described8,29 CTL-EN factor-dependent cell line. Surface expression of GMRα and βc subunits was examined by IF as previously shown.9 CTL-EN cells are maintained in murine IL-2 but are also able to respond to human GM-CSF when expressing human GM-CSF receptors.
Mutation of Tyr577 in βc enhances colony formation by primary hematopoietic cells in response to GM-CSF
We first examined the response of primary hematopoietic cells to GM-CSF using a colony assay in which FL cells from βc−/−/βIL-3−/− mice were transduced with wtβc or Tyr577Phe mutant receptor. As shown in Figure 1A, the number of colonies observed in cells expressing the Tyr577Phe mutant was significantly higher (P < .01) than that in cells expressing the wtβc. We also compared colony growth with mutants in which either all 8 of the tyrosine residues mutated to phenylalanine (F8) or the serine at position 585 were replaced with a glycine residue (Ser585Gly; Figure 1A). There was no significant difference in the colony numbers from the Ser585Gly mutant when compared with wtβc (P > .05). In contrast, the F8 mutant, in which no tyrosine residues remain, showed essentially no colony formation in response to GM-CSF. With each receptor mutant, we observed no colony growth in the absence of cytokine; similar levels of colony formation were observed in the presence of a cocktail of IL-6, SCF, and Epo (Figure 1A). The increased colony formation for the Tyr577Phe mutant was consistently seen over 8 different experiments (Figure 1B), and a titration of GM-CSF (Figure 1C) showed that at all concentrations of GM-CSF tested, the Tyr577Phe mutant promoted a higher number of colonies than the wtβc. In addition, we transduced BM cells from βc−/−/βIL-3−/− mice with the same constructs and assessed colony formation in response to GM-CSF. As shown in Figure 1D, there were also enhanced colony numbers in response to GM-CSF stimulation in those cells transduced with the Tyr577Phe mutant compared with wtβc.
Mutation of Tyr577 in βc of the GM-CSF/IL-3/IL-5 receptors enhances hematopoietic colony formation in response to GM-CSF. FL cells from βc−/−/βIL-3−/− mice were transduced with a retrovirus encoding the GM-CSF receptor α chain and either wild type βc or mutants of βc for 72 hours in a cocultivation system with ψ2 retroviral producer cells. Following transduction and quantification of transduction efficiency by immunofluorescence, 100 000 transduced cells were plated in 35-mm dishes containing 0.3% agar with human GM-CSF (100 ng/mL) or a cocktail of IL-6 (50 ng/mL), SCF (100 ng/mL), and Epo (4U/mL). (A) Comparison of wtβc with mutants in which all 8 tyrosines had been replaced by phenylalanine (F8), and the single-residue mutants Tyr577Phe or Ser585Gly in mediating colony formation in response to GM-CSF. This experiment is representative of 5 similar experiments. Error bars represent SEM from 3 to 5 replicate plates. ▨ depicts average colony formation of all groups in response to the cocktail stimulation. (B) TheTyr577Phe mutation (□) consistently enhanced colony formation from FL cells in response to 100 ng/mL GM-CSF compared with wtβc (■). Error bars represent SEM from 3 to 5 replicate plates. (C) Titration of human GM-CSF for its ability to stimulate colony formation in cells transduced with wtβc (■) or Tyr577Phe mutant (squlo]). Pooled data from 3 separate colony assays are shown. Error bars represent SEM from 3 to 11 plates per group. (D) Bone marrow (BM) cells from βc−/−/βIL-3−/− mice were transduced and plated in agar as above. Colony formation was stimulated with GM-CSF or the IL-6, SCF, and Epo cocktail as described in “Biological assays”. Colony formation was assessed at day 7, and in the experiment shown, error bars represent SEM from 6 replicate plates from 1 experiment that was representative of 3 performed. (E) FL cells transduced with either Tyr577Phe βc (□ and  ) or wt βc (■/▨) were cultured for the indicated times in liquid media of IMDM plus 15% HI-FCS supplemented with GM-CSF at 100 ng/mL (□ and ■) or a cytokine cocktail (50 ng/mL IL-6, 100 ng/mL SCF, and 10 ng/mL G-CSF;
) or wt βc (■/▨) were cultured for the indicated times in liquid media of IMDM plus 15% HI-FCS supplemented with GM-CSF at 100 ng/mL (□ and ■) or a cytokine cocktail (50 ng/mL IL-6, 100 ng/mL SCF, and 10 ng/mL G-CSF;  or ▨). At the indicated days, cells were counted, assessed for viability, and plated in agar for a further 10 to 14 days in cocktail containing SCF, Epo, and IL-6. Error bars represent SEM from 3 to 5 replicate plates.
or ▨). At the indicated days, cells were counted, assessed for viability, and plated in agar for a further 10 to 14 days in cocktail containing SCF, Epo, and IL-6. Error bars represent SEM from 3 to 5 replicate plates.
Mutation of Tyr577 in βc of the GM-CSF/IL-3/IL-5 receptors enhances hematopoietic colony formation in response to GM-CSF. FL cells from βc−/−/βIL-3−/− mice were transduced with a retrovirus encoding the GM-CSF receptor α chain and either wild type βc or mutants of βc for 72 hours in a cocultivation system with ψ2 retroviral producer cells. Following transduction and quantification of transduction efficiency by immunofluorescence, 100 000 transduced cells were plated in 35-mm dishes containing 0.3% agar with human GM-CSF (100 ng/mL) or a cocktail of IL-6 (50 ng/mL), SCF (100 ng/mL), and Epo (4U/mL). (A) Comparison of wtβc with mutants in which all 8 tyrosines had been replaced by phenylalanine (F8), and the single-residue mutants Tyr577Phe or Ser585Gly in mediating colony formation in response to GM-CSF. This experiment is representative of 5 similar experiments. Error bars represent SEM from 3 to 5 replicate plates. ▨ depicts average colony formation of all groups in response to the cocktail stimulation. (B) TheTyr577Phe mutation (□) consistently enhanced colony formation from FL cells in response to 100 ng/mL GM-CSF compared with wtβc (■). Error bars represent SEM from 3 to 5 replicate plates. (C) Titration of human GM-CSF for its ability to stimulate colony formation in cells transduced with wtβc (■) or Tyr577Phe mutant (squlo]). Pooled data from 3 separate colony assays are shown. Error bars represent SEM from 3 to 11 plates per group. (D) Bone marrow (BM) cells from βc−/−/βIL-3−/− mice were transduced and plated in agar as above. Colony formation was stimulated with GM-CSF or the IL-6, SCF, and Epo cocktail as described in “Biological assays”. Colony formation was assessed at day 7, and in the experiment shown, error bars represent SEM from 6 replicate plates from 1 experiment that was representative of 3 performed. (E) FL cells transduced with either Tyr577Phe βc (□ and  ) or wt βc (■/▨) were cultured for the indicated times in liquid media of IMDM plus 15% HI-FCS supplemented with GM-CSF at 100 ng/mL (□ and ■) or a cytokine cocktail (50 ng/mL IL-6, 100 ng/mL SCF, and 10 ng/mL G-CSF;
) or wt βc (■/▨) were cultured for the indicated times in liquid media of IMDM plus 15% HI-FCS supplemented with GM-CSF at 100 ng/mL (□ and ■) or a cytokine cocktail (50 ng/mL IL-6, 100 ng/mL SCF, and 10 ng/mL G-CSF;  or ▨). At the indicated days, cells were counted, assessed for viability, and plated in agar for a further 10 to 14 days in cocktail containing SCF, Epo, and IL-6. Error bars represent SEM from 3 to 5 replicate plates.
or ▨). At the indicated days, cells were counted, assessed for viability, and plated in agar for a further 10 to 14 days in cocktail containing SCF, Epo, and IL-6. Error bars represent SEM from 3 to 5 replicate plates.
In order to determine the effect of the Tyr577Phe mutation on the primitive progenitor population, we cultured the transduced FL cells in liquid culture prior to plating them in the colony assay. Following up to 10 days incubation in liquid culture with 100 ng/mL GM-CSF, cells were plated in a colony assay with a cocktail of SCF, IL-6, and Epo. This cocktail of cytokines was selected as a positive control to stimulate all cells, rather than just transduced FL cells. Figure 1E reveals enhanced colony formation from cells transduced with the Tyr577Phe mutant compared with wtβc stimulated with GM-CSF for 2 to 7 days in liquid culture. As a positive control, we cultured cells in a cytokine cocktail of SCF (100 ng/mL), IL-6 (50 ng/mL), and G-CSF (10 ng/mL) prior to the colony assay. No significant difference in colony formation was observed between the wtβc and the Tyr577Phe mutant for these positive controls (Figure 1E).
To observe the morphology of cultured FL cells, we transferred colonies from the assays shown in Figure 1 to slides and stained with hematoxylin. In addition, we cultured wtβc- or Tyr577Phe-expressing FL cells in 100 ng/mL GM-CSF or the cytokine cocktail of SCF, IL-6 and G-CSF. Cells from these cultures were either stained with lineage-specific antibodies or were cytospun and stained with hematoxylin. All of these approaches indicated that after 14 days in culture in GM-CSF, more than 90% of cells expressing either wtβc or the Tyr577Phe mutant receptor were identified as monocyte or macrophage (data not shown). Thus, the increased colony formation observed for the Tyr577Phe mutant in Figure 1 was not due to altered differentiation of cells in response to GM-CSF. As colony formation relies on the ability of GM-CSF to coordinately regulate cell survival and proliferation, we investigated whether the increased colony formation observed in FL cells expressing the Tyr577Phe mutant was due to increased cell survival. Although equivalent levels of cell survival were observed in FL cells transduced with the wtβc and the Tyr577Phe mutant in response to GM-CSF, strikingly, we found increased survival in cells expressing the Tyr577Phe mutant in the absence of factor. This ability of the Tyr577Phe mutant to enhance survival in the absence of GM-CSF was observed in cultures containing either 10% HI-FCS (P < .05) or 0.5% HI-FCS (P < .05; Figure 2). We also examined the ability of the Tyr577Phe mutant to regulate cell proliferation in response to GM-CSF. For these experiments, we used CTL-EN cells expressing either wtβc or the Tyr577Phe mutant. The ED50 for GM-CSF–mediated proliferation of cells expressing the Tyr577Phe βc mutant (0.0033 ng/mL) was 7.5-fold lower compared with that observed for the wtβc receptor (0.025 ng/mL) (data not shown).
Mutation of Tyr 577 in βc enhances GM-CSF–responsive survival. FL cells transduced with α chain and wtβc or Tyr577Pheβc were washed and plated at 1.5 × 105/mL in IMDM containing either 0.5% HI-FCS or 10% HI-FCS. Groups contained either no added factor (□), 50 ng/mL hGM-CSF (■), or a prosurvival cytokine cocktail containing IL-6 (50 ng/mL), SCF (100 ng/mL), and G-CSF (10 ng/mL) (▧). After 48 hours, cells were stained with the 4H1 anti-GMRα monoclonal antibody followed by an anti-mouse IgG-PE antibody, and with annexin V–FLUOS. Viability analysis was performed by flow cytometry as described in “Materials and methods.” The average of duplicate samples (± SD) is indicated. *P < .001; **P = .001. This experiment is representative of 3 performed.
Mutation of Tyr 577 in βc enhances GM-CSF–responsive survival. FL cells transduced with α chain and wtβc or Tyr577Pheβc were washed and plated at 1.5 × 105/mL in IMDM containing either 0.5% HI-FCS or 10% HI-FCS. Groups contained either no added factor (□), 50 ng/mL hGM-CSF (■), or a prosurvival cytokine cocktail containing IL-6 (50 ng/mL), SCF (100 ng/mL), and G-CSF (10 ng/mL) (▧). After 48 hours, cells were stained with the 4H1 anti-GMRα monoclonal antibody followed by an anti-mouse IgG-PE antibody, and with annexin V–FLUOS. Viability analysis was performed by flow cytometry as described in “Materials and methods.” The average of duplicate samples (± SD) is indicated. *P < .001; **P = .001. This experiment is representative of 3 performed.
Mutation of Tyr577 of βc enhances tyrosine phosphorylation of Gab2 and PI3K activation
In order to understand the mechanisms underlying the enhanced survival, proliferation, and colony formation responses of the Tyr577Phe mutant, we examined the regulation of key receptor-proximal and downstream signaling events regulated by the GM-CSF receptor. GM-CSF stimulation of cells expressing either wtβc or Tyr577Phe mutant receptor resulted in βc tyrosine phosphorylation (as detected by the 4G10 mAb) while, as expected, no phosphorylation of Tyr577 was detected in cells expressing the Tyr577Phe mutant (as detected by the anti–phospho-Tyr577 pAb; Figure 3A). There was a slight difference in kinetics of down-regulation of βc tyrosine phosphorylation between the FL and the CTL-EN cells, but both cell types demonstrated rapid maximal βc tyrosine phosphorylation within 15 minutes of cytokine stimulation. Consistent with previous reports from our laboratory and others,8-10,32,33 we found that mutation of Tyr577 prevented the tyrosine phosphorylation of Shc in response to GM-CSF. Previous studies by Gu et al10 suggested that Tyr577 is the major site required for Gab2 phosphorylation in response to GM-CSF. However, our results in FL cells showed that mutation of Tyr577 resulted both in increased basal Gab2 phosphorylation and also in response to GM-CSF stimulation (Figure 3A). This was not simply due to higher expression of the Tyr577Phe mutant compared with the wtβc in FL cells, as these were expressed at similar levels in 12 independent transductions both in terms of the number of cells transduced and in the levels of protein expressed (Figures 3B, 4C). In contrast to the tyrosine phosphorylation of Gab2, we did not detect any defect in SHP2 phosphorylation in cells expressing the Tyr577Phe mutant (Figure 3A).
FL cells transduced with Tyr577Pheβc show enhanced Gab2 tyrosine phosphorylation in the absence of Shc phosphorylation. (A) FL cells transduced with either wtβc or Tyr577Phe were cultured for 12 hours in RPMI containing 0.5% HI-FCS and stimulated with GM-CSF for 5 minutes prior to lysis. Cell lysates were cleared and immunoprecipitated with antibodies to βc, Shc, SHP2, or Gab2. Immunoprecipitates were subjected to SDS-PAGE and immunoblotting with the antibodies as shown in Figure 1A. (B) Mutation of Tyr577 does not abolish SHP2 coimmunoprecipitation with βc. CTL-EN cells expressing either wtβc or Tyr577Phe were factor deprived for 12 hours before stimulation with 50 ng/mL GM-CSF for 1, 2, 5, 15, 30, or 45 minutes. Following stimulation, βc was immunoprecipitated with the anti-βc antibody 1C1, and the immunoprecipitates were subjected to SDS-PAGE and immunoblotted with anti-phosphoTyr577, antiphosphotyrosine 4G10, anti-βc, or anti-SHP2 antibodies. A vertical line has been inserted to indicate where a gel lane was cut. These were from separate gels run from a single experiment. (C) FL cells transduced with either wtβc or Tyr577Phe were cultured for 12 hours before stimulation with 50 ng/mL GM-CSF for 5, 15, or 30 minutes. Following stimulation, the cells were lysed, and the lysates were subjected to SDS-PAGE and immunoblotted with phosphospecific antibodies to JAK2, STAT5A, Akt, and Erk. An anti-Erk antibody was used as a loading control. The data shown is representative of 3 separate experiments performed using transduced FL cells.
FL cells transduced with Tyr577Pheβc show enhanced Gab2 tyrosine phosphorylation in the absence of Shc phosphorylation. (A) FL cells transduced with either wtβc or Tyr577Phe were cultured for 12 hours in RPMI containing 0.5% HI-FCS and stimulated with GM-CSF for 5 minutes prior to lysis. Cell lysates were cleared and immunoprecipitated with antibodies to βc, Shc, SHP2, or Gab2. Immunoprecipitates were subjected to SDS-PAGE and immunoblotting with the antibodies as shown in Figure 1A. (B) Mutation of Tyr577 does not abolish SHP2 coimmunoprecipitation with βc. CTL-EN cells expressing either wtβc or Tyr577Phe were factor deprived for 12 hours before stimulation with 50 ng/mL GM-CSF for 1, 2, 5, 15, 30, or 45 minutes. Following stimulation, βc was immunoprecipitated with the anti-βc antibody 1C1, and the immunoprecipitates were subjected to SDS-PAGE and immunoblotted with anti-phosphoTyr577, antiphosphotyrosine 4G10, anti-βc, or anti-SHP2 antibodies. A vertical line has been inserted to indicate where a gel lane was cut. These were from separate gels run from a single experiment. (C) FL cells transduced with either wtβc or Tyr577Phe were cultured for 12 hours before stimulation with 50 ng/mL GM-CSF for 5, 15, or 30 minutes. Following stimulation, the cells were lysed, and the lysates were subjected to SDS-PAGE and immunoblotted with phosphospecific antibodies to JAK2, STAT5A, Akt, and Erk. An anti-Erk antibody was used as a loading control. The data shown is representative of 3 separate experiments performed using transduced FL cells.
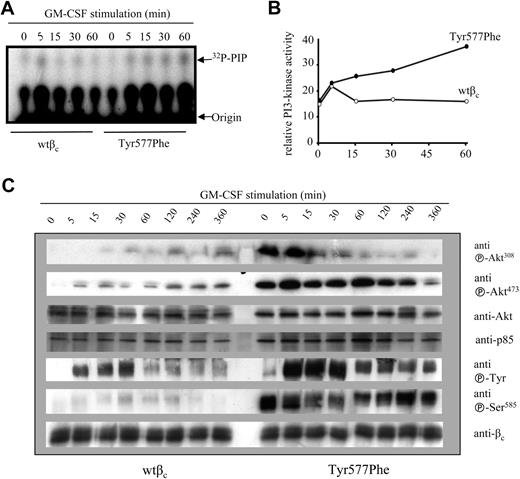
Enhanced and prolonged PI3K activation in CTL-EN cells expressing βc Tyr577Phe in response to GM-CSF. CTL-EN cells expressing either wtβc or Tyr577Phe were factor deprived for 12 hours in RPMI containing 0.5% HI-FCS before stimulation with 50 ng/mL GM-CSF for 0, 15, 30, or 60 minutes. The cells were then lysed, and the lysates cleared and subjected to immunoprecipitation with anti-βc antibodies. (A) Immunoprecipitates were then tested in an in vitro kinase assay. (B) Quantification of counts from panel A as measured in a liquid scintillation counter. Experiment shown is representative of 3 separate assays that were performed. (C) FL cells transduced with either wtβc or Tyr577Phe were stimulated with GM-CSF for 0, 5, 15, 30, 60, 120, 240, or 360 minutes before lysis. Cell lysates were cleared and immunoprecipitated with antibodies to βc. Immunoprecipitates were subjected to SDS-PAGE and immunoblotting with the antibodies as shown. Anti-βc antibody 1C1 and anti-p85 were used as a loading controls for immunoprecipitations and lysates, respectively.
Enhanced and prolonged PI3K activation in CTL-EN cells expressing βc Tyr577Phe in response to GM-CSF. CTL-EN cells expressing either wtβc or Tyr577Phe were factor deprived for 12 hours in RPMI containing 0.5% HI-FCS before stimulation with 50 ng/mL GM-CSF for 0, 15, 30, or 60 minutes. The cells were then lysed, and the lysates cleared and subjected to immunoprecipitation with anti-βc antibodies. (A) Immunoprecipitates were then tested in an in vitro kinase assay. (B) Quantification of counts from panel A as measured in a liquid scintillation counter. Experiment shown is representative of 3 separate assays that were performed. (C) FL cells transduced with either wtβc or Tyr577Phe were stimulated with GM-CSF for 0, 5, 15, 30, 60, 120, 240, or 360 minutes before lysis. Cell lysates were cleared and immunoprecipitated with antibodies to βc. Immunoprecipitates were subjected to SDS-PAGE and immunoblotting with the antibodies as shown. Anti-βc antibody 1C1 and anti-p85 were used as a loading controls for immunoprecipitations and lysates, respectively.
We then compared the ability of the wtβc and Tyr577Phe receptors to signal via the JAK/STAT, Ras/MAPK, and PI3K pathways. No major differences in JAK2, STAT5A, or Erk phosphorylation were observed in FL cells expressing either wtβc or the Tyr577Phe mutant (Figure 3C). However, while the recruitment of PI3K activity to the wtβc was rapid and transient, sustained PI3K activity was associated with the Tyr577Phe mutant in response to GM-CSF. Quantitation of these results showed that although cells expressing the Tyr577Phe mutant showed a similar initial amplitude of PI3K activation at 5 minutes, the PI3K activity associated with the Tyr577Phe mutant failed to be down-regulated (Figures 4A,B). In line with these results, we also observed enhanced basal and sustained Ser473 and Thr308 phosphorylation of Akt (protein kinase B) in cells expressing the Tyr577Phe mutant (Figure 4C). Interestingly, we observed increased basal and induced phosphorylation of Ser585 in cells expressing the Tyr577Phe mutant (Figure 4C). We treated CTL-EN cells with cyclohexamide to determine whether the half-life of the wtβc and Tyr577Phe receptors were the same. There were no observable differences in βc or p85 levels between the wtβc and Tyr577Phe βc for up to 12 hours of treatment (see Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Mutation of Tyr577 uncouples SHIP from the GM-CSF receptor
SHIP1 and SHIP2 are important negative regulators of cytokine-activated PI3K signaling in hematopoietic cells.17 In order to determine whether Tyr577 is responsible for the recruitment of SHIP, we immunoprecipiated Shc and probed for associated phospho-SHIP. As shown in Figure 5A, there is an induction of association of phospho-SHIP with Shc at 1 minute, which is sustained for the 45 minutes of GM-CSF stimulation. In contrast, the recruitment of phospho-SHIP to Shc in response to GM-CSF was reduced in cells expressing the Tyr577Phe mutant compared with wtβc. As shown by the bottom panel in Figure 5A, there isequal loading of Shc at all time points. To test whether defective SHIP activity may contribute to the enhanced PI3K signaling observed for the Tyr577Phe mutant, we examined the recruitment of SHIP activity to the wtβc and Tyr577Pheβc in response to GM-CSF. CTL-EN cells were stimulated with GM-CSF, βc was immunoprecipitated, and the immunoprecipitates were subjected to an in vitro PI(3,4,5)P3 5-phosphatase assay. We found that while the wtβc was able to recruit 5′-phosphatase activity, no such recruitment was observed for the Tyr577Phe mutant (Figure 5). Quantitation of the data in Figure 5B is shown in Figure 5C. Our results indicated that activation of the GM-CSF receptor in the absence of Tyr577 phosphorylation leads to hyperactivation of the PI3K pathway, leading to enhanced cell survival and proliferation.
Mutation of Tyr577Phe in βc abolishes the hydrolysis of PI-(3,4,5)P3 in response to GM-CSF. CTL-EN cells expressing either wtβc or Tyr577Phe were factor deprived for 12 hours in RPMI containing 0.5% HI-FCS before stimulation with either 0 or 50 ng/mL GM-CSF. Following stimulation, the cells were lysed, and the lysates were cleared and subjected to immunoprecipitation with anti-Shc antibody or anti-βc antibody. (A) Shc immunoprecipitates were subjected to SDS-PAGE and immunoblotting with antibody to phospho-SHIP. (B) βc immunoprecipitates subjected to a PtdIns(3,4,5)P3 5-phosphatase assay. (C) Quantification of PtdIns(3,4,5)P3 5-phosphatase activity in each immunoprecipitate. The bars represent the mean (± SD) from 2 independent experiments.
Mutation of Tyr577Phe in βc abolishes the hydrolysis of PI-(3,4,5)P3 in response to GM-CSF. CTL-EN cells expressing either wtβc or Tyr577Phe were factor deprived for 12 hours in RPMI containing 0.5% HI-FCS before stimulation with either 0 or 50 ng/mL GM-CSF. Following stimulation, the cells were lysed, and the lysates were cleared and subjected to immunoprecipitation with anti-Shc antibody or anti-βc antibody. (A) Shc immunoprecipitates were subjected to SDS-PAGE and immunoblotting with antibody to phospho-SHIP. (B) βc immunoprecipitates subjected to a PtdIns(3,4,5)P3 5-phosphatase assay. (C) Quantification of PtdIns(3,4,5)P3 5-phosphatase activity in each immunoprecipitate. The bars represent the mean (± SD) from 2 independent experiments.
Discussion
While others have shown that the Shc adaptor protein binds Tyr577 of βc via its PTB domain and that Tyr577 of βc is necessary for the tyrosine phosphorylation of Shc in response to GM-CSF, the significance of these signaling events in terms of biological response has remained unresolved.9,32-34 In fact, studies using Ba/F3 cells have shown that despite the clear defect in Shc tyrosine phosphorylation in cells expressing a Tyr577Phe mutant, no significant defect in GM-CSF (or IL-3) biological function was apparent.33 We have established model systems using both primary hematopoietic cells derived from mouse FL and the murine CTL-EN cell line to examine multiple GM-CSF biological activities in a β-subunit–null background. We show that Tyr577 of βc serves important, nonredundant functions to restrain GM-CSF–mediated biological responses by negatively regulating signaling through the PI3K pathway. Primary FL cells expressing the βc Tyr577Phe mutant consistently demonstrated increased numbers of colonies in agar assays in response to GM-CSF when compared with cells expressing the wtβc (Figure 1). We found that underlying the increased colony numbers observed, the Tyr577 mutant promoted not only increased cell proliferation, but also cell survival in response to GM-CSF (Figure 2).
Examination of βc tyrosine phosphorylation as well as signaling via the JAK/STAT, Ras/MAPK, and PI3K pathways indicated that the increased magnitude of biological responses by the Tyr577Phe mutant was not simply due to an overall enhancement of multiple βc signaling pathways. We observed no clear differences between wtβc and the Tyr577Phe mutant in terms of βc tyrosine phosphorylation or the phosphorylation of JAK2, STAT5, Erk, or SHP2 (Figure 3). However, clear differences between wtβc and the Tyr577Phe mutant in terms of PI3K signaling were observed. We found that PI3K signaling was deregulated in the Tyr577Phe mutant, resulting from (1) the sustained recruitment of PI3K activity to the mutant βc in response to GM-CSF (Figure 4), and (2) the failure of the Tyr577Phe mutant receptor to recruit the 5′ inositol phosphatase SHIP in response to GM-CSF (Figure 5). The sustained recruitment of PI3K to the membrane by the Tyr577Phe mutant in the absence of SHIP would be expected to lead to overproduction of PIP(3,4) and PIP(3,4,5) and enhanced activation of downstream PI3K effectors. In fact, we have shown that the phosphorylation of both Thr308 and Ser473 in Akt is increased in cells expressing the Tyr577Phe mutant when compared with cells expressing wtβc (Figure 4C). Although deregulation of PI3K observed for the Tyr577Phe mutant is consistent with the enhanced biological responses mediated by this mutant, it is also possible that loss of Tyr577 phosphorylation uncouples the receptor from other negative regulators of cytokine function, such as Cbl and the SOCS family of proteins.35,36 While we cannot exclude such a possibility, Tyr577 does not lie within a suitable motif for the binding of SOCS or Cbl SH2 domains, and we were unable to coimmunoprecipitate βc with either SOCS3 or Cbl (data not shown). Other possibilities for enhanced signal duration may come from reduced endocytosis and/or proteosome degradation. SOCS3 plays a major role in endocytosis of the G-CSF receptor via ubiquitination of lysine residues, and the loss of SOCS3 recruitment prevents lysosomal routing.37 Truncations in the G-CSF receptor, in which internalization motifs are removed, cause hyperproliferation and sustained STAT activation from prolonged surface expression. These have been demonstrated in patients with severe congenital neutropenia (SCN).38
Our findings that the Tyr577Phe mutant promoted enhanced GM-CSF–mediated cell proliferation and survival in primary cells was somewhat surprising, as previous experiments examining the biological effects of mutating Tyr577 had not detected any significant perturbation in GM-CSF or IL-3 activities.9,11,12,33 A possible reason for this is that experiments using a single, saturating dose of GM-CSF may not have revealed an enhanced biological response.9,11 In this regard, it is significant that no defect in MAPK activation was observed following stimulation of embryonic fibroblasts derived from ShcA−/− mice with high concentrations of either epidermal growth factor (EGF) or platelet-derived growth factor (PDGF).39 However, dose-response analysis of MAPK activation revealed that at submaximal growth factor concentrations, 50-fold higher concentrations of EGF and 25-fold higher concentrations of PDGF were required to attain the same level of MAPK activation when comparing ShcA−/− to wt fibroblasts.39 Thus, Shc may function to set the threshold of signaling in response to growth factors and cytokines, and such functions would only be revealed by performing comprehensive dose-response experiments such as those described herein. Another possibility is that, unlike other studies that examined βc signaling and function by transfecting Ba/F3 cells with wtβc and mutant receptors, we expressed human βc in a murine βc–null background. It has been shown in our laboratory40 and others41 that ectopically expressed human βc subunits can heterodimerize with endogenous murine GM-CSFRα and βc subunits, leading to anomalous results. Such a phenomenon has been observed in other receptor systems, such as the EGF receptor family, and has been proposed to lead to spurious results.42 Thus recruitment of endogenous βc subunits containing an intact Shc-binding site into activated receptor complexes containing transfected Tyr577Phe mutant βc may reconstitute the negative regulatory pathway and obscure any enhanced GM-CSF activity.
Shc has been shown to be recruited to a broad spectrum of cell-surface receptors, and has been widely proposed to be a key regulator of Ras/MAPK signaling.43 The PTB domain of Shc has been shown to bind phosphotyrosine residues within NXXY motifs (where Y is a phosphotyrosine), and this represents a key mechanism by which Shc is recruited to a range of cell-surface receptors, including IL-2Rβ,44 neu/c-ErbB2,45 Ret,46 c-Mpl,47 and TrkA.48 Similarly, Tyr577 of βc lies within a NXXY motif and is known to be important for the recruitment of Shc via its PTB domain.6,49 Once bound to the receptor, Shc itself can be phosphorylated on Tyr239/240 and Tyr317, providing docking sites for the SH2 domain of Grb2.49 In this manner, Shc is able to recruit Grb2/Sos complexes, leading to the activation of the Ras/MAPK pathway.50 However, in the case of the GM-CSF receptor, Ras/MAPK signaling occurs via multiple functionally redundant tyrosine residues. While Tyr577 has been shown to regulate Ras/MAPK signaling via the recruitment of Shc-Grb2-Sos complexes,10 Tyr612 and Tyr695 are also able to regulate Ras/MAPK signaling via an independent mechanism. It is proposed that the binding of SHP2 to Tyr612 and Tyr695 allows the recruitment of Grb2-Sos and the regulation of Ras/MAPK signaling.51 In fact, we observed no obvious defect in Erk phosphorylation for the Tyr577Phe mutant (Figure 3), underscoring the functionally redundant roles served by Tyr577, Tyr612, and Tyr695 in regulating Ras/MAPK signaling.
In contrast to Ras/MAPK signaling, our results show that Tyr577 of βc serves unique and nonredundant functions in terms of PI3K signaling. The recruitment of PI3K activity to the Tyr577Phe mutant receptor was not only increased, but the kinetics of PI3K activation were also sustained compared with wtβc (Figure 4). While mutating Tyr577 de-represses a PI3K-binding site on the βc subunit, facilitating enhanced PI3K signaling, none of the remaining 5 βc tyrosines lie within a suitable context for binding the SH2 domains of p85. Rather, our results support the notion that Ser585 phosphorylation of βc is de-repressed in the context of the Tyr577Phe mutant and recruits PI3K. Our previous studies have shown that Ser585 provides an important docking site for the 14–3-3 family of scaffold proteins and is important for the recruitment of PI3K to the receptor.8 We have also shown that the phosphorylation of Tyr577 sterically interferes with the phosphorylation of Ser585, with the phosphorylation of Ser585 and of Tyr577 being mutually exclusive.29 The results presented herein suggest that the block in Ser585 phosphorylation normally exerted by the phosphorylation of Tyr577 is lost in the Tyr577Phe mutant, leading to the deregulation of Ser585 phosphorylation and enhanced PI3K signaling. In line with these results, we have also shown that pharmacologic blockade of Tyr577 phosphorylation using a JAK2 inhibitor also leads to constitutive Ser585 phosphorylation (data not shown).
Our results showing that Gab2 tyrosine phosphorylation and PI3K signaling were enhanced in the Tyr577Phe mutant were unexpected, as Gu et al have shown that mutation of Tyr577 results in decreased Gab2 tyrosine phosphorylation in response to GM-CSF.10 Nevertheless, our findings do highlight the correlation between enhanced biological activity, Gab2 phosphorylation, and PI3K activity, and are consistent with a major role of PI3K in GM-CSF function.8,9 Gab2 is the major phosphorylated binding protein for p85 of PI3K responsible for more than 50% of cytokine-mediated PI3K activity.10 It remains to be seen, however, how Gab2 is recruited to the Tyr577Phe mutant, particularly since Shc phosphorylation appears to be a prerequisite itself for Gab2 phosphorylation.10 A possibility is that Gab2 is recruited through binding to SHP-2,52 which is still associated to the GM-CSF receptor in the Tyr577Phe mutant (Figure 3A), presumably through its alternate binding sites, Tyr612 and Tyr695.4,10,12,32,33 A second possibility is that loss of phosphotyrosine substrates (such as Shc) due to the Tyr577Phe mutation could result in the compensatory increase in tyrosine phosphorylation of other substrates.
The failure of the Tyr577Phe mutant to recruit SHIP would further deregulate the PI3K signaling axis and enhance PI3K effector functions such as cell proliferation and survival. Shc is known to recruit SHIP following cytokine and antigen receptor activation;53-55 this interaction can occur via the SH2 domain of SHIP binding Tyr317 of Shc.49,54,56 Alternatively, it is also conceivable that the PTB domain of Shc binds NXXY motifs in the C-terminus of SHIP, raising the possibility that NXXY motifs in SHIP and βc compete for the binding of Shc. In line with this proposal are the findings of Velazquez et al, where proteomic approaches suggested that βc-Shc complexes were distinct from Shc-SHIP complexes.49 Such a mode of signaling has been reported for other cell-surface receptors where the PTB domain of Shc has been found to compete with other PTB-domain proteins for binding NXXY motifs. For example, both Shc and IRS-1 compete for the binding of Tyr1062 of the Ret receptor, and Shc and FRS2 compete for the binding of Tyr490 of the TrkA receptor.46,48
While 3′-inositol phosphatases such as PTEN are thought to control basal levels of PIP(3,4) and PIP(3,4,5) in the plasma membrane, the 5′-inositol phosphatases such as SHIP are proposed to negatively regulate growth factor– and cytokine-induced production of PIP(4,5) and PIP(3,4,5).57 This concept is supported by the observation that hematopoietic cells derived from SHIP−/− mice have increased and sustained PI3K signaling in response to cytokines.20 Thus, it is interesting to note that 2 key features of the Tyr577Phe mutant βc have also been reported for primary hematopoietic cells derived from SHIP−/− mice. First, IL-3 promoted enhanced PI3K signaling in SHIP−/− hematopoietic cells; second, these cells were hypersensitive to IL-3 and GM-CSF.19,20 In fact, there is considerable overlap in the phenotypes displayed by SHIP−/− mice and mice overexpressing GM-CSF, including a myeloproliferative-like phenotype accompanied by infiltration of peripheral tissues by myeloid cells.19,20,58 In a similar manner to the Tyr577Phe mutant described in our studies, C-terminally truncated G-CSF receptors promote sustained and enhanced proliferation in myeloid cells,59,60 suggesting that such dominant loss-of-function mutations affecting Shc signaling may underlie some hematopoietic disorders. Further work will be required to determine whether analogous mutations also exist in the βc subunit of the GM-CSF receptor.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (RO1-AI-50744–02), the National Health and Medical Research Council of Australia, and the Cancer Council of South Australia. M.A.G. is a Peter Nelson Leukemia Research Fellow.
National Institutes of Health
Authorship
Contribution: H.S.R. and M.A.G. designed and performed research, and wrote the paper; F.C.S., E.F.B., and L.O. performed research; C.A.M. designed research; C.G.B. designed research and contributed vital reagents; and A.F.L. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angel Lopez, Cytokine Receptor Laboratory, Division of Human Immunology, Institute of Medical and Veterinary Science, Hanson Institute, Frome Road, Adelaide, South Australia, SA 5000 Australia; e-mail:angel.lopez@imvs.sa.gov.au.

![Figure 1. Mutation of Tyr577 in βc of the GM-CSF/IL-3/IL-5 receptors enhances hematopoietic colony formation in response to GM-CSF. FL cells from βc−/−/βIL-3−/− mice were transduced with a retrovirus encoding the GM-CSF receptor α chain and either wild type βc or mutants of βc for 72 hours in a cocultivation system with ψ2 retroviral producer cells. Following transduction and quantification of transduction efficiency by immunofluorescence, 100 000 transduced cells were plated in 35-mm dishes containing 0.3% agar with human GM-CSF (100 ng/mL) or a cocktail of IL-6 (50 ng/mL), SCF (100 ng/mL), and Epo (4U/mL). (A) Comparison of wtβc with mutants in which all 8 tyrosines had been replaced by phenylalanine (F8), and the single-residue mutants Tyr577Phe or Ser585Gly in mediating colony formation in response to GM-CSF. This experiment is representative of 5 similar experiments. Error bars represent SEM from 3 to 5 replicate plates. ▨ depicts average colony formation of all groups in response to the cocktail stimulation. (B) TheTyr577Phe mutation (□) consistently enhanced colony formation from FL cells in response to 100 ng/mL GM-CSF compared with wtβc (■). Error bars represent SEM from 3 to 5 replicate plates. (C) Titration of human GM-CSF for its ability to stimulate colony formation in cells transduced with wtβc (■) or Tyr577Phe mutant (squlo]). Pooled data from 3 separate colony assays are shown. Error bars represent SEM from 3 to 11 plates per group. (D) Bone marrow (BM) cells from βc−/−/βIL-3−/− mice were transduced and plated in agar as above. Colony formation was stimulated with GM-CSF or the IL-6, SCF, and Epo cocktail as described in “Biological assays”. Colony formation was assessed at day 7, and in the experiment shown, error bars represent SEM from 6 replicate plates from 1 experiment that was representative of 3 performed. (E) FL cells transduced with either Tyr577Phe βc (□ and ) or wt βc (■/▨) were cultured for the indicated times in liquid media of IMDM plus 15% HI-FCS supplemented with GM-CSF at 100 ng/mL (□ and ■) or a cytokine cocktail (50 ng/mL IL-6, 100 ng/mL SCF, and 10 ng/mL G-CSF; or ▨). At the indicated days, cells were counted, assessed for viability, and plated in agar for a further 10 to 14 days in cocktail containing SCF, Epo, and IL-6. Error bars represent SEM from 3 to 5 replicate plates.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/10/10.1182_blood-2007-01-070391/6/m_zh80210708730001.jpeg?Expires=1765886933&Signature=tRo8qph14lhbSavvTrG4uhoktWSHjdeTh4fpfz8fmU33hvS1sSwymXjw2ARicOuauxUr6KiqlbZyB-pTdyoRD0Ls1B39ROE~UA5FXFAIRl7rPV9JtLL1WZZRx-yY~nKLvJ8h0FWkttPhPELo2XOEImECV3JCE-~zL9ipmvl9d0PzjIpuqQM4PbEOLP8aci6JvGY~0aj~nTYj2ckfVAOTHh5h7nwX3i7scUuiw8fiSQp9VfIY2AwUFoBLiIE5yl7bA5GCCK3V4FEd6z2lnfac-O71OPwrm-mBw-49xMky4nTU-qiTf97rzYXsyYoG0DLwvFlbeXmKcfba16VG-vSoLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal