The CD4 coreceptor is crucial in the activation of major histocompatibility complex (MHC) class II restricted CD4 + T lymphocytes by binding the same MHC class as the T-cell receptor (TCR) and by potentiating TCR-dependent signaling. CD4 is also expressed by invariant natural killer T cells (iNKT), which recognize natural and synthetic lipid antigens, such as α-galactosyl ceramide (α-GalCer), in association with the MHC class I–like CD1d molecule. Human iNKT cells can be divided into 2 major subsets depending on CD4 expression: CD4 + iNKT preferentially produce T-helper (Th)0/Th2 cytokines, whereas CD4− iNKT cells produce Th1 cytokines after antigenic activation. Cytokines produced by iNKT may have immunomodulatory roles in various physiopathologic contexts, but their mode of regulation by iNKT cells remains ill-defined. Using blocking reagents neutralizing CD4 binding, experimental systems where MHC class II molecules are absent and recombinant α-GalCer/CD1d complexes, we show that CD4 potentiates human iNKT cell activation by engaging CD1d molecules. These results indicate that the CD4 coreceptors may contribute to the fine tuning of iNKT cells reactivity.
Introduction
On recognition of antigenic peptides bound to major histocompatibility complex (MHC) molecules, the T cell receptor (TCR) complex generates essential biochemical signals for T-cell activation.1 Sensitivity of T cells to TCR-dependent activation is greatly enhanced when CD4 or CD8 proteins are engaged at the same time.2 These proteins have been referred to as coreceptors,3 because they are supposed to bind the same MHC molecule engaged by the TCR, and are supposed to drag the associated tyrosine kinase Lck in contact with the TCR-associated CD3 zeta chains to initiate the phosphorylation events leading to T-cell activation.4 While CD8 binds MHC class I molecules, the CD4 coreceptor binds MHC class II molecules, and augments signaling via the TCR by up to 100-fold.2 Structure-function studies and crystallographic analysis of CD4–MHC class II complexes indicate that CD4 interacts with its residues on the D1 domain with both α2 and β2 domains of the MHC class II αβ heterodimer.5,,,–9
Invariant natural killer T cells (iNKT) cells are a subset of nonconventional T lymphocytes present in most mammalian species studied so far that share several phenotypic features with natural killer cells, such as surface expression of CD161.10 Human iNKT cells express an invariant Vα24-JαQ TCRα chain paired preferentially with junctionally diverse Vβ11DβJβ regions.11,12 This semi-invariant TCR (invTCR) is strikingly conserved in evolution, and recognizes lipids and glycolipids presented by CD1d, a member of the CD1 family of antigen-presenting molecules exhibiting an MHC-class I-like structure.13,–15 The invTCR display an exquisite specificity for the xenogeneic glycosphingolipid α-GalCer, presented by CD1d, which can be used to selectively activate iNKT cells in vitro and in vivo.16,17 A number of more physiologic CD1d-restricted antigens recognized by iNKT cells have been recently identified.18,,,,,–24 These include bacterial glycolipids, endogenous glycolipids, tumor-derived glycoplipids, and phospholipids, as well as nonlipidic molecules. iNKT cells can be found in various peripheral organs including spleen, lymph nodes, and liver. Peripheral iNKT cells display a memory activated phenotype and can rapidly secrete large amounts of cytokines including IFN-γ, TNF-α, IL-4, and IL-13 on antigenic activation. Production of cytokines by activated iNKT cells can play a critical role in the pathogenesis of various infectious, autoimmune, and allergic diseases, as well as in the control of tumor progression25 (see this reference for a recent review).
Mature iNKT cells are either CD4 +, CD8αα +, or CD4−CD8− double negative (DN).10 The very low frequency of CD8αβ + cells among mature iNKT cells has been related to the early deletion of CD8 + iNKT cells during thymic development,12,26 presumably caused by high-avidity interactions between TCR/CD8 and self-CD1. Although direct interaction between CD8 and CD1d has not been formally demonstrated, these data suggest that CD8 functions as a coreceptor in iNKT cells by binding CD1d. In humans, CD4 + and DN iNKT cells display distinct functions, the former subset being T-helper (Th)0/Th2 like, with the latter being Th1.27,28 A recent study directly demonstrated the existence of functionally distinct CD4 + and CD4− iNKT subsets in mice.29 The reasons for the association between CD4 expression and iNKT cell functional specialization are still unclear. CD4 + and DN iNKT subsets endowed with distinct functions may correspond to distinct maturational stages along linear differentiation of iNKT cells as suggested by recent studies.30,–32 Like mainstream MHC class II-restricted T cells, CD4 engagement may also generate key signals leading to potentiation of Th2 effector functions of iNKT cells.
We presently studied the role of CD4 molecules in the activation of iNKT cells. We found that CD4 contributed to the activation of a sizeable fraction of CD4 + iNKT cell clones in vitro, and obtained binding and functional data supporting direct CD4-CD1d interactions.
Materials and methods
Antibodies and reagents
The following antibodies were used: C15-FITC (AV24S1), C21-PE (BV11S1), UCHT1-PC5 (CD3), and BMA031-PC5 (TCR Pan αβ), purchased from Immunotech, Marseille, France. For CD4 stainings and blocking assays, the following were used: 6D10 (CLB, Amsterdam, The Netherlands), 13B8.2 (Immunotech), RPA-T4 (BD Pharmingen, San Diego, CA), BL4 (Immunotech), SIM.4 (NIH AIDS Research and Reference Reagent Program), S3.5 (IgG2a, Caltag Laboratory, Burlingame, CA), and mouse-matched isotype controls (IgG1, IgG2a and IgG2b). Soluble recombinant glycosylated monovalent gp120 from HIV was kindly provided by S. Abrignani (Novartis Vaccines, Siena, Italy). Blocking DRβ (134-152, NGQEEKAGVVSTGLIQNGD)33 and CD1d (241-259, GEQEQQGTQPGDILPNADE) peptides were synthesized and purified at Sigma Genosys, Saint Quentin Fallavier, France. Antihuman IFN-γ and antihuman IL-4 used for intracellular cytokine staining experiments were obtained from BD Pharmingen. α-GalCer (KRN 7000) was obtained from Kirin Brewery Company Ltd, Gunma, Japan. Antihuman CD1d mAb (51.1) was kindly provided by S. Porcelli (Albert Einstein College of Medicine, New York, NY).
Generation of iNKT cell clones and cell culture
Peripheral blood mononuclear cells (PBMCs) of healthy donors were stained with phycoerythrin (PE)-conjugated anti-CD3, fluorescein isothiocyanate (FITC) conjugated anti-Vβ11 (IgG2a), biotinylated anti-Vα24 (IgG1) monoclonal antibodies (mAbs) and cychrome-streptavidin, and triple-positive cells were sorted and cloned by limiting dilution. CD4 + iNKT cell clones (LP16DC1, LP16DC7, LP17DC2, LP16DCP6, and CD4CL1) co-expressing Vα24 and Vβ11 TCR were used for this study. Canonical invVα24-JαQ TCR expression by Vα24/Vβ11 + T-cell clones was determined by polymerase chain reaction heteroduplex.34 Human iNKT cell clones 20.22 and 20.49 were from a PBMC-derived AV24BV11 T-cell line.17 Nucleotide sequence of Vα24-Jα18 (TCRα) and Vβ11-Dβ1-Jβ2 (TCRβ) junctional transcripts derived from the clones was determined as described in the online supplementary information. B7, B9, and MAD11 iNKT cell polyclonal populations (< 95%) were generated from PBMCs of healthy donors as described.35 T cells stained with CD4-specific mAbs were sorted using immunomagnetic beads (Dynal Biotech SA, Compiègne, France).36 iNKT cell clones and lines were maintained and restimulated in RPMI 1640 supplemented with 10% normal human serum (NHS) (RPMI-NHS) and hrIL-2 (300 IU/mL) as described.35 Flow cytometry was performed on a FACSCalibur apparatus using the Cellquest software (Becton Dickinson Biosciences, Le Pont de Claix, France). HeLa CD1d transfected cells, kindly provided by Dr L. Brossay, Brown University, Providence, RI, were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum. C1R-hCD1d and RMA/S-CD1d transfectant cell lines37 were maintained in RPMI 1640 supplemented with 5% fetal calf serum, 50 μM β-mercaptoethanol, and 0.5 mg/mL G418.
Generation of mouse T-hybridoma cells engineered with human invTCR and CD4
The 58αβ-hybridoma cells were cotransfected with the cDNA coding for the human invVα24-JαQ and Vβ11 using electroporation as described.38 The generation of the TCR containing vectors is detailed in the supporting information. Transfected 58αβ cells were selected to express a functional human invTCR. Several clones were super-transfected with hCD4 cDNA, and cells co-expressing invTCR and CD4 were selected by flow cytometry and used for functional studies.
iNKT cell activation assays
iNKT cell clones LP16DC1 and LP16DC7 were used for in vitro activation assays with either C1R-hCD1d or Hela-hCD1d transfected cells.17 Transfected or mock transfected C1R and Hela cells were loaded for 1 hour at 37°C with 30 ng/mL α-GalCer in RPMI tissue culture medium supplemented with 10% NHS. C1R or Hela cells were seeded in 96 flat well plates, respectively, at concentrations of 106/well and 105/well in RPMI-NHS and hrIL-2 (5 U/mL). iNKT clones were added (2.5 × 105 cells/well), with or without 10 μg/mL of anti-CD4 blocking monoclonal antibodies SIM.4 or S3.5 or mouse-matched isotype controls. Culture supernatants were harvested after 48 hours at 37°C, and iNKT cells activation was evaluated by enzyme-linked immunosorbent assay quantitative analysis of secreted cytokines.
Soluble recombinant CD1d molecules were produced as described in the supporting information. hrCD1d were pulsed with a 5-fold molar excess of α-GalCer (diluted in 0.5% PolySorbate 20) for 14 to 16 hours at 23°C, and coated onto wells of 96 flat wells plates at different concentrations for 2 hours at 37°C. iNKT cell clones LP17DC2 and LP16DCP6 (1.5 × 104 cells/well) or iNKT cell transfectomas (105 cells/well) were then added in RPMI-NHS with or without 10 μg/mL blocking antihuman CD4 SIM-4, or S3.5, 6D10,39 IgG1 and IgG2a isotype controls, or with 10 μg/mL of soluble recombinant glycosylated monovalent gp120 from HIV. After 16 hours (iNKT transfectoma) or 48 hours (iNKT cell clones), cell activation was quantified by enzyme-linked immunosorbent assay determination of the amount of secreted IL-2 and IFN-γ, respectively.
For IFN-γ and IL-4 release assays, iNKT cell clones 20.22 and 20.49 (5 × 104 cells/well) were incubated for 20 hours with HeLa CD1d cells (2.5 × 104 cells/well), prepulsed for 12 hours with sonicated DMSO or α-GalCer (1 to 10−5 μg/mL) diluted in phosphate-buffered saline. Cytokine quantification in culture supernatants was performed by enzyme-linked immunosorbent assay. For CD4 blocking assays, iNKT cells were pre-incubated with anti-CD4 mAb RPA-T4, diluted at 2 and 4 μg/mL, for 15 minutes on ice before being incubated for 20 hours with target cells. Blocking peptides and iNKT cell clones were used pre-incubated at grading doses (from 12.5 to 200 μg/mL) for 1 hour at 37°C; T cells were extensively washed and cocultured for 20 hours with target cells. The CD4 + iNKT cell clone CL1CD4 was used for activation assay in vitro with human LCL C1R or murine T Lymphoma RMA/S cell lines engineered with hCD1d cDNA. Cell lines were cocultured with NKT cell clone at 1:5 iNKT cell/antigen-presenting cell (APC) ratio in the presence of 20 U/mL hrIL-2. Cultures were incubated either with isotype matched control IgG or anti-CD4 mAb S3.5 at 20 μg/mL. After 48 hours, IFN-γ secreted in the supernatant was quantified by enzyme-linked immunosorbent assay.
CD3 downregulation
HeLa CD1d cells (2.5 × 104) were pulsed for 12 hours with α-GalCer (0.1 μg/mL) and used to activate iNKT cell clones 20.22 and 20.49 (5 × 104 cells/well) for different times in RPMI-NHS. CD3 downregulation was calculated by measuring CD3 fluorescence intensity in activated and unstimulated samples following a standard cell surface staining procedure.
Tetrameric α-GalCer/CD1d complexes
Tetramers of human CD1d molecules were produced as described.40 Tetramer stainings (2 to 126 nM) were performed in phosphate-buffered saline/bovine serum albumin 0.1% for 1 hour at room temperature. Anti-CD3 or anti-TCRαβ mAbs were used to normalize the intensity of tetramer staining. The cells were washed twice in phosphate-buffered saline/bovine serum albumin 0.1% before analysis by flow cytometry. For CD4 blocking assays, cells were pre-incubated for 15 minutes on ice in the presence of CD4-specific mAb (2-15 μg/mL) or corresponding isotype controls. For peptide blocking experiments, iNKT cells were pre-incubated for 1 hour at 37°C with 100 μg/mL of DR or CD1 peptides. For tetramer staining decay, iNKT cell clones were stained for 1 hour at room temperature with 6 nM α-GalCer tetramer and anti-TCRαβ mAb. The cells were washed twice and 30 μg/mL anti-CD1d mAb were added to prevent rebinding of the tetramers. At various time points, an aliquot was washed twice and analyzed by flow cytometry.
Results
CD4 blocking inhibits antigen-dependent activation of CD4 + iNKT cell clones
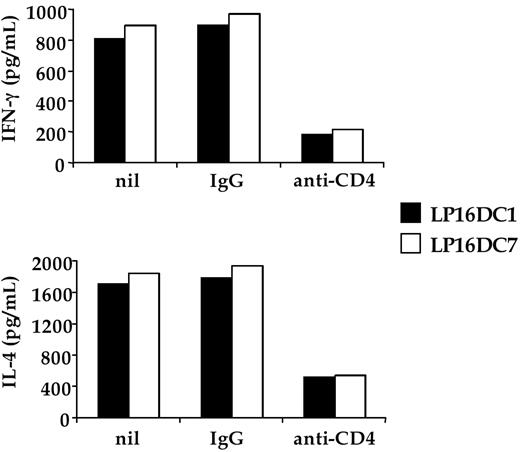
We first asked whether CD4 expressed by human iNKT cells played any functional role in their antigen-dependent activation. A set of CD4 + Vα24/Vβ11 iNKT cells clones was assayed for recognition of CD1d-expressing APC, pulsed with α-GalCer, in the presence or absence of mAbs known to block CD4 coreceptor function. Addition of anti-CD4 blocking mAb to the assay markedly inhibited the antigen-dependent activation of CD4 + iNKT cell clones, leading to a substantial reduction in their production of both IFN-γ and IL-4 cytokines (Figure 1). In keeping with its crucial role in the activation of MHC class II–restricted CD4 + T cells, CD4 appears to contribute also to the activation of CD1d-restricted CD4 + iNKT cells.
Blocking CD4 inhibits antigen-specific activation of CD4 + iNKT cell clones. The CD4+ iNKT cell clones LP16DC1 and LP16DC7 were cultured in duplicate wells at 2:1 ratio with C1R-CD1d transfectants, pulsed with α-GalCer. Cultures received nothing (nil), isotype-matched control IgG (IgG), or the anti-CD4 mAb S3.5 (anti-CD4). After 48 hours, supernatants were collected and tested by enzyme-linked immunosorbent assay for the content of IFN-γ or IL-4. One representative experiment of 3 is shown. Similar data were obtained by using the anti-CD4 6D10 blocking mAb.
Blocking CD4 inhibits antigen-specific activation of CD4 + iNKT cell clones. The CD4+ iNKT cell clones LP16DC1 and LP16DC7 were cultured in duplicate wells at 2:1 ratio with C1R-CD1d transfectants, pulsed with α-GalCer. Cultures received nothing (nil), isotype-matched control IgG (IgG), or the anti-CD4 mAb S3.5 (anti-CD4). After 48 hours, supernatants were collected and tested by enzyme-linked immunosorbent assay for the content of IFN-γ or IL-4. One representative experiment of 3 is shown. Similar data were obtained by using the anti-CD4 6D10 blocking mAb.
Because the primary coreceptor function of CD4 is to bind MHC class II molecules, CD4 could enhance CD4 + iNKT cell activation by interacting with MHC-II molecules, expressed on the C1R-CD1d LCL APCs used in the assay described. We thus tested the effect of CD4 blocking on the activation of CD4 + iNKT cell clones in an APC-free system, in which the antigenic signal was provided solely by plastic-bound, recombinant CD1d molecules loaded with α-GalCer. Blocking anti-CD4 mAb drastically inhibited IFN-γ production by α-GalCer–CD1d-stimulated CD4 + iNKT cell clones in the absence of MHC class II-expressing APCs (Figure 2). Because human-activated T and iNKT cells express MHC class II, CD4 and MHC class II expressed on different iNKT cells could interact in trans, resulting in the measured coreceptor effect. To address this, we transfected the invVα24-JαQ and Vβ11 TCR chains, derived from a human iNKT cell clone, into the murine TCR-negative T-cell hybridoma line 58αβ, which is completely devoid of MHC class II molecules, like any mouse T cell.41 A clone derived from this cell line, expressing a functional human Vα24/Vβ11 TCR on the membrane (not shown), was super-transfected with full-length human CD4 cDNA. Subclones expressing both human invTCR and CD4 (Figure 3A) were tested for recognition of plastic-bound recombinant α-GalCer–CD1d complexes in the presence or absence of CD4-blocking reagents. As shown in Figure 3B, activation of the invTCR transfectants by α-GalCer–CD1d was inhibited by CD4-blocking mAb, confirming that CD4 played a coreceptor role for CD1d recognition in the complete absence of MHC class II molecules.
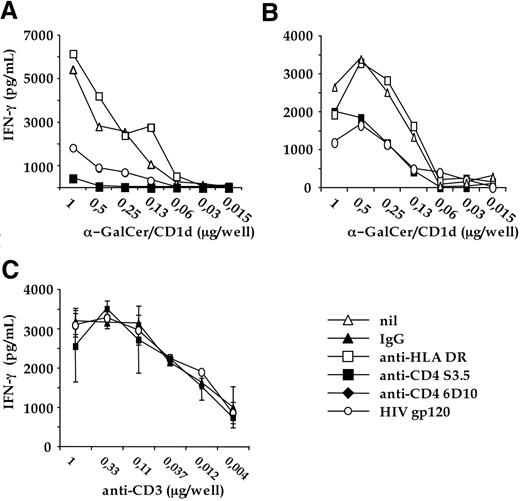
The CD4-CD1d interaction is critical for the antigen-specific activation of CD4 + iNKT cells clones. The CD4 + iNKT clones LP17DC2 (A) and LP16DCP6 (B) were cultured with increasing doses of plastic-bound soluble recombinant human CD1d, pulsed with α-GalCer. Cultures received nothing (nil), isotype-matched IgG1 (IgG), anti-CD4 S3.5 blocking mAb (anti-CD4), or recombinant glycosylated monomeric HIV gp120 (HIV gp120). IFN-γ contained in the culture was determined by enzyme-linked immunosorbent assay. One representative experiment of 3 is shown. Similar data were obtained by using the anti-CD4 6D10 blocking mAb. (C) LP16DCP6 clone was cultured in triplicated wells containing increasing doses of plastic-bound antihuman CD3 mAb TR66, in the presence of: isotype-matched control IgG1 (IgG); recombinant glycosylated momomeric HIV gp120; anti-CD4 S3.5 blocking mAb. One representative experiment of 3 is shown. Similar experiments performed with other CD4 + iNKT cell clones gave comparable results.
The CD4-CD1d interaction is critical for the antigen-specific activation of CD4 + iNKT cells clones. The CD4 + iNKT clones LP17DC2 (A) and LP16DCP6 (B) were cultured with increasing doses of plastic-bound soluble recombinant human CD1d, pulsed with α-GalCer. Cultures received nothing (nil), isotype-matched IgG1 (IgG), anti-CD4 S3.5 blocking mAb (anti-CD4), or recombinant glycosylated monomeric HIV gp120 (HIV gp120). IFN-γ contained in the culture was determined by enzyme-linked immunosorbent assay. One representative experiment of 3 is shown. Similar data were obtained by using the anti-CD4 6D10 blocking mAb. (C) LP16DCP6 clone was cultured in triplicated wells containing increasing doses of plastic-bound antihuman CD3 mAb TR66, in the presence of: isotype-matched control IgG1 (IgG); recombinant glycosylated momomeric HIV gp120; anti-CD4 S3.5 blocking mAb. One representative experiment of 3 is shown. Similar experiments performed with other CD4 + iNKT cell clones gave comparable results.
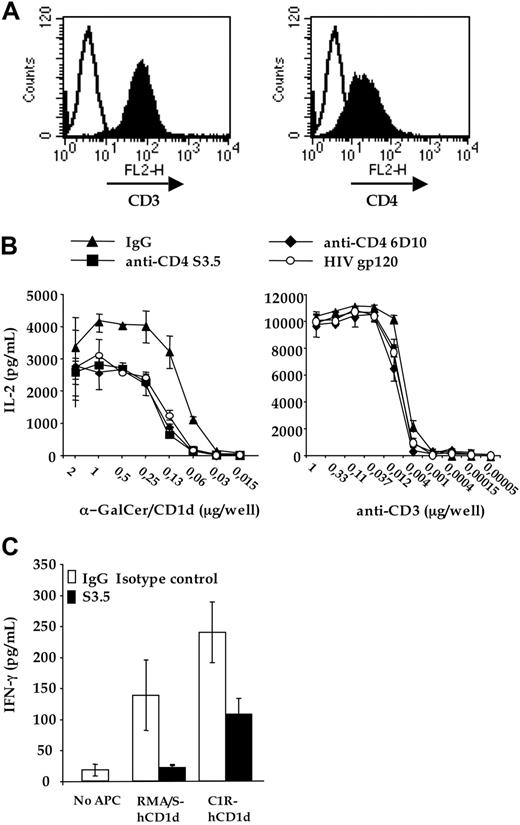
The CD4-CD1d interaction is critical for the antigen-specific activation of CD4 + invTCR transfectants. (A) 58αβ-mouse T-cell hybridoma was transfected with the invVα24-JαQ/Vβ11 TCR (left panel) and human CD4 (right panel). (B) CD4 + invTCR transfectants were activated with increasing doses of either plastic-bound soluble recombinant human CD1d, pulsed with α-GalCer (left panel) or plastic-bound antimouse CD3 2C11 (right panel), in the presence of: isotype matched control IgG (IgG1 + IgG2a); recombinant glycosylated momomeric HIV gp120 (gp120); anti-CD4 S3.5 (S3.5) or 6D10 (6D10) blocking mAbs. IL-2 contained in the culture was determined by enzyme-linked immunosorbent assay. (C) Human CD4 + iNKT cell clone CL1CD4 was cocultured in duplicate wells with RMA/S-hCD1d or C1R-CD1d, with or without either isotype-matched control IgG or anti-CD4 mAb S3.5. After 48 hours, culture supernatants were collected and tested by enzyme-linked immunosorbent assay for secreted IFN-γ. Nontransfected C1R and RMA/S cells were not recognized by the clone (data not shown). One representative of 3 independent experiments is shown.
The CD4-CD1d interaction is critical for the antigen-specific activation of CD4 + invTCR transfectants. (A) 58αβ-mouse T-cell hybridoma was transfected with the invVα24-JαQ/Vβ11 TCR (left panel) and human CD4 (right panel). (B) CD4 + invTCR transfectants were activated with increasing doses of either plastic-bound soluble recombinant human CD1d, pulsed with α-GalCer (left panel) or plastic-bound antimouse CD3 2C11 (right panel), in the presence of: isotype matched control IgG (IgG1 + IgG2a); recombinant glycosylated momomeric HIV gp120 (gp120); anti-CD4 S3.5 (S3.5) or 6D10 (6D10) blocking mAbs. IL-2 contained in the culture was determined by enzyme-linked immunosorbent assay. (C) Human CD4 + iNKT cell clone CL1CD4 was cocultured in duplicate wells with RMA/S-hCD1d or C1R-CD1d, with or without either isotype-matched control IgG or anti-CD4 mAb S3.5. After 48 hours, culture supernatants were collected and tested by enzyme-linked immunosorbent assay for secreted IFN-γ. Nontransfected C1R and RMA/S cells were not recognized by the clone (data not shown). One representative of 3 independent experiments is shown.
Of note, monomeric recombinant glycosylated HIV gp120, a viral protein that binds CD4 without cross-linking it, strongly inhibited the activation of CD4 + iNKT cells activated by plastic bound α-GalCer–CD1d complexes (Figures 2A,B, and 3B), suggesting that inhibition of iNKT cell activation was caused by disruption of the CD4–CD1d interaction, rather than to a negative signal delivered into CD4 + iNKT cells on CD4 cross-linking. As control experiments, we studied the effect of CD4 blocking on the activation of CD4 + iNKT cells by CD3 cross-linking. As shown in Figures 2C and 3B (right panel), neither CD4-blocking mAb nor HIV gp120 inhibited cytokine production by CD4 + iNKT cells on CD3 cross-linking.
Human iNKT cells are also intrinsically autoreactive, that is to say they recognize APC expressing CD1d without the addition of exogenous antigens, presumably because of the presentation of endogenous lipids by CD1d.37 To evaluate the possible role of CD4 as coreceptor in self-ligands recognition by iNKT cells, the CD4 + CD4CL1 human iNKT cell clone was cocultured in the absence of α-GalCer, with or without blocking anti-CD4 mAb, with murine T lymphoma RMA/S cells engineered with hCD1d (RMA/S-CD1d) or with human C1R-CD1d cells (Figure S1, available on the Blood website; see Supplemental Materials link at the top of the online article). The amount of secreted IFN-γ by iNKT cells in response to self-ligands presented by RMA/S-CD1d or C1R-CD1d cell lines was significantly lowered by the anti-CD4 mAb (Figure 3C). This observation strongly suggested that CD4 molecule was involved as coreceptor in iNKT cells recognition of murine and human self-ligand(s) as well. Furthermore, because RMA-/S hCD1d are completely MHC class II–negative, it was possible to further rule out the influence of the interaction between CD4 and MHC class II expressed by APC in the recognition of murine self-antigens presented by transfected hCD1d.
CD4 coreceptor potentiates αGalCer/CD1d-induced activation of CD4 + iNKT cells
These experiments relied on CD4 blockade to demonstrate a functional role of CD4 in iNKT cell activation. As a complementary approach to assess the contribution of CD4 coreceptors to the potentiation of iNKT cell effector functions, we compared the functional characteristics of CD4 + and CD4− iNKT cell clones expressing identical TCR. Such clones, which were fortuitously isolated from PBMCs of a healthy donor along a repertoire analysis of peripheral iNKT cells,17 showed a stable CD4bright, CD4dim, and CD4CD8 DN phenotype, despite expression of TCRα and TCRβ chains with identical nucleotide sequences (Figure S2). When incubated with Hela-CD1 transfectants loaded with α-GalCer, the CD4bright iNKT clones produced larger amounts of IFN-γ and IL4 than their DN counterparts, although both clones produced similar amounts of these cytokines on stimulation by PMA and calcium ionophore (Figure 4A and Figure S3). Furthermore TCR/CD3 down-modulation induced on exposure to various α-GalCer concentrations was more pronounced and lasted longer on the CD4 + than on the DN iNKT clone, particularly at suboptimal antigen doses (Figure 4B). Finally, the addition of blocking anti-CD4 mAb to the assay inhibited IFN-γ production (Figure 4C), cytotoxic activity (Figure S4), and antigen-induced TCR down-modulation (not shown) of the CD4 + but not the DN iNKT clones. Altogether these results are consistent with a TCR/CD4 co-engagement by CD1d/α-GalCer, leading to sustained TCR signaling and potentiation of effector responses.
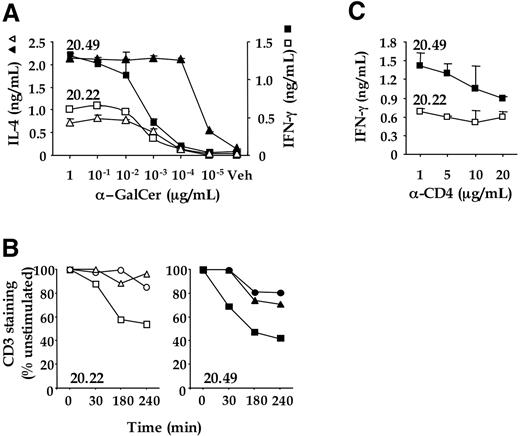
DN and CD4 + NKT cell clones expressing identical TCR are differentially activated after α-GalCer/CD1d activation. (A) Cytokine expression profiles of DN (20.22) and CD4 + (20.49) NKT cell clones. HeLa CD1d cells were pulsed with α-GalCer (1 to 10−5 μg/mL) or DMSO (Veh) and used to stimulate the NKT cell clones 20.22 (open symbols) and 20.49 (closed symbols). After 12 hours, IFN-γ (squares) and IL-4 (triangles) in supernatants was measured by enzyme-linked immunosorbent assay. Data represent the average value of duplicate samples ± SD and are representative of at least 3 independent experiments. (B) Time course of CD3 down-regulation in NKT cell clones 20.22 (open symbols) and 20.49 (closed symbols) stimulated with HeLa CD1d cells pre-pulsed with α-GalCer at 10−3 (squares), 10−4 (triangles) and 10−5 (circles) μg/mL. Data are representative of at least 3 independent experiments. (C) Effect of anti-CD4 mAb on cytokine production. HeLa CD1d cells were pulsed with 10−5 μg/mL of α-GalCer, washed and used to stimulate the iNKT cell clones 20.22 (open squares) and 20.49 (closed squares) in the presence of increasing doses of anti-CD4 mAb RPA-T4 (α-CD4). After 12 hours, IFN-γ in the supernatants was measured by enzyme-linked immunosorbent assay. Data represent the average value of duplicate samples ± SD and are representative of at least 3 independent experiments.
DN and CD4 + NKT cell clones expressing identical TCR are differentially activated after α-GalCer/CD1d activation. (A) Cytokine expression profiles of DN (20.22) and CD4 + (20.49) NKT cell clones. HeLa CD1d cells were pulsed with α-GalCer (1 to 10−5 μg/mL) or DMSO (Veh) and used to stimulate the NKT cell clones 20.22 (open symbols) and 20.49 (closed symbols). After 12 hours, IFN-γ (squares) and IL-4 (triangles) in supernatants was measured by enzyme-linked immunosorbent assay. Data represent the average value of duplicate samples ± SD and are representative of at least 3 independent experiments. (B) Time course of CD3 down-regulation in NKT cell clones 20.22 (open symbols) and 20.49 (closed symbols) stimulated with HeLa CD1d cells pre-pulsed with α-GalCer at 10−3 (squares), 10−4 (triangles) and 10−5 (circles) μg/mL. Data are representative of at least 3 independent experiments. (C) Effect of anti-CD4 mAb on cytokine production. HeLa CD1d cells were pulsed with 10−5 μg/mL of α-GalCer, washed and used to stimulate the iNKT cell clones 20.22 (open squares) and 20.49 (closed squares) in the presence of increasing doses of anti-CD4 mAb RPA-T4 (α-CD4). After 12 hours, IFN-γ in the supernatants was measured by enzyme-linked immunosorbent assay. Data represent the average value of duplicate samples ± SD and are representative of at least 3 independent experiments.
CD4 coreceptor affects the binding of soluble α-GalCer/CD1d multimers to iNKT cell clones
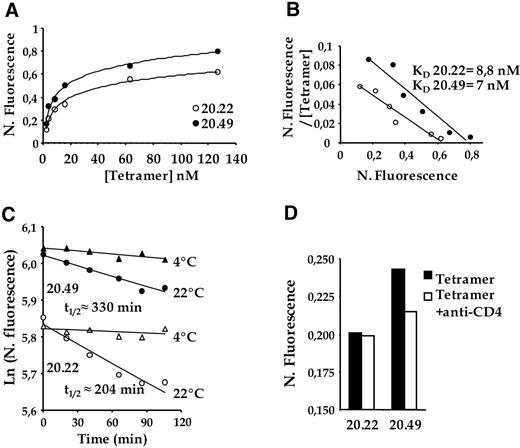
To directly document interactions between CD1d and CD4, we studied the binding of soluble tetrameric α-GalCer–CD1d complexes (CD1d tetramers) to CD4 + and DN iNKT cell clones expressing identical TCR. While unloaded CD1d tetramers did not bind to iNKT clones (not shown), a saturable binding of α-GalCer-loaded CD1d tetramers was obtained on both CD4 + and DN clones when using a concentration range of tetramer from 3 to 126 nM (Figure 5A). When normalized to TCR expression, CD1d tetramer staining intensity was higher on the CD4 + than on the DN clone. Scatchard transformation of tetramer binding indicated a slightly higher binding avidity of CD1d tetramers to the CD4 + than to the DN clone (apparent KD of, respectively, 7 nM and 8.8 nM; Figure 5B).
Differential binding of α-GalCer–loaded CD1d tetramers on CD4 + and DN human iNKT cell clones expressing identical TCR. (A) iNKT cell clones 20.22 (open symbols) and 20.49 (closed symbols) were incubated with increasing concentrations of α-GalCer–loaded CD1d tetramer. For each point, tetramer fluorescence is normalized to the level of surface expressed TCR (N. Fluorescence). (B) Scatchard transformation of the binding isotherm of α-GalCer–loaded CD1d tetramer to the NKT cell clones 20.22 (open symbols) and 20.49 (closed symbols). The ratio of normalized fluorescence to the tetramer concentration is plotted against the normalized fluorescence. (C) Decay plots of the natural logarithm of binding to the NKT cell clones 20.22 (open symbols) and 20.49 (closed symbols) of α-GalCer-loaded CD1d tetramers. Normalized fluorescence is plotted versus time after addition of anti-CD1d blocking mAb. Cells were incubated with α-GalCer–loaded CD1d tetramer and TCR mAb then brought to either + 4°C or + 22°C. Blocking anti-CD1d (30 μg/mL) was added and, at consecutive time points, an aliquot was analyzed by flow cytometry. Half-lives of tetramer stainings are indicated (t1/2). (D) α-GalCer–loaded CD1d tetramer binding to iNKT cell clones 20.22 and 20.49 in the absence (filled histograms) or presence of anti-CD4 mAbs (open histograms). One representative experiment (of 3) is shown.
Differential binding of α-GalCer–loaded CD1d tetramers on CD4 + and DN human iNKT cell clones expressing identical TCR. (A) iNKT cell clones 20.22 (open symbols) and 20.49 (closed symbols) were incubated with increasing concentrations of α-GalCer–loaded CD1d tetramer. For each point, tetramer fluorescence is normalized to the level of surface expressed TCR (N. Fluorescence). (B) Scatchard transformation of the binding isotherm of α-GalCer–loaded CD1d tetramer to the NKT cell clones 20.22 (open symbols) and 20.49 (closed symbols). The ratio of normalized fluorescence to the tetramer concentration is plotted against the normalized fluorescence. (C) Decay plots of the natural logarithm of binding to the NKT cell clones 20.22 (open symbols) and 20.49 (closed symbols) of α-GalCer-loaded CD1d tetramers. Normalized fluorescence is plotted versus time after addition of anti-CD1d blocking mAb. Cells were incubated with α-GalCer–loaded CD1d tetramer and TCR mAb then brought to either + 4°C or + 22°C. Blocking anti-CD1d (30 μg/mL) was added and, at consecutive time points, an aliquot was analyzed by flow cytometry. Half-lives of tetramer stainings are indicated (t1/2). (D) α-GalCer–loaded CD1d tetramer binding to iNKT cell clones 20.22 and 20.49 in the absence (filled histograms) or presence of anti-CD4 mAbs (open histograms). One representative experiment (of 3) is shown.
CD4 coreceptor is thought to potentiate conventional T-cell responses through stabilization of TCR/peptide-MHC interactions.42 To address this, we compared CD1d tetramer binding decay on CD4 and DN iNKT cell clones in the presence of saturating amounts of anti-CD1d mAb (which prevents rebinding of tetramers to the cells; Figure 5C). In agreement with previous studies using CD1d tetramers,40 a very low decay of the tetramer staining was observed for both the CD4 and DN NKT cell clones at 4°C. By contrast at 22°C, the tetramer staining decay was higher for the DN clone than for the CD4 clone as indicated by their respective t1/2 of 204 minutes and 330 minutes. This decay occurred stochastically as attested by the linear decay plot of the natural logarithm of the normalized fluorescence. Consistent with a direct contribution of CD4 coreceptors to the differential binding of CD1d tetramers to CD4 versus DN iNKT clones expressing identical TCR, tetramer binding to the CD4 clone was decreased by blocking anti-CD4 Ab, and was brought back to levels obtained with the DN clone (Figure 5D). To test the generality of these observations, we studied the effect of anti-CD4 mAb on CD1d tetramer binding to freshly expanded polyclonal Vα24Vβ11 iNKT cell lines enriched for either DN or CD4 + cells. Tetramer staining intensities obtained with CD4 + iNKT cell lines derived from 2 distinct donors were brighter than those obtained with donor-matched DN cell lines (MFI of 242 versus 180 in donor A; Figure S5). Moreover anti-CD4 mAb reduced CD1d tetramer binding to CD4 (but not DN) cell lines in both donors, while they did not affect CD3 staining intensity.
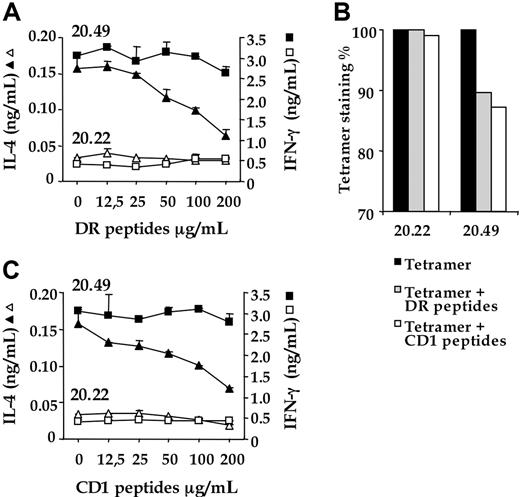
To further investigate the interactions between CD4 and CD1d molecules, we determined whether DR and CD1 synthetic peptides corresponding respectively to the putative CD4 interaction sites of human MHC class II and CD1d could affect iNKT effector responses and invTCR/α-GalCer–CD1d interactions. To this end, we designed a DR peptide corresponding to a critical CD4 binding region in the β2 loop of MHC class II DR1 peptide (β chain, position 134-152)33 and a CD1 peptide corresponding to a putative CD4 binding region in CD1d, deduced from comparative analysis of the crystal structures and primary amino acid sequences of CD1d and MHC class II molecules (see “Material and methods” and “Discussion”). As shown in Figure 6A, both peptides specifically inhibited, in a dose-dependent manner, IL-4 secretion of CD4 + iNKT cells (clone 20.49). Moreover, these peptides decreased the binding of CD1d tetramers to CD4 + iNKT cells but not to their DN counterparts (Figure 6B).
Peptides corresponding to the MHC class II and the putative CD1 regions binding to CD4 affect effector responses and TCR engagement of CD4 + iNKT cells. (A) iNKT cell clones CD4 + 20.49 (closed symbols) and DN 20.22 (open symbols) were pre-incubated with grading doses of DR or CD1 peptides for 1 hour at 37°C, washed, and subsequently cocultured with HeLa CD1d cells pre-loaded with α-GalCer (10−1 μg/mL). After 20 hours, IL-4 (triangles) and IFN-γ (squares) released in the supernatants were titrated by enzyme-linked immunosorbent assay. Data represent the average value of duplicate samples ± SD and are representative of at least 3 independent experiments. (B) α-GalCer–loaded CD1d tetramer binding to iNKT cell clones 20.22 and 20.49 in the absence (filled histograms) or presence of DR (shaded histograms) and CD1 peptides (open histograms). One representative experiment (of 3) is shown.
Peptides corresponding to the MHC class II and the putative CD1 regions binding to CD4 affect effector responses and TCR engagement of CD4 + iNKT cells. (A) iNKT cell clones CD4 + 20.49 (closed symbols) and DN 20.22 (open symbols) were pre-incubated with grading doses of DR or CD1 peptides for 1 hour at 37°C, washed, and subsequently cocultured with HeLa CD1d cells pre-loaded with α-GalCer (10−1 μg/mL). After 20 hours, IL-4 (triangles) and IFN-γ (squares) released in the supernatants were titrated by enzyme-linked immunosorbent assay. Data represent the average value of duplicate samples ± SD and are representative of at least 3 independent experiments. (B) α-GalCer–loaded CD1d tetramer binding to iNKT cell clones 20.22 and 20.49 in the absence (filled histograms) or presence of DR (shaded histograms) and CD1 peptides (open histograms). One representative experiment (of 3) is shown.
Discussion
We showed that CD4 contributes to CD4 + iNKT cell activation after invTCR recognition of α-GalCer–CD1d complex. The blocking reagents used to neutralize CD4 function bind the outermost D1 and D2 domains of the coreceptor, involved in both MHC class II and gp120 binding.8,33,43,44 The same CD4 domains may thus be involved in the interaction with CD1d as well, although the fine molecular anatomy of this binding remains to be determined in comparison with that of class II.
As expected from its coreceptor function, CD4 was particularly important at suboptimal antigen levels, where the activation signals emanating from TCR require potentiation from the coreceptor.45 Furthermore, although a sizeable fraction (4/7) of the CD4 + iNKT cell clones tested were affected by CD4 blocking reagents, several CD4 + clones turned out to be “CD4-independent” (data not shown), probably because of their higher TCR affinity for α-GalCer–CD1d complexes, a representation similar to that of class II–restricted CD4 + T cells.39 These results are in line with a recent study showing that Vα24-JαQ independent α-GalCer–CD1d reactive NKT cells are never DN, but instead express either CD8αβ or CD4 coreceptors.46 One possible interpretation is that activation of T cells expressing noncanonical TCR by α-GalCer–CD1d systematically requires engagement of CD4 or CD8 coreceptors to compensate for a weaker affinity of Vα24-negative TCR for α-GalCer–CD1d complexes, compared with invTCR.
Several observations suggest that CD4 potentiates iNKT activation by engaging CD1d rather than MHC class II molecules. First, CD4 enhanced iNKT cell effector functions in experimental systems where MHC class II molecules were absent. In particular, the mouse T hybridoma cells engineered with invTCR were completely negative for MHC class II expression, yet they responded better to recombinant α-GalCer–CD1d stimulation when they expressed CD4, suggesting direct interactions between CD4 and CD1d. Furthermore, CD1d tetramers bound better to CD4 + than DN iNKT clones expressing identical TCR, and their binding to CD4 clones was inhibited by CD4 blocking reagents. Interestingly, CD4 + iNKT cells are present in MHC class II–deficient mice,47 indicating that CD4 expression on these cells is independent of the expression of MHC class II molecules during thymic selection. We cannot discount, however, the possibility that CD4 expressed on iNKT cells binds both CD1d and MHC class II molecules coexpressed on APCs, resulting in a synergistic stimulus for T-cell activation. CD1d is indeed found on APCs that are typically MHC class II–positive, such as B cells, monocytes, and dendritic cells,48 and CD4 could bind both antigen presenting molecules on them.
The functional evidence for a direct CD4–CD1d interaction raises the question as to whether the CD1d structure is compatible with CD4 binding. Resolution of the crystal structure of mouse CD1d14 revealed that its α3 domain retains some structure similarity with the highly acidic loop (residues 220-229) in the α3 domain from class I, implicated in CD8 binding,49 as well as with its equivalent CD4 binding sites in the α2 and β2 domains (residues 137-142 and 134-152 in I-Ad and HLA-DR4, respectively) of class II.8 CD1d would thus display a structure compatible with coreceptor binding, although one should be aware that the same degree of similarity in the α3 domain is found even in the neonate Fc receptor FcRn, a molecule with an MHC class I–like folding that does not bind accessory molecules.14
CD1d displays hybrid features found on either MHC class I or class II products. Like MHC class I, CD1 is associated with β2-microglobulin.14 Furthermore, the relative paucity of peripheral iNKT cells expressing a CD8 αβ coreceptor, despite differentiation through a CD4CD8 double-positive stage, and the in vivo deletion of iNKT cells on forced expression of CD8 coreceptors,13 suggest that, like MHC class I, CD8 binds CD1d. However, CD1d displays several MHC class II features50 (see reference for a recent review) such as (1) intracellular routing and glycolipid loading in endosomal and late lysosomal compartments enriched for MHC class II51 ; (2) association between CD1d and the Ii invariant chain52 ; and (3) control of CD1d-restricted iNKT cell development by cathepsin L, a key protease involved in MHC class II peptide processing and Ii cleavage.53 Along this line, the fact that CD1 proteins show comparable levels of similarity and divergence to both families of MHC proteins14,15 implies that CD1 may have diverged from a primordial common ancestral antigen-presenting molecule at least at the same time of the divergence of MHC class I and class II molecules. It is therefore possible that CD1 has maintained throughout evolution a kind of “primordial” capacity to bind both CD4 and CD8 coreceptors, possibly at lower affinity than class II and I, respectively, a property lost during evolution by the more “specialized” MHC proteins. Moreover, it is possible that CD4 interacts with the other CD1 proteins, which display structurally conserved α3 domains,15 and CD4 + T cells specific for any of the other CD1 molecules have been described.15
Collectively, our findings indicate that CD4 coreceptor could play a relevant role in the glycolipid-CD1d recognition process by CD4 + iNKT cells. iNKT cells are designed to respond rapidly to self or foreign antigens presented by CD1d after tissue damage and cellular stress. The requirement for CD4 coreceptor may be important for the fine-tuning of their reactivity in thymus and periphery. As for conventional MHC class II–restricted T cells, CD4 may also directly take part in the acquisition of particular effector functions by mature iNKT cells, such as Th2 cytokine production. This could explain the correlation found between Th2 polarization and CD4 expression, which has been reported for human iNKT cells.27,28 Therefore, on an applied standpoint, our results raise the possibility to elicit iNKT cell responses with restricted functional features through direct in vivo modulation of CD4/CD1d interactions.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Grazia Galli, Sergio Abrignani, Massimo Degano, Jim Kaufman, and Louis Gastinel for discussion.
This work was supported by grants from by the Associazione Italiana per la Ricerca sul Cancro (AIRC), the Human Frontiers Science Program (HFSP), European Union, Compagnia di San Paolo (P.D. and G.C.), FISM (G.B.), and institutional grants from Institut National de la Santé et de la Recherche Médicale (INSERM), European Union, and Association pour la Recherche sur le Cancer (ARC) (E.S. and M.B.).
Authorship
Contribution: A.T. and C.L. contributed equally to this study as first co-authors. P.D., M.B., E.S., and G.C. contributed equally to the study concept and design. A.T., C.L., S.M., L.C., C.G., and G.B. performed research and analyzed data. S.S. provided tetramer reagents and contributed to the design and the analysis of the tetramer binding experiments. All authors participated in the analysis and interpretation of the data and critical revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giulia Casorati or Paolo Dellabona, Experimental Immunology Unit, Cancer Immunotherapy and Gene Therapy Program, DIBIT, H San Raffaele Scientific Institute, 20132 Milano, Italy; e-mail: casorati.giulia@hsr.it, dellabona.paol@hsr.it; Emmanuel Scotet or Marc Bonneville, INSERM U601, Département de Recherche en Cancérologie, Institut de Biologie, 9 quai Moncousu, 44093 Nantes cedex, France; e-mail: Emmanuel.Scotet@nantes.inserm.fr, bonnevil@nantes.inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal