p62dok and Dok-3 are members of the Dok family of adaptors found in B cells, with the former cloned as a substrate of the p210bcr/abl oncoprotein in Ph + chronic myelogenous leukemia. A role for p62dok in FcγRIIB–mediated negative regulation of B-cell proliferation had been established previously. Here, we generated Dok-3−/− mice to assess the function of Dok-3 in B cells. Mice lacking Dok-3 have normal B-cell development but possess higher level of IgM antibodies in their sera. In comparison to wild-type mice, Dok-3−/− mice mounted significantly enhanced humoral immune responses to T cell–independent type I and II antigens. Dok-3–deficient B cells hyperproliferated, exhibited elevated level of calcium signaling as well as enhanced activation of NF-κB, JNK, and p38MAPK in response to B-cell receptor (BCR) engagement. In the absence of Dok-3, the localization of the inhibitory phosphatase SHIP-1 to the plasma membrane is intact while its phosphorylation is compromised, suggesting that Dok-3 could function to facilitate or sustain the activation of SHIP-1. The phenotype and responses of Dok-3−/− mice and B cells could be differentiated from those of the Dok-1−/− counterparts. Hence, we propose that Dok-3 plays a distinct and nonredundant role in the negative regulation of BCR signaling.
Introduction
B-cell development occurs in the adult bone marrow where precursor B cells mature through discrete stages into surface immunoglobulin (Ig)-positive immature B cells that subsequently transit to the peripheral lymphoid organs to develop into long-lived mature B cells. In the periphery, further differentiation of B cells could occur upon specific antigen recognition.1 Once activated, B cells may directly differentiate into IgM-secreting plasma cells or participate in germinal center reactions to generate either Ig class–switched plasma or memory B cells.2
B cell receptor (BCR) signaling plays a pivotal role in the generation and activation of B lymphocytes.3 Engagement of the BCR is known to activate various cytoplasmic tyrosine kinases such as Syk, Lyn, Blk, and Bruton tyrosine kinase (Btk), and this leads to the activation of transcription factors such as NF-AT and NF-κB that regulate the expression of distinct genes, and, ultimately, drive various aspects of B cell responses and differentiation. Genetic deletions of key signaling molecules in the BCR signal transduction pathway have indicated that the magnitude and duration of BCR signaling is important in driving B-cell differentiation and responses. For example, mutations in BCR and its associated signaling molecules could either impair primary B lymphopoiesis and result in immunodeficiencies, or generate excessive B cells and cause autoimmunity.4,5
Given the sensitivity of B cells to the strength of BCR signaling, and the need to prevent overt activation of B lymphocytes that may result in harm to the organism, several classes of signaling molecules act to inhibit or down-regulate BCR signaling following B-cell activation. These include cell surface receptors such as FcγRIIB, tyrosine kinases such as Csk, as well as intracellular phosphatases such as SHP-1 and SHIP-1.6,,,–10 Other signaling proteins that may play an inhibitory role in BCR signaling are adaptor molecules that do not have enzymatic activities on their own but could recruit other effector molecules to shut down the BCR signaling cascade.
The Dok proteins are examples of adaptor molecules that have been implicated in inhibitory signaling.11,12 Members of this family include p62dok (Dok-1), Dok-2 (Dok-R/FRIP), Dok-3 (Dok-L), Dok-4, 5, 6 and 7. The Dok family of adaptors shares a similar structure that is characterized by an amino-terminal PH domain followed by a central PTB domain and a proline- and tyrosine-rich carboxy-terminal tail.13,–15 Dok-1, 2 and 3 are expressed in hematopoietic cells while the other Dok members are preferentially expressed in cells of the nervous or muscular system.16,,,–20 Dok-1 was first cloned as a substrate of the p210bcr/abl oncoprotein in Ph + chronic myelogenous leukemia, and it was shown recently that Dok-1 and 2 played a role in myeloid homeostasis and leukemia suppression.21,22
B lymphocytes express both Dok-1 and 3.12,17 Dok-1 is phosphorylated following activation of various cytokine receptors, receptor protein tyrosine kinases, and the BCR, and is known to bind p120rasGAP, Nck, c-abl, and other SH2-containing proteins.14,23,24 An inhibitory role for Dok-1 in B cell signaling was demonstrated by the generation of Dok-1−/− mice.12 Dok-1–deficient B cells were shown to be hyperproliferative to BCR stimulation by intact whole antibodies but not IgM F(ab′)2 fragments. Indeed, Dok-1 is involved in FcγRIIB-mediated negative regulation of cell proliferation and is shown to suppress MAP kinase activation.12,25,26
Dok-3 was cloned by virtue of its binding to Csk, SHIP-1, and Dok-3 c-abl, and differs from Dok-1 in that it does not directly bind p120rasGAP.16,17 A recent report, however, suggests that Dok-3 could bind p120rasGAP via Grb2.27 Overexpression of Dok-3 dampens B-cell proliferation and cytokine secretion induced by BCR cross-linking.28 Biochemical studies of Dok-3 in the A20 B cell line indicate that it might play an inhibitory role in BCR signaling, in particular in the inhibition of JNK activation, and this inhibitory action of Dok-3 was likely mediated through the binding of SHIP-1.28
Although in vitro overexpression systems have suggested that Dok-3 might play a role in BCR signaling, its physiological role in B cell biology remains to be determined. Dok-1 mutation did not impair B-cell development and it remains to be determined if Dok-3 would play a role in B cell differentiation. It is also currently not clear why B lymphocytes express both Dok-1 and Dok-3, given that the 2 adaptor molecules appear to play similar inhibitory roles in cell signaling. It is plausible that Dok-1 and Dok-3 effect different inhibitory signals and therefore regulate different aspects of B-cell activation. Alternatively, the presence of the 2 Dok molecules could suggest some degree of redundancy of Dok proteins in BCR signaling. However, that Dok-1−/− mice exhibited a phenotype suggests that Dok-1 is not redundant in B cell signaling. But this has yet to be established for Dok-3. To attempt to address these various questions and to understand the role of Dok-3 in B cell development and activation, we have generated a null mutation in the mouse Dok-3 gene.
Materials and methods
Generation of Dok-3−/− mice
A targeting vector was assembled to disrupt exons 1 and 2 of Dok-3. To screen for homologous recombinants, genomic DNA from vector-transfected G418-resistant E14.1 ES cell clones were digested with HindIII and analyzed by Southern blotting with a 303bp exon 5 probe. Clones containing disrupted Dok-3 were injected into C57BL/6 blastocysts to generate chimera, which were bred for germ-line transmission of the mutated allele. All mice were maintained in a specific-pathogen free facility and approved for use according to institutional and national guidelines.
Flow cytometry
Single cell suspensions were obtained from spleen and lymph nodes by dissociation of tissues with a plastic mesh and rubber-stopper from a 5-mL syringe, and by injecting RPMI containing 10% fetal calf serum (FCS) into the peritoneal cavities, femurs, and tibiae of mice. All cells were treated with erythrocyte lysing solution (0.15 M NH4Cl, 1 mM KHCO3 and 0.1 mM Na2EDTA). For fluorescence activated cell sorting (FACS) analyses, cells were stained with FITC-, PE- and biotin-conjugated monoclonal antibodies (mAb) for 10 minutes on ice and washed 3 times. Biotin-conjugated mAb staining was subsequently revealed with streptavidin-CyChrome. Flow cytometry was performed on a FACScan (Becton Dickinson, Mountain View, CA) and data analyzed with CellQuest software (BD Biosciences, San Jose, CA). Abs used in the FACS analyses were anti-B220 (RA3–6B2), anti-BP-1, anti-IgM (331.12), anti-CD2, anti-CD3, anti-CD5, anti-CD11b, anti-CD25 and anti-CD43.
Measurement of basal immunoglobulin levels
NUNC Maxisorp 96-well plates were coated with 2 μg/mL of antimouse Igκ antibody (BD Biosciences). Serially diluted serum (50 μL) was added to each well and incubated at 4°C overnight. Samples were washed 5 times in PBS containing 0.05% Tween-20 before incubation with biotinylated rat antimouse IgM, IgG1, IgG2a, IgG2b or IgG3 detection antibodies (BD Biosciences). Detection antibodies were washed off before the addition of streptavidin-coupled horseradish peroxidase secondary antibody. Finally BD OptEIA detection reagent (Becton Dickinson) was applied and colorimetric changes were quantified by measuring absorbance at 450 nm with a TECAN Genios multiplate reader.
Immunizations of Dok-3−/− mice
Mice were immunized intraperitoneally with 10 μg of 4-hydroxy-3-nitrophenyl acetyl (NP) coupled to lipopolysaccride (NP1-LPS) and 10μg NP25-Ficoll to examine responses against T cell-independent type I and II antigens, respectively. Sera were collected 7 days after immunizations to determine NP-specific IgM and IgG3 antibodies via enzyme-linked immunosorbent assay (ELISA) using NP17-BSA (5 μg/mL) as substrate. For T cell-dependent immune response, mice were immunized with 100 μg of NP30-CG (chicken globulin). Primary immune response was quantitated by ELISA using sera collected at 7 and 14 days after immunizations. For secondary immune response, mice were rechallenged at day 30 with 5 μg of NP30-CG, and antibody titers determined 5 days later.
In vitro cell stimulations and measurement of proliferation and calcium signaling
Splenic B cells were isolated to more than 90% purity from wild-type and mutant mice using anti-CD43 mAb-coupled magnetic beads in a magnetic activated cell sorting (MACS) negative selection protocol (Miltenyl Biotech, Bergisch Gladbach, Germany). Cells (2 × 105) were cultured in each well of a 96-well flat-bottomed plate in RPMI supplemented with 10% FCS, 0.055 mM β-mercaptoethanol, 2 mM l-glutamine and penicillin/streptomycin and stimulated with 10 μg/mL IgM F(ab′)2 fragment alone or together with 0.5 μg/mL CD40Ligand. For proliferation assays, cells were stimulated for 18 hours at 37°C before addition of 1 μCi of H3-thymidine (Amersham Bioscience, Buckinghamshire, United Kingdom) for another 6 hours and harvested with a TOMTEC cell harvester. The incorporation of radioactivity was measured on a Wallac Trilux 1450 Microbeta liquid scintillation and luminescence counter (PerkinElmer Life Sciences, Waltham, MA). To measure calcium flux, B cells (5 × 106) were loaded with Indo-1 AM (2 μM; Molecular Probes, Carlsbad, CA) in PBS containing 1 mM CaCl2, 1 mM MgCl2 and 1% FCS for 45 minutes at 37°C and stimulated with anti-IgM F(ab′)2 fragment or anti-IgG F(ab′)2 fragment (Jackson Immuno Research, West Grove, PA). Calcium flux was monitored on an LSRII flow cytometer (Becton Dickinson) in real time for 15 minutes.
Western blotting
Splenic B cells were lysed on ice for 10 minutes in phospholysis buffer containing 1% NP40, 10 mM Tris-HCl pH8.0, 150 mM NaCl, 1 mM EDTA, 0.2 mM Na3VO4, and a cocktail of protease inhibitors (Roche, Basel, Switzerland) and sonicated. Cell homogenates were centrifuged at 4300g for 15 minutes at 4°C and supernatants were recovered for protein quantification using a BCA protein assay kit (Pierce, Rockford, IL). For Western blot analyses, cell lysates from stimulated cells were probed with antibodies against various signaling molecules. Antibodies used from Santa Cruz (Santa Cruz, CA) were anti-Dok-1, anti-Dok-3 (D-18), anti-phospho-ERK, anti-ERK2, anti-JNK1, anti-p38MAPK, anti-IκBα, anti-SHIP-1, and anti-Lyn; from Cell Signaling Technology (Danvers, MA), anti-phospho-SHIP-1 (Tyr1020), anti-phospho-SAPK/JNK (Thr183/Tyr185) and anti-phospho-p38 (Thr180/Tyr182).
Isolation of subcellular fractions
To isolate plasma membrane fractions, splenic B cells were lysed on ice for 20 minutes in a hypotonic buffer containing 15 mM Tris-HCl pH7.5, 5 mM KCl, 1.5 mM MgCl2, 0.1 mM EGTA, 0.2 mM Na3VO4, and protease inhibitor cocktail (Roche). Cell lysates were passed through a 26G needle and centrifuged at 500g. Supernatants were recovered and transferred to polycarbonate tubes for ultracentrifugation at 20 800g for 1 hour at 4°C. The resulting pellet containing the plasma membrane fraction was solubilized in Triton X-100 lysis buffer (150 mM NaCl, 15 mM Tris-HCl pH7.5, 5 mM EDTA, 1% Triton X-100, 0.2 mM Na3VO4, and protease inhibitor cocktail). To isolate the nuclear fraction, cells were treated with NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce) according to the manufacturer's protocol. Samples were quantitated before adding to radiolabeled oligonucleotides bearing the NFκB consensus sequence in gel-shift assays.
Results
Generation of Dok-3−/− mice and analyses of mutant B cell development
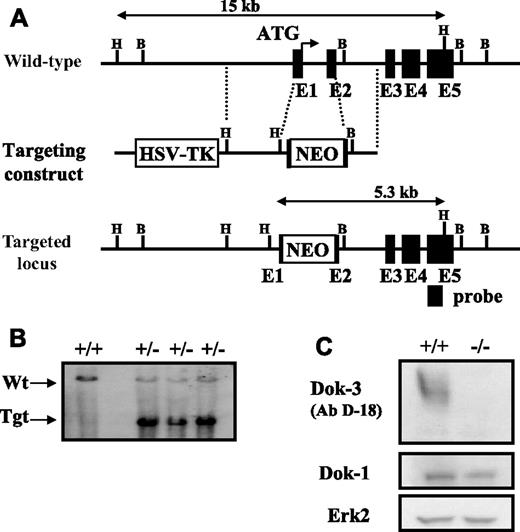
We generated Dok-3−/− mice by inserting a neomycin-resistance gene into the Dok-3 locus in mouse embryonic stem cells. The targeting strategy removed the transcriptional start codon in exon 1 and partially replaced exon 2 of Dok-3 (Figure 1A). The inactivation of Dok-3 was verified by Southern blotting of targeted mouse ES cell DNA using an external probe, which distinguished the 15kb wildtype and 5.3kb targeted loci (Figure 1B). Western blot analyses of mouse splenic whole cell lysates using 2 different commercial anti-Dok-3 antibodies, one of which is shown in Figure 1C, and another homemade anti-Dok-3 (data not shown), also indicated that no full-length or truncated protein was produced in the mutant. Furthermore, Western blot analyses of mutant whole-cell lysates with an anti-Dok-1 antibody indicated that Dok-1 protein expression was still intact. Thus, we have specifically and effectively disrupted dok-3.
Inactivation of dok-3 gene locus. (A) Partial restriction maps of wild-type dok-3 locus, targeting construct, and inactivated locus after homologous recombination. Restriction enzymes used to define the loci are: B, BamHI and H, HindIII. The external probe used to screen HindIII-digested ES cells, which distinguishes the 15-kb wild-type and 5.3-kb targeted loci, is indicated. (B) Southern blot analysis of HindIII-digested wild-type and G418-resistant ES cell clones depicting the wild-type and inactivated dok-3 loci. (C) Western blot analysis of whole cell lysates obtained from wild-type and Dok-3−/− mouse spleens. The blots were first probed with anti-Dok-3 antibodies and reprobed with anti-Dok-1 and anti-ERK2 antibodies to control for equal loading of whole cell lysates.
Inactivation of dok-3 gene locus. (A) Partial restriction maps of wild-type dok-3 locus, targeting construct, and inactivated locus after homologous recombination. Restriction enzymes used to define the loci are: B, BamHI and H, HindIII. The external probe used to screen HindIII-digested ES cells, which distinguishes the 15-kb wild-type and 5.3-kb targeted loci, is indicated. (B) Southern blot analysis of HindIII-digested wild-type and G418-resistant ES cell clones depicting the wild-type and inactivated dok-3 loci. (C) Western blot analysis of whole cell lysates obtained from wild-type and Dok-3−/− mouse spleens. The blots were first probed with anti-Dok-3 antibodies and reprobed with anti-Dok-1 and anti-ERK2 antibodies to control for equal loading of whole cell lysates.
Mice lacking Dok-3 were born in a Mendelian ratio, suggesting that Dok-3 was not essential for organism development. Since Dok-3 was reportedly expressed mainly in hematopoietic cells, and in particular B cells,17 we decided to focus the current study on the effect of a lack of Dok-3 on B cell development, function, and activation.
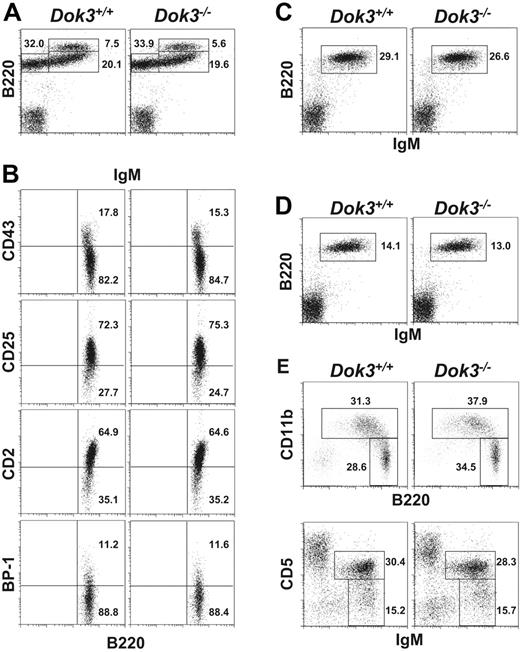
B cells first develop in the adult bone marrow, starting from B220 +IgM− pro/pre B cells and progressing to B220 +IgMhigh immature and B220 +IgM + mature B cells.1 Flow cytometry analysis of B cell fractions in the bone marrow of Dok-3−/− mice indicated that there was no significant difference in the fractions (Figure 2A) or absolute number (Table 1) of these cells in the mutant mice compared with wild-type controls. In addition, detailed analyses of the early B220 +IgM− B cells with various markers such as CD2, CD25, CD43 and BP-1 that further differentiate pro-B and pre-B cells also revealed no perturbation in the early stage development of mutant B cells (Figure 2B).
Normal B cell development in Dok-3−/− mice. Flow cytometry analyses of B cell populations in the bone marrow (A and B), spleen (C), lymph nodes (D) and peritoneal cavity (E) of wild-type and Dok-3−/− mice. Bone marrow B220 +IgM− cells were further analyzed for their expression of CD43, CD2, CD25 and BP-1 (B). Numbers indicate percent of cells in the lymphocyte gate for (A, C, D and E) and percent of B220 +IgM− cells for (B). Data shown are representative of 3 independent experiments.
Normal B cell development in Dok-3−/− mice. Flow cytometry analyses of B cell populations in the bone marrow (A and B), spleen (C), lymph nodes (D) and peritoneal cavity (E) of wild-type and Dok-3−/− mice. Bone marrow B220 +IgM− cells were further analyzed for their expression of CD43, CD2, CD25 and BP-1 (B). Numbers indicate percent of cells in the lymphocyte gate for (A, C, D and E) and percent of B220 +IgM− cells for (B). Data shown are representative of 3 independent experiments.
Enumeration of total cells and lymphocyte populations in various tissues of wild-type and Dok-3−/− mice
| Tissue . | Total cells, × 107 . | CD3+, × 106 . | B220+ IgM+, × 106 . | CD5+ IgM+, × 106 . | B220+ IgM−, × 106 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| +/+ . | −/− . | +/+ . | −/− . | +/+ . | −/− . | +/+ . | −/− . | +/+ . | −/− . | |
| Spleen | 10.32 ± 1.13 n=6 | 10.83 ± 0.88 n=6 | 23.00 ± 4.20 n=6 | 27.30 ± 5.00 n=6 | 18.30 ± 2.00 n=6 | 21.50 ± 1.50 n=6 | ND | ND | ND | ND |
| Bone marrow | 3.78 ± 0.58 n=6 | 3.46 ± 0.41 n=6 | ND | ND | 1.50 ± 0.30 n=6 | 1.60 ± 0.20 n=6 | ND | ND | 1.90 ± 0.50 n=6 | 2.50 ± 0.60 n=6 |
| Peritoneum | 0.39 ± 0.03 n=10 | 0.45 ± 0.07 n=9 | ND | ND | 1.08 ± 0.22 n=6 | 0.68 ± 0.11 n=7 | 0.40 ± 0.05 n=7 | 0.40 ± 0.06 n=7 | ND | ND |
| Lymph node | 1.10 ± 0.40 n=4 | 1.15 ± 0.39 n=4 | 4.37 ± 1.43 n=4 | 4.25 ± 1.19 n=4 | 1.60 ± 0.73 n=4 | 1.65 ± 0.71 n=4 | ND | ND | ND | ND |
| Tissue . | Total cells, × 107 . | CD3+, × 106 . | B220+ IgM+, × 106 . | CD5+ IgM+, × 106 . | B220+ IgM−, × 106 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| +/+ . | −/− . | +/+ . | −/− . | +/+ . | −/− . | +/+ . | −/− . | +/+ . | −/− . | |
| Spleen | 10.32 ± 1.13 n=6 | 10.83 ± 0.88 n=6 | 23.00 ± 4.20 n=6 | 27.30 ± 5.00 n=6 | 18.30 ± 2.00 n=6 | 21.50 ± 1.50 n=6 | ND | ND | ND | ND |
| Bone marrow | 3.78 ± 0.58 n=6 | 3.46 ± 0.41 n=6 | ND | ND | 1.50 ± 0.30 n=6 | 1.60 ± 0.20 n=6 | ND | ND | 1.90 ± 0.50 n=6 | 2.50 ± 0.60 n=6 |
| Peritoneum | 0.39 ± 0.03 n=10 | 0.45 ± 0.07 n=9 | ND | ND | 1.08 ± 0.22 n=6 | 0.68 ± 0.11 n=7 | 0.40 ± 0.05 n=7 | 0.40 ± 0.06 n=7 | ND | ND |
| Lymph node | 1.10 ± 0.40 n=4 | 1.15 ± 0.39 n=4 | 4.37 ± 1.43 n=4 | 4.25 ± 1.19 n=4 | 1.60 ± 0.73 n=4 | 1.65 ± 0.71 n=4 | ND | ND | ND | ND |
Cell counts were calculated based on total cell numbers and percentage of the different lymphocyte populations as determined by FACS staining. Cell numbers are presented as mean ± SEM. Statistical significance was determined by paired 2-tailed Student t test.
n indicates the sample size; ND, not determined.
Analysis of spleen (Figure 2C) and lymph nodes (Figure 2D) also did not yield any defect in the peripheral B cell populations in Dok-3−/− mice. The fractions corresponding to transitional stage I (IgMhighCD21−CD23−), transitional stage II (IgMhighCD21 +CD23 +), marginal zone (IgMhighCD21highCD23−) or mature follicular (IgMlowCD21lowCD23 +) B cells were also not affected by a Dok-3-deficiency (data not shown). Moreover, the absolute number of peripheral B lymphocytes (Table 1) was comparable in wild-type and Dok-3−/− mice. Finally, analysis of the peritoneal cavity of Dok-3−/− mice (Figure 2E) suggested that the total B1 cell population (B220lowCD11b +) and in particular, B-1a (IgM +CD5 +CD23−) and B-1b (IgM +CD5−CD23−) cells, could also develop normally in these animals. Taken together, the data indicated that the lack of Dok-3 did not disrupt primary B lymphopoiesis or perturb the homeostasis of peripheral B cell populations.
Enhanced basal level of serum IgM and T cell-independent immune responses in Dok-3−/− mice
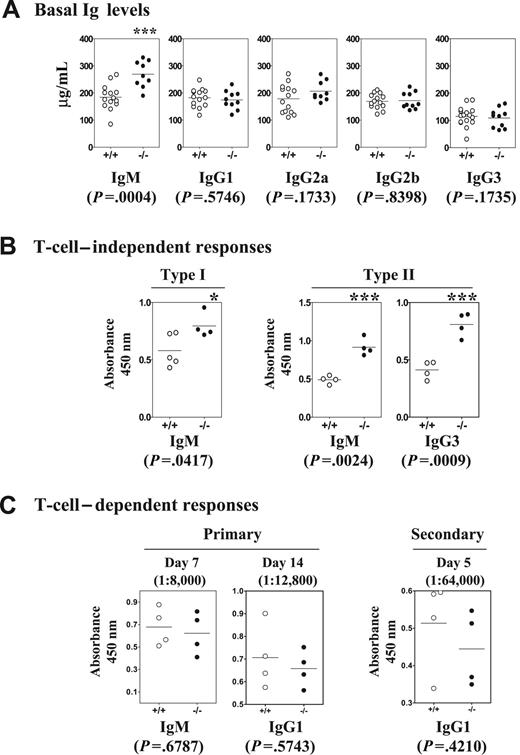
Since Dok-3 deficiency did not disrupt B-cell generation and maturation, we next measured the basal levels of the various classes of serum immunoglobulins in wild-type and Dok-3−/− mice to determine if the absence of Dok-3 would affect B-cell function. As shown in Figure 3A, Dok-3−/− mice had significantly higher level of IgM antibodies in their sera compared with wild-type mice, although the levels of other Ig classes were comparable. These data were the first indication that Dok-3 could play a role in B-cell activation.
Enhanced basal sera immunoglobulin levels and T-cell–independent immune responses in Dok-3−/− mice. Wild-type (open circles) and Dok-3−/− (black circles) mice were analyzed via ELISA for (A) basal serum Ig levels, (B) humoral immune responses to T-cell–independent type I and II antigens NP-LPS and NP-Ficoll, respectively, and (C) humoral immune response to T-cell–dependent antigen, NP-CG. NP-specific antibodies were measured 7 days after immunization for the T-cell–independent immune responses and sera were diluted 2000-fold. For T-cell–dependent immune response, sera were examined at days 7 and 14 for antigen-specific IgM and IgG1 antibodies in the primary response. For secondary immune response, mice were rechallenged 30 days later with the same antigen and examined 5 days thereafter for NP-specific IgG1 antibodies. Sera were diluted as indicated. In all graphs, each data point denoted a single mouse analyzed. Statistical significance was determined by paired 2-tailed Student t test. Horizontal bars indicate the mean responses.
Enhanced basal sera immunoglobulin levels and T-cell–independent immune responses in Dok-3−/− mice. Wild-type (open circles) and Dok-3−/− (black circles) mice were analyzed via ELISA for (A) basal serum Ig levels, (B) humoral immune responses to T-cell–independent type I and II antigens NP-LPS and NP-Ficoll, respectively, and (C) humoral immune response to T-cell–dependent antigen, NP-CG. NP-specific antibodies were measured 7 days after immunization for the T-cell–independent immune responses and sera were diluted 2000-fold. For T-cell–dependent immune response, sera were examined at days 7 and 14 for antigen-specific IgM and IgG1 antibodies in the primary response. For secondary immune response, mice were rechallenged 30 days later with the same antigen and examined 5 days thereafter for NP-specific IgG1 antibodies. Sera were diluted as indicated. In all graphs, each data point denoted a single mouse analyzed. Statistical significance was determined by paired 2-tailed Student t test. Horizontal bars indicate the mean responses.
To further assess the role of Dok-3 in B-cell function, we immunized wild-type and Dok-3−/− mice with T-cell–independent type I and II antigens such as NP-LPS and NP-Ficoll, which elicited antibody responses of the IgM and IgM and IgG3 classes, respectively. As seen in Figure 3B, Dok-3−/− mice secreted significantly greater amounts of antigen-specific IgM antibodies 7 days after NP-LPS challenge compared with wild-type animals, suggesting that the mutant mice had enhanced T-cell–independent type 1 immune responses. Similarly, the mutant mice also secreted significantly higher levels of antigen-specific IgM and IgG3 antibodies 7 days after immunization with NP-Ficoll (Figure 3B), again demonstrating a heightened immune response to T-cell–independent type II antigens. Thus, the lack of Dok-3 leads to enhanced T-cell–independent immune responses in B cells.
Given that Dok-3 deficiency leads to exaggerated B cell responses to T-cell–independent antigens, we were interested to determine if mutant B cell responses to T-cell–dependent antigens would be similarly affected. To test this, wild-type and mutant mice were immunized with NP conjugated to chicken globulin (NP17-CG) and assayed for the secretion of NP-specific IgM and IgG1 antibodies. As shown in Figure 3C, both wild-type and mutant mice mounted comparable anti-NP IgM and IgG1 antibody responses 7 and 14 days after the primary challenge, respectively. There was also no significant difference in the titer of antigen-specific IgG1 antibodies between wild-type and mutant mice during the secondary immune response to NP. In addition, B cell follicles and germinal centers found in wild-type and Dok-3−/− mice at the height of the secondary T-cell–dependent immune response to NP-CG were comparable in terms of numbers, size and structure (data not shown). Thus, it appeared that the absence of dok-3 did not affect T-cell–dependent humoral immune responses.
Dok-3–deficient B cells are hyperresponsive to BCR stimulation
In vivo analyses of basal serum Ig levels and T-cell–independent immune responses in Dok-3−/− mice suggested that B cells lacking Dok-3 could be hyperresponsive. To determine if this was indeed the case and if the phenomenon was B-cell intrinsic, we directly stimulated wild-type and Dok-3−/− B cells through their BCR and examined their responses in vitro.
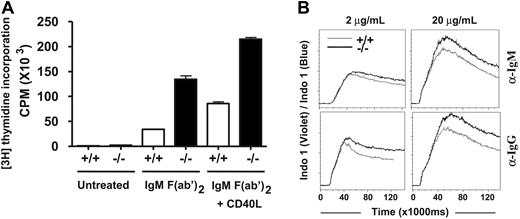
B cells can be activated by anti-IgM F(ab′)2 fragments that engage the BCR. As shown in Figure 4A, wild-type B cells were activated and induced to proliferate upon cross-linking of their BCR by anti-IgM (Fab')2 fragments and the extent of their cellular proliferation was further enhanced by costimulation through CD40. Likewise, Dok-3−/− B cells were also activated upon stimulation of their BCR by anti-IgM F(ab′)2 fragments or stimulation through both BCR and CD40, but under both circumstances they exhibited multiple-fold increase in proliferation compared with wild-type B cells. Furthermore, flow cytometry analysis indicated that there was no difference in the survival of Dok-3−/− B cells compared with wild-type B cells in vitro, suggesting that the measured difference in cell proliferation was not due to the mutant cells being more resistant to apoptosis (data not shown). Thus, Dok-3−/− B cells are hyper-proliferative in response to BCR stimulation.
Dok-3−/− B cells are hyperresponsive to BCR stimulation in vitro. (A) Purified wild-type (white column) and Dok-3−/− (black column) splenic B cells were not treated or treated with anti-IgM F(ab′)2 alone or with anti-IgM F(ab′)2 and CD40 ligand and the proliferation of cells was measured via incorporation of thymidine. Statistical significance was determined by paired 2-tailed Student t test. Figure shown is representative of more than 3 independent experiments. (B) Enhanced calcium signaling in Dok-3−/− B cells. Purified wild-type (gray line) and Dok-3−/− (black line) B cells were stimulated with either 2 or 20 μg/mL of anti-IgM or anti-IgG antibodies and the elicited intracellular calcium flux was monitored in real time via flow cytometry. Figure shown is representative of 2 independent experiments.
Dok-3−/− B cells are hyperresponsive to BCR stimulation in vitro. (A) Purified wild-type (white column) and Dok-3−/− (black column) splenic B cells were not treated or treated with anti-IgM F(ab′)2 alone or with anti-IgM F(ab′)2 and CD40 ligand and the proliferation of cells was measured via incorporation of thymidine. Statistical significance was determined by paired 2-tailed Student t test. Figure shown is representative of more than 3 independent experiments. (B) Enhanced calcium signaling in Dok-3−/− B cells. Purified wild-type (gray line) and Dok-3−/− (black line) B cells were stimulated with either 2 or 20 μg/mL of anti-IgM or anti-IgG antibodies and the elicited intracellular calcium flux was monitored in real time via flow cytometry. Figure shown is representative of 2 independent experiments.
We next examined intracellular calcium signaling triggered by BCR cross-linking of Dok-3−/− B cells. As shown in Figure 4B, treatment with either a low (2 μg/mL) or high (20 μg/mL) dose of anti-IgM or anti-IgG F(ab′)2 fragments triggered intracellular calcium signaling in wild-type B cells and the magnitude of the calcium flux corresponded with the dosage of the agonists given. Interestingly, at both low and high dose of stimulations, Dok-3−/− B cells showed a much higher level of calcium signaling compared with wild-type control, suggesting that a Dok-3-deficiency directly impacted upon the induction of calcium fluxes. Taken together, the data suggested that Dok-3 might play a role in inhibiting BCR signaling as its absence led to a greater induction of B cell proliferation and intracellular calcium signaling.
Enhancement of NFκB, JNK, and p38 MAPK activation in BCR-stimulated Dok-3−/− B cells
The data in the previous section indicated that Dok-3 directly participated in BCR signaling by inhibiting its activation. It is well known that BCR engagement activates multiple downstream signaling pathways, including those leading to NFκB and MAPK activation.3 We were thus interested to elucidate the BCR signaling pathways affected by a Dok-3-deficiency that could result in a hyperresponsive B cell phenotype.
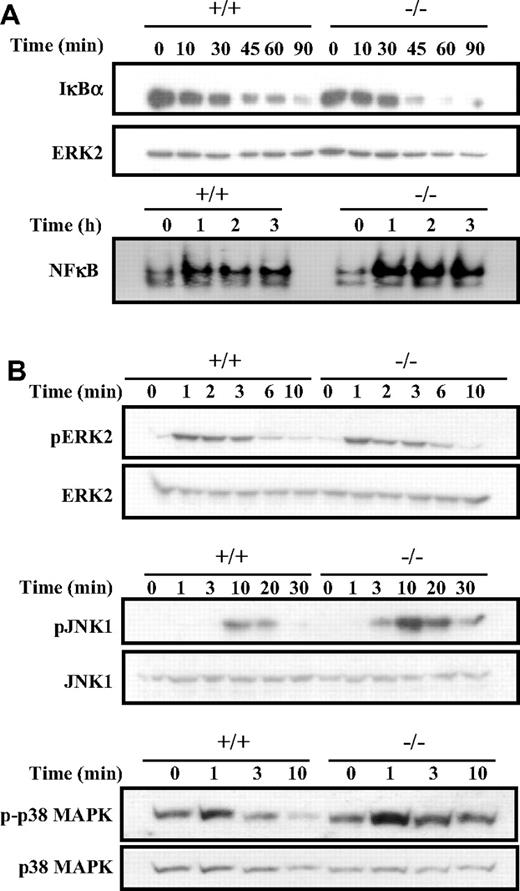
We first examined the NFκB signaling pathway that is activated by BCR engagement and known to contribute to cell proliferation. Cross-linking of the BCR leads to the phosphorylation and degradation of the IκB subunits, and this allows the nuclear localization and DNA-binding of the various NFκB homodimers and heterodimers.29 Since Dok-3−/− B cells show a higher level of cellular proliferation, one would therefore expect this pathway to be affected by a Dok-3 mutation. Indeed, as shown in Figure 5A and compared with wild-type cells, Dok-3−/− B cells showed enhanced IκBα degradation in response to BCR stimulation. Significant degradation of the IκBα subunits was already apparent at the 45-minute time point in the mutant cells, compared with wild-type cells. As a consequence, there was also greater induction of NFκB DNA-binding activities in Dok-3−/− B cells compared with wild-type B cells upon BCR engagement. The greater induction of NFκB activities in Dok-3−/− B cells in response to BCR stimulation correlates with the higher proliferation capacity of these mutant B cells and is again consistent with an inhibitory role for Dok-3 in BCR signaling.
Enhanced NFκB, JNK and p38 MAPK activation in BCR-activated Dok-3−/− B cells. (A) Enhanced NFκB activation in BCR-stimulated Dok-3−/− B cells. Purified splenic B cells were stimulated via their BCR and the activation of NFκB was assessed via degradation of IκBα in Western blot analysis (upper panel) and induction of DNA-binding activity in gel-shift assay (lower panel). The anti-ERK2 blot was included as control for equal loading of whole cell lysates in the Western blot analysis. Equal amount of nuclear extracts as determined by BCA assay was used in the EMSA (electrophoretic mobility shift assay). Data shown is representative of 3 experiments. (B) Enhanced activation of JNK and p38 MAPK signaling in BCR-stimulated Dok-3−/− B cells. Purified splenic B cells were stimulated via their BCR and examined for various MAPK activation using specific antibodies that recognized the phosphorylated forms of the various MAPKs. Anti-ERK2, anti-JNK1, and anti-p38 blots were included as controls for equal loading of cell lysates. Figure shown is representative of at least 3 independent experiments.
Enhanced NFκB, JNK and p38 MAPK activation in BCR-activated Dok-3−/− B cells. (A) Enhanced NFκB activation in BCR-stimulated Dok-3−/− B cells. Purified splenic B cells were stimulated via their BCR and the activation of NFκB was assessed via degradation of IκBα in Western blot analysis (upper panel) and induction of DNA-binding activity in gel-shift assay (lower panel). The anti-ERK2 blot was included as control for equal loading of whole cell lysates in the Western blot analysis. Equal amount of nuclear extracts as determined by BCA assay was used in the EMSA (electrophoretic mobility shift assay). Data shown is representative of 3 experiments. (B) Enhanced activation of JNK and p38 MAPK signaling in BCR-stimulated Dok-3−/− B cells. Purified splenic B cells were stimulated via their BCR and examined for various MAPK activation using specific antibodies that recognized the phosphorylated forms of the various MAPKs. Anti-ERK2, anti-JNK1, and anti-p38 blots were included as controls for equal loading of cell lysates. Figure shown is representative of at least 3 independent experiments.
Next, we examined the activation of the various MAPK in Dok-3−/− B cells in response to BCR stimulation. BCR signaling is known to trigger all 3 classes of MAPK. As shown in Figure 5B, Dok-3−/− B cells exhibited greater activation of JNK1, as revealed by its phosphorylation status, compared with wild-type B cells upon BCR cross-linking. In addition, the mutant B cells also showed greater and more sustained activation of p38 MAPK. Interestingly, in contrast to JNK and p38 MAPK activation, the phosphorylation and activation of ERK was not much different between that of wild-type and Dok-3−/− B cells. Nevertheless, taken together, the biochemical analyses suggested that the absence of Dok-3 could lead to greater induction of NFκB, JNK, and p38 MAPK signaling in antigen-receptor–activated B cells.
Dok-3-deficiency impairs SHIP-1 activation but not SHIP-1 localization
Our biochemical data had shown that Dok-3 negatively regulates BCR signaling by influencing calcium signaling and NFκB and MAPK activation (Figures 4 and 5), and this could result in the hyperactive phenotype of Dok-3−/− B cells. We were therefore interested to examine the mechanism by which Dok-3 exerted its inhibitory effect.
B lymphocytes possess both Dok-1 and Dok-3. It is plausible that deficiency in Dok-3 could compromise the expression and function of Dok-1. We thus checked for the presence and function of Dok-1 in Dok-3–deficient B cells. As shown in Figure 1C, the expression of Dok-1 was normal in Dok-3−/− cells. Furthermore, Dok-1 could be phosphorylated and localized to the plasma membrane in BCR-stimulated Dok-3−/− B cells (data not shown), suggesting that the absence of Dok-3 did not compromise the expression, plasma membrane localization or activation of Dok-1 in Dok-3−/− B cells. Hence, Dok-3 is nonredundant to Dok-1 in its negative regulation of B cell activation.
Dok-3 has been shown to bind 3 other signaling molecules, namely c-abl,16 Csk, and SHIP-117 ; of these, Csk and SHIP-1 are known to negatively regulate BCR signaling. Previous work by Robson et al28 further narrowed the inhibitory role of Dok-3 to its interaction with SHIP-1. We thus focused on the effect of a lack of Dok-3 on SHIP-1 activation. Upon BCR cross-linking, SHIP-1 is recruited to the plasma membrane and is activated to dephosphorylate downstream targets and thereby attenuate B-cell activation.31 However, it is currently not known whether SHIP-1 first localizes to the plasma membrane and subsequently gets activated or is first activated and subsequently localizes to the plasma membrane of B cells.28
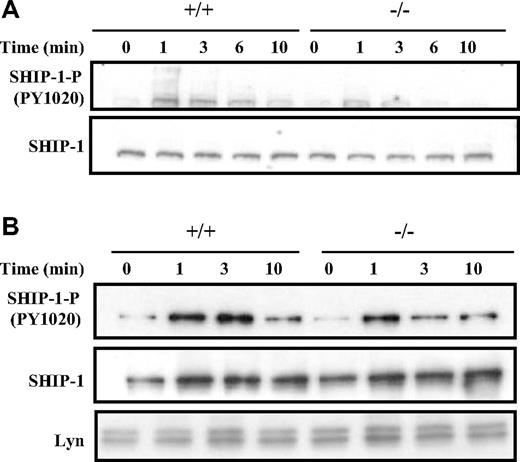
As shown in Figure 6A, Western blot analysis of whole-cell lysates suggested that the activation of SHIP-1 following BCR cross-linking, as indicated by the phosphorylation of the key tyrosine 1020 residue, was compromised in Dok-3−/− B cells compared with the wild-type control. SHIP-1 was phosphorylated at the 1-minute time point in both wild-type and Dok-3−/− B cells, but the level of SHIP-1 phosphorylation was not sustained in the mutant cells at the 3-minute time point. This suggested that the absence of Dok-3 impaired SHIP-1 activation.
Dok-3 deficiency leads to less sustained SHIP-1 activation after BCR stimulation. (A) Western blot analysis of whole-cell lysates obtained from purified BCR-stimulated wild-type and Dok-3−/− B cells using anti-SHIP-1 and antiphospho-SHIP-1 antibodies. Data shown are representative of 5 independent experiments. (B) Analyses of SHIP-1 recruitment to plasma membrane after BCR stimulation in Dok-3−/− B cells. Plasma membrane fractions were obtained from wild-type and mutant B cells at various time points after BCR engagement and probed for the presence of phospho-SHIP-1 and SHIP-1 proteins. The anti-Lyn blot was included as control for equal loading of plasma membrane fractions. Data shown are representative of 3 independent experiments.
Dok-3 deficiency leads to less sustained SHIP-1 activation after BCR stimulation. (A) Western blot analysis of whole-cell lysates obtained from purified BCR-stimulated wild-type and Dok-3−/− B cells using anti-SHIP-1 and antiphospho-SHIP-1 antibodies. Data shown are representative of 5 independent experiments. (B) Analyses of SHIP-1 recruitment to plasma membrane after BCR stimulation in Dok-3−/− B cells. Plasma membrane fractions were obtained from wild-type and mutant B cells at various time points after BCR engagement and probed for the presence of phospho-SHIP-1 and SHIP-1 proteins. The anti-Lyn blot was included as control for equal loading of plasma membrane fractions. Data shown are representative of 3 independent experiments.
The impairment in SHIP-1 activation in Dok-3−/− B cells could be due either to impairment in its phosphorylation per se or to the localization of SHIP-1 to the plasma membrane upon B-cell activation. To distinguish these possibilities, we directly isolated the plasma membrane fraction of BCR-stimulated wild-type and Dok-3−/− B cells. As seen in Figure 6B, the amount of SHIP-1 recruited to the plasma membranes of wild-type and Dok-3−/− B cells after BCR cross-linking was equivalent, suggesting that SHIP-1 had no problem localizing to the plasma membrane in the absence of Dok-3. However, similar to the finding in whole cell lysates, the phosphorylation and, hence, activation of membrane-bound SHIP-1 in Dok-3−/− B cells was compromised. Again, the level of initial SHIP-1 phosphorylation was equivalent in both BCR-stimulated wild-type and Dok-3−/− B cells at the 1-minute time point, but thereafter, the phosphorylation level of SHIP-1 was not sustained in the mutant cells. Thus, it appeared that Dok-3 does not recruit SHIP-1 to the plasma membrane but rather, facilitates and sustains the phosphorylation and activation of SHIP-1.
Discussion
We have generated Dok-3−/− mice to study the function of Dok-3 in B cells. Our analyses indicate that Dok-3 plays a role in the negative regulation of BCR signaling as mutant mice had higher level of basal IgM antibodies in their sera and exhibited enhanced responses to both T-cell–independent type I and II antigens. Consistent with these phenotypes, Dok-3-deficient B cells also hyperproliferated and showed higher levels of calcium flux and enhanced NFκB, JNK, and p38 MAPK activation upon BCR stimulation. Moreover, in the absence of Dok-3, the membrane localization of the inhibitory SHIP-1 was intact while its activation was compromised upon BCR engagement. These data are consistent with the postulated role of Dok-3 as an inhibitory signaling molecule that possibly acts through SHIP-1 to attenuate BCR signaling.
B cells possess both Dok-1 and Dok-3. The genetic disruption of Dok-3 in our current study did not affect Dok-1 expression or its localization to the plasma membrane and activation upon BCR cross-linking (data not shown). Thus, the phenotype of the Dok-3−/− mice documented in this report was not due to loss of Dok-1 expression or function or concurrent defects in Dok-1 and Dok-3. More importantly, the analyses of Dok-1−/−12 and Dok-3−/− (this report) mice indicate that the 2 molecules are not redundant with respect to each other in their negative regulation of BCR signaling as both single mutant mice exhibited a phenotype. Close comparison of the 2 types of mutant mice further suggests that Dok-1 and Dok-3 may have distinct functions in B cells. Dok-3 appears to play a more direct role in inhibiting BCR signaling, while Dok-1 appears to negatively regulate BCR signaling via FcγRIIB. This is demonstrated by their differential proliferative responses to stimulation by anti-IgM F(ab′)2 fragments. While Dok-1−/− B cells proliferated to the same extent as wild-type B cells when stimulated with anti-IgM F(ab′)2 fragments, Dok-3−/− B cells exhibited an increase in cellular proliferation when stimulated similarly (Figure 4A). This indicates that Dok-3 but not Dok-1 could play a direct inhibitory role in BCR signaling. Dok-1−/− and Dok-3−/− mice also differ in their humoral immune responses to antigens. Dok-1−/− mice have normal T-cell–independent and T-cell–dependent immune responses to antigens.12 On the other hand, Dok-3−/− mice have enhanced T-cell–independent humoral immune responses to both T-cell–independent type I and II antigens but normal IgM responses to T-cell–dependent antigens. Hence, the data seemed to suggest that Dok-3 might play an important role in the direct negative regulation of BCR activation.
The mechanism of negative regulation of BCR signaling by Dok-3 remains to be determined. Dok-3 is an adaptor protein and is not likely to directly inhibit BCR activation, but could do so via interaction with other signaling molecules with enzymatic activities. So far, Dok-3 has been shown to bind c-abl, Csk, and SHIP-1,16,17 and various reports have indicated that SHIP-1 could be the main interaction partner for Dok-3. Consistent with this notion, we demonstrated that the sustained activation of SHIP-1, as reflected by its phosphorylation status, was compromised in Dok-3−/− B cells following BCR cross-linking. However, the localization of SHIP-1 to the plasma membrane following BCR stimulation remains intact in Dok-3−/− B cells. Taken together, the data indicate that Dok-3 is upstream of SHIP-1 in the signaling cascade, that SHIP-1 could independently localize to the plasma membrane upon B-cell activation, and that Dok-3 probably facilitates the sustained activation of SHIP-1 but not its localization to the plasma membrane. It will thus be interesting to determine the identities of other interaction partners for Dok-3, some of which could be kinases involved in phosphorylating and activating SHIP-1.
We note that the phenotype of the Dok-3 mutant mice (described in this report) was quite identical to that of the SHIP-1−/− mice.30,31 That the activation of SHIP-1 was compromised in Dok-3−/− mice supports the notion that the 2 molecules act in a common signaling axis. However, we also note that the phenotype of the Dok-3 mutant mice was less severe compared with that of the SHIP-1−/− mice. The finding that the activation of SHIP-1 was compromised but not completely impaired in Dok-3−/− B cells is also consistent with the notion that SHIP-1 mutations would be more dominant than those of Dok-3. Perhaps the sustained activation of SHIP-1 is dependent on Dok-3 and another signaling molecule. One possibility would, of course, be Dok-1. It will be interesting to determine if combined deficiencies in Dok-1 and 3 mirror more closely the deficiency in SHIP-1. In addition, it will also be important to assess the combined deficiencies of these 2 adaptors in B cell development and activation.
Last but not least, given that Dok-3 mutant B cells are hyperactive in their responses to stimulant, it remains possible that Dok-3 mutant mice could succumb to autoimmunity. Preliminary testing for anti-DNA reactivity using sera from 11-month-old Dok-3−/− mice has so far proven negative (data not shown). Tests are currently underway to determine if older Dok-3 mutant mice would contain autoantibodies in their sera and if the lack of Dok-3 in conjunction with other mutations could tip the signaling threshold toward autoimmunity.
An Inside Blood analysis of this article appears at the front of this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of the LAM laboratory for insightful discussions.
This work was supported by the Biomedical Research Council of the Agency for Science, Technology and Research, Singapore.
Authorship
Contribution: C.-H. N. and S. X. performed the experiments and K.-P. L. conceptualized the project and wrote the paper.
Conflict-of-interest disclosure: The authors declare no conflicting financial interests.
Correspondence: Kong-Peng Lam, Laboratory of Molecular and Cellular Immunology, Biomedical Sciences Institutes, Agency for Science, Technology and Research (A*STAR) & Singapore Immunology Network, 61 Biopolis Drive, Lab 6–15, Proteos, Singapore 138673, Singapore; e-mail: mcblamkp@imcb.a-star.edu.sg.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal