Although receptor activator of nuclear factor (NF)–κB ligand (RANKL) signaling has been shown to prolong the survival of mature dendritic cells (DCs), the association of RANKL pathway with Fas-mediated apoptosis is obscure. Here, we found that bone marrow–derived DCs (BMDCs) from the Fas-deficient strain MRL/lpr mice, could survive much longer than normal DCs. The expressions of Bcl-x and Bcl-2 and the nuclear transport of NF-κB of RANKL-stimulated BMDCs from MRL/lpr mice were significantly up-regulated. By contrast, Fas expression of BMDCs from normal C57BL/6 and MRL+/+ mice was increased by RANKL stimulation, and an enhanced DC apoptosis was found when stimulated with both RANKL and anti-Fas mAb, which was associated with activation of caspase-3 and caspase-9. Furthermore, the expression of FLIPL, an inhibitory molecule against Fas-mediated apoptosis, in normal DCs was significantly decreased by RANKL and anti-Fas mAb. Indeed, the adoptive transfer of RANKL-stimulated DCs resulted in rapid acceleration of autoimmunity in MRL/lpr recipients. These findings indicate that the crosstalk between RANKL and Fas signaling in DCs might control immune tolerance.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells that reside in peripheral tissues in an immature state for optimal antigen uptake and respond to inflammatory signals.1,2 After capture of pathogen-derived antigens and activation by pathogens, DCs migrate from tissues to the lymphoid organs where DCs become mature and initiate antigen-specific T cells.1,2 While many subpopulations of DCs have been reported to be heterogeneous in their phenotype and localized in lymphoid tissues, there is general agreement that DCs originate from a hematopoietic progenitor, and that there are 3 DC subtypes, including myeloid DCs (CD11c+CD11b+CD8α−), lymphoid DCs (CD11c+CD11b−CD8α+), and plasmacytoid DCs (CD11c+B220+Gr-1+), all of which have been recently identified.3,,–6
The lifespan of activated antigen-bearing DCs has been estimated to be as short as 3 days.7,8 Limiting the lifespan of DCs by means of apoptosis may serve to regulate the availability of antigen for T cells and to control immune responses. An important and well-characterized mechanism for apoptosis in immune cells is mediated by Fas, the death receptor.9 Although Fas has been implicated in the apoptosis of DCs,10,11 DCs are known to be resistant to Fas-induced cell death.12 In addition, it has been reported that the resistance to Fas-induced apoptosis in DCs correlates with the consititutive expression of the Fas-associated death domain–like IL-1β–converting enzyme (FLICE)–inhibitory protein (FLIP) ligand.13 However, the molecular mechanism for maintenance of DCs through Fas-mediated signals is still unclear.
Receptor activator of nuclear factor (NF)–κB ligand (RANKL),14,15 a type II membrane protein of the tumor necrosis factor (TNF) family, is expressed on osteoblasts, stromal cells, and activated T cells,15 and binds to the signaling receptor RANK and the decoy receptor osteoprotegerin (OPG).15,16 Mice lacking RANKL or RANK display dramatically reduced osteoclastogenesis, show defects in early differentiation of T and B cells, systemic lymph nodes, and fail to develop mammary glands.17,18 Moreover, recent data have showed that the interaction of Fas ligand and Fas expressed on osteoclast precursors increases RANKL-induced osteoclastogenesis.19 On the other hand, RANKL-RANK interaction has been shown to prolong the survival of mature DCs, whereas the association of the RANKL pathway with Fas-mediated signals in the activation or function of DCs has been obscure.15,20
In this study, to define the molecular mechanism for the autoimmunity by immune dysregulation of DCs through Fas and the RANKL pathway, the MRL/lpr mouse strain, an autoimmune-prone strain that has a mutated Fas gene, was used to analyze the immune functions of the DCs and autoimmunity such as rheumatoid lesions.
Materials and methods
Mice
MRL/Mp-lpr/lpr (MRL/lpr; aged 4-12 weeks; n = 105), MRL+/+ mice (aged 4-12 weeks; n = 55), and C57BL/6 (B6; n = 50) mice were purchased from Charles River Japan Inc. (Atsugi, Japan). All mice were maintained in specific pathogen–free conditions in our animal facility, and the experiments were approved by an animal ethics board of Tokushima University (Tokushima, Japan).
Generation of murine BMDCs
A crude population of DCs was generated in vitro from mouse bone marrow as described with some modifications.21,22 Briefly, bone marrow was flushed from the long bones of the limbs. Cells were plated at a density of 1 × 106 and were cultured for 7 days in 5% CO2 at 37°C in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, 10 ng/mL recombinant mouse granulocyte-monocyte colony-stimulating factor (GM-CSF; PeproTech, Le Perray-en-Yvelines, France), and 5 ng/mL mouse IL-4 (PeproTech). DCs (CD11c+ cells, < 70%) including mainly immature DCs (50% CD11c+CD86− cells, < 50%) were routinely used for the experiments.
Flow cytometric analysis
Bone marrow–derived DCs (BMDCs) were stained with fluorescein isothiocyanate (FITC)–conjugated anti-CD11c mAb, along with rat anti– major histocompatibility complex class II (MHC class II; I-Ak) as primary Ab and phycoerythin (PE)–conjugated anti-rat IgG as secondary Ab. BMDCs were also stained with PE–anti-CD86, PE–anti-CD80, or anti–Bcl-2, anti–Bcl-xL, and PE–anti-rat IgG. For each staining, isotype-matched mAb was used as a control. All mAbs were obtained from BD Biosciences (San Diego, CA). The cells were analyzed with an EPICS flow cytometer (Beckman Coulter Inc, Miami, FL), and data were analyzed with FlowJo FACS analysis software (Tree Star Inc, Ashland, OR).
Detection of apoptotic cells
Cells were treated with or without 100 ng/mL Fas-activating antibody (Jo-2; BD Biosciences) for 24 to 72 hours and were detected with an EPICS flow cytometer using an Annexin V–FITC apoptosis detection kit (Genzyme, Cambridge, MA). Briefly, after cultured cells were washed in phosphate-buffered saline (PBS), the cells were incubated with FITC-conjugated Annexin-V and propidium iodide (PI) for 10 minutes at room temperature in the dark. Binding buffer was added, and apoptotic cells were detected by flow cytometric analysis with an EPICS flow cytometer.
Measurement of cytokine production
Cytokine production was tested by a 2-step sandwich enzyme-linked immunosorbent assay (ELISA) using a mouse IL-12p40, IL-10, and interferon (IFN)-γ, IL-2, and IL-4 kit (Genzyme, Cambridge, MA). In brief, the culture supernatants from DCs or T cells were added to microtiter plates precoated with anti-IL-12p40, IL-10, IFN-γ, IL-2, and IL-4 capture Ab and incubated overnight at 4°C. After adding biotinylated detecting Ab and incubating at room temperature for 45 minutes, streptavidin-peroxidase was added and incubated again at room temperature for an additional 30 minutes. Finally, an 2,2′-azino-di-3-ethylbenzthiazoline sulfonate substrate containing H2O2 was added, and the colorimetric reaction was read at an absorbance of 450 nm using an automatic microplate reader (Bio-Rad Laboratories Inc, Hercules, CA). The concentration was calculated according to the standard curves produced by various concentrations of recombinant cytokines.
MTT assay
The effect of RANKL on proliferation of BMDCs was determined by the MTT dye uptake method. Briefly, the cells (2000/well) were incubated in triplicate in a 96-well plate in the presence or absence of RANKL in a final volume of 0.1 mL for the indicated time periods at 37°C. Then, 0.025 mL MTT solution (5 μg/mL in PBS) was added to each well. After incubation at 37°C for 2 hours, 0.1 mL of the extraction buffer (20% sodium dodecyl sulfide [SDS], 50% dimethylformamide) was added. Incubation was continued overnight at 37°C, and then the optical density (OD) was measured at 450 nm using an automatic microplate reader (Flow, McLean, VA), with the extraction buffer as blank.
Western blot analysis
The cell extracts from the nucleus and cytoplasm of DCs were prepared using the Nuclear/Cytosol Fractionation Kit (Bio Vision, Mountain View, CA). Cells were briefly washed, collected in ice-cold PBS in the presence of phosphatase inhibitors, and centrifuged at 230g for 5 minutes. The pellets were resuspended in a hypotonic buffer, treated with detergent, and centrifuged at 14 000g for 30 seconds. After collection of the cytoplasmic fraction, the nuclei were lysed and nuclear proteins were solubilized in lysis buffer containing protease inhibitors. A total of 50 μg of each sample per well was used for SDS–polyacrylamide gel electrophoresis. After blocking with 5% nonfat milk, the membrane was incubated with primary antibodies for TRAF6, IKK, p-IκBa, NF-κB p65 (RelA), and p50 (Santa Cruz Biotechnology, Santa Cruz, CA). Antibody-antigen complexes were detected using a horseradish peroxidase–conjugated secondary antibody. Protein binding was visualized with enhanced chemiluminescence Western blotting reagent (Amersham Biosciences, Arlington Heights, IL). Western blotting using anti–Bcl-2, anti–Bcl-xL, anti-Bax, anti-Bid (BD Bioscience), anti–caspase-3, anti–caspase-8, and anti–caspase-9 Abs (Cell Signaling Technology, Beverly, MA), and anti-FLIPL (Santa Cruz Biotechnology) was performed essentially as described for the lysis buffer. Control for protein loading was provided by anti-mouse histone, or GAPDH mAb (Sigma Chemical Co, St Louis, MO).
siRNA of Fas and RANK
For small interfering RNA (siRNA) of Fas and RANK, a siTrio Full Set (B-Bridge International, Sunnyvale, CA) was used for analyzing the function of DCs. Briefly, each cocktail including the 3 RNA oligonucleotides listed here was transfected into cells with a Silencer siRNA Transfection II kit (Ambion, Austin, TX). Sequences of the oligonucleotide sets are as follows: Fas, GGGAAGGAGUACAUGGACATT (sense), UGUCCAUGUACUCCUUCCCTT (antisense), CGAAAGUACCGGAAAAGAATT (sense), UUCUUUUCCGGUACUUUCGTT (antisense), CCAGAAGGACCUUGGAAAATT (sense), UUUUCCAAGGUCCUUCUGGTT (antisense); and RANK, CCAAGGAGGCCCAGGCUUATT (sense), UAAGCCUGGGCCUCCUUGGTT (antisense), GGGAAAGCGCUGACAGCUATT (sense), UAGCUGUCAGCGCUUUCCCTT (antisense), CUGAAAAGCACCUGACAAATT (sense), UUUGUCAGGUGCUUUUCAGTT. Transfected cells were incubated with or without RANKL, and flow cytometric analysis was performed.
Confocal microscopic analysis
Immature DCs were seeded onto glass-bottom culture dishes (MatTek, Ashland, MA) at a density of 500 cells/well and stimulated for 30, 60, or 120 minutes with RANKL. Subsequently, cells were washed and fixed in cold 3% paraformaldehyde (PFA) in PBS for 10 minutes, then permeabilized with 0.2% Triton-X in PBS for 2 minutes. The slides were then washed thoroughly with PBS and incubated with optimal dilutions of the primary Ab in PBS containing 1% bovine serum albumin (BSA)–2.5% FBS in PBS for 1 hour at room temperature. Cells were stained with optimal dilutions of the primary Abs for 1 hour. After 3 washes with 0.0001% Triton-X in PBS, the cells were stained with Alexa Fluor 488 goat anti-rabbit IgG (H + L; Molecular Probes, Eugene, OR) or Texas red–conjugated goat anti-mouse (Molecular Probes) as the second Abs for 30 minutes and washed with PBS. For each fluorochrome label, negative control Abs were added. Finally, the slides were incubated with 4′, 6-diamidino-2-phenylindole (DAPI) for 5 minutes to label the nuclei and mounted in Vectashield (Vector Labs, Burlingame, CA) for analysis by confocal microscopy (LSM5 PASCAL, Carl Zeiss, Gottingen, Germany). A 63 × 1.4 oil DIC objective lens was used. Quick Operation Version 3.2 (Carl Zeiss) for imaging acquisition and Adobe Photoshop CS2 (Adobe Systems, San Jose, CA) for image processing were used.
Transfer of DCs
BMDCs were stimulated in vitro for 48 hours with 100 ng/mL RANKL and 50 μg/mL bovine type II collagen (CII). RANKL-stimulated BMDCs were transferred into the base of the tail by subcutaneous injections in 200 μL PBS at the age of 4 weeks.
Histopathology
All organs were removed from the mice, fixed with 4% phosphate-buffered formaldehyde (pH 7.2), and prepared for histologic examination. The sections (4 μm in thickness) were stained with hematoxylin and eosin (HE). Histologic grading of inflammatory arthritis was performed according to the methods by Edwards et al23 as follows: a 1-point score indicates hyperplasia/hypertrophy of synovial cells, fibrosis/fibroplasia, proliferation of cartilage and bone, destruction of cartilage and bone, and mononuclear cell infiltrate. Imaging was analyzed by microscope (BX50, Olympus, Tokyo, Japan) at 20 × 10.70 objective lens. Viewfinder Lite Version 1.0 (Olympus) for image acquisition and Adobe Photoshop CS2 for image processing were used.
Measurement of anti-dsDNA Ab, RF, and CII Ab levels
Anti–double-stranded DNA (dsDNA) Abs, rheumatoid factor (RF), and anti-CII Ab were detected by ELISA as described previously.11 Briefly, flat-bottom plates (Nalge Nunc International, Roskilde, Denmark) were coated with 1.5 μg/mL of native calf thymus DNA (Life Technologies, Rockville, MD) in buffer containing 0.1 M sodium bicarbonate and 0.05 M citric acid at 4°C overnight. Serum samples were serially diluted (starting at 1:200) and added to the plates for 1 hour of incubation at 37°C. After washing, peroxidase-conjugated goat anti-mouse IgG, or IgM (Southern Biotechnology Associates, Birmingham, AL) was added and incubated for 1 hour at 37°C. Ab binding was visualized using orthophenylenediamine (Sigma Chemical). For the measurement of IgG and IgM RF, human IgG and IgM (Chemicon International, Temecula, CA) were coated onto plates at 10 μg/mL (pH 9.6). The microtiter plate was coated with 100 μL CII antigen solution. After washing 3 times, 100 μL/well of serum samples that had been serially diluted in PBS/Tween 20/1% BSA and control serum samples were added and incubated for 1 hour at 37°C. After washing, peroxidase-conjugated goat anti-mouse IgG (at 1.4 μg/mL, 100 μL/well; Organon Teknika, Durham, NC) was added and incubated for 1 hour at 37°C. A total of 100 μL o-phenylenediamine (0.5 mg/mL) dissolved in 0.1 M citrate buffer (pH 5.0) containing 0.012% H2O2 was added, and the reaction was stopped using 8 N H2SO4 (20 μL/well).
Statistics
The Student t test was used for statistical analysis. P values greater than .05 were considered significant.
Results
DC numbers and subtypes of MRL/lpr mice
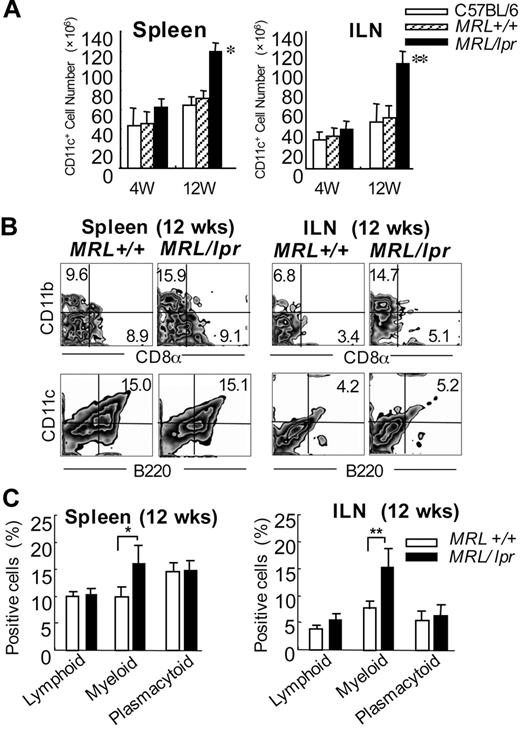
To examine the number of DCs from MRL/lpr mice, the spleen cells and inguinal lymph node (ILN) cells were analyzed at 4 and 12 weeks of age. We found that the DC number of both spleen and ILNs from MRL/lpr mice was significantly higher than that from control mice at 12 weeks of age (Figure 1A). We next analyzed the increased subset of DCs from MRL/lpr mice. A significant increase of the myeloid (CD11b+CD11c+CD8α−) DC subset of both spleen and ILNs from MRL/lpr mice was observed in contrast to the population from control MRL+/+ mice, whereas there was no difference in the lymphoid (CD11c+CD11b−CD8α+) and plasmacytoid (B220+CD11c+) DCs between MRL/lpr and control mice (Figure 1B-C). Based on these findings, we recognized the possibility that myeloid DCs from MRL/lpr mice may have any influence on the immune disorder of the mice.
Myeloid dendritic cells in the spleen and inguinal lymph nodes from MRL/lpr mice. (A) The CD11c+ DC cell number of the spleen and ILNs is shown as means ± SD of 5 to 7 MRL/lpr and control mice at 4 and 12 weeks of age.(B) Mononuclear cells were isolated from the spleen and ILNs at 12 weeks of age, and the proportions were determined using flow cytometry. Shown are representative plots of CD8α+, CD11b+, B220+, and CD11c+ cells. Percentages in each region indicate the frequency of lymphoid (CD11c+CD11b−CD8α+), myeloid (CD11c+CD11b+CD8α−), and plasmacytoid (CD11c+B220+) DCs. Data are representative of 5 to 7 mice in each group. (C) Graph shows the mean frequency of lymphoid, myeloid, and plasmacytoid DCs. *P > .05; **P > .01 MRL/lpr versus MRL+/+ mice. Results are representative of 3 independent experiments.
Myeloid dendritic cells in the spleen and inguinal lymph nodes from MRL/lpr mice. (A) The CD11c+ DC cell number of the spleen and ILNs is shown as means ± SD of 5 to 7 MRL/lpr and control mice at 4 and 12 weeks of age.(B) Mononuclear cells were isolated from the spleen and ILNs at 12 weeks of age, and the proportions were determined using flow cytometry. Shown are representative plots of CD8α+, CD11b+, B220+, and CD11c+ cells. Percentages in each region indicate the frequency of lymphoid (CD11c+CD11b−CD8α+), myeloid (CD11c+CD11b+CD8α−), and plasmacytoid (CD11c+B220+) DCs. Data are representative of 5 to 7 mice in each group. (C) Graph shows the mean frequency of lymphoid, myeloid, and plasmacytoid DCs. *P > .05; **P > .01 MRL/lpr versus MRL+/+ mice. Results are representative of 3 independent experiments.
Activation of RANKL-stimulated DCs from MRL/lpr mice
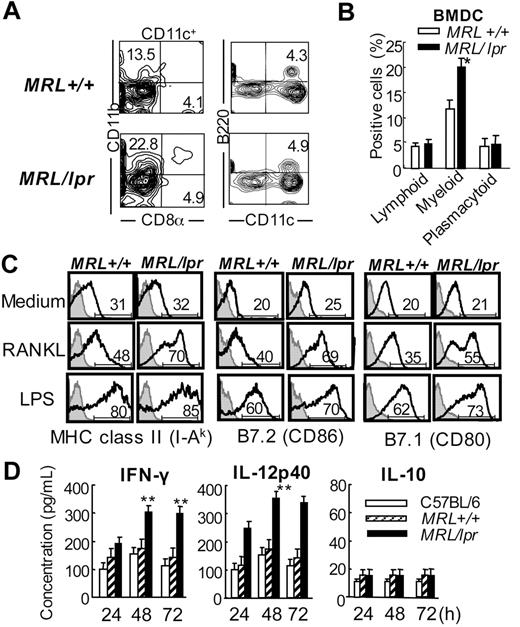
To determine the function of DCs from MRL/lpr mice, we generated BMDCs by using the culture of bone marrow cells with recombinant mouse GM-CSF and IL-4. When the cell-surface markers on BMDCs after the culture for 7 days were determined, the myeloid subset DCs from MRL/lpr mice had increased significantly compared with that from MRL+/+ mice, resembling the phenotype of the spleen and ILN from MRL/lpr mice (Figure 2A-B).
Surface phenotypes and cytokine productions in RANKL-stimulated DCs from MRL/lpr mice. (A) BMDCs were generated from MRL/lpr and MRL+/+ mice by using the culture of bone marrow cells with GM-CSF and IL-4 for 7 days; the cell-surface markers of BMDCs were then analyzed. BMDCs from MRL/lpr and MRL+/+ mice were analyzed by flow cytometry. Shown are representative plots of CD8α+, CD11b+, B220+, and CD11c+ cells. Percentages in each region indicate the frequency of lymphoid, myeloid, and plasmacytoid DCs. Results are representative of 3 independent experiments. (B) The graph shows the mean frequency of lymphoid, myeloid, and plasmacytoid DCs. Data are means ± SD of 5 to 7 mice in each group. (C) BMDCs were stimulated with 100 ng/mL mouse recombinant RANKL or 100 ng/mL LPS for 48 hours, and then the surface expressions of MHC class II and costimulatory molecules were detected by flow cytometric analysis. The result is representative of 3 independent experiments. (D) The secretions of IFN-r, IL-12p40, and IL-10 in culture supernatants from the BMDCs stimulated by RANKL were detected by ELISA. Data are means ± SD of triplicate samples and are representative of 4 independent experiments. *P > .05; **P > .01, MRL/lpr versus control mice.
Surface phenotypes and cytokine productions in RANKL-stimulated DCs from MRL/lpr mice. (A) BMDCs were generated from MRL/lpr and MRL+/+ mice by using the culture of bone marrow cells with GM-CSF and IL-4 for 7 days; the cell-surface markers of BMDCs were then analyzed. BMDCs from MRL/lpr and MRL+/+ mice were analyzed by flow cytometry. Shown are representative plots of CD8α+, CD11b+, B220+, and CD11c+ cells. Percentages in each region indicate the frequency of lymphoid, myeloid, and plasmacytoid DCs. Results are representative of 3 independent experiments. (B) The graph shows the mean frequency of lymphoid, myeloid, and plasmacytoid DCs. Data are means ± SD of 5 to 7 mice in each group. (C) BMDCs were stimulated with 100 ng/mL mouse recombinant RANKL or 100 ng/mL LPS for 48 hours, and then the surface expressions of MHC class II and costimulatory molecules were detected by flow cytometric analysis. The result is representative of 3 independent experiments. (D) The secretions of IFN-r, IL-12p40, and IL-10 in culture supernatants from the BMDCs stimulated by RANKL were detected by ELISA. Data are means ± SD of triplicate samples and are representative of 4 independent experiments. *P > .05; **P > .01, MRL/lpr versus control mice.
Next, activation of the BMDCs from MRL+/+ and MRL/lpr mice stimulated with RANKL or lipopolysaccaride (LPS) was estimated by analyzing the surface phenotypes, including MHC class II, B7.2 (CD86) and B7.1 (CD80). BMDCs were stimulated with 100 ng/mL mouse recombinant RANKL or 100 ng/mL LPS for 48 hours, and then the surface expressions were detected by flow cytometric analysis as shown in Figure 2C. The expressions of MHC class II, B7.1, and B7.2 on BMDCs from MRL/lpr mice in response to RANKL were enhanced compared with those from MRL+/+ mice, but no significant difference in the expressions on the BMDCs stimulated with LPS from between MRL+/+ and MRL/lpr mice (Figure 2C).
This finding indicates that activation of BMDCs from MRL/lpr mice via RANKL might be enhanced. In addition, to define the secretion of some cytokines such as IFN-γ, IL-12p40, and IL-10 from RANKL-stimulated BMDCs of MRL/lpr mice, BMDCs from normal B6 mice, MRL+/+, and MRL/lpr mice were stimulated with RANKL for 24 to 72 hours, and the cytokine secretion of the culture supernatants was analyzed by ELISA. Significantly increased secretions of IFN-γ and IL-12p40, not IL-10, of BMDCs from MRL/lpr mice were observed compared with those from C57BL/6 (B6) and MRL+/+ mice (Figure 2D). These data suggest that the Fas molecule may influence the activation and function of DCs through RANKL signaling.
Signaling molecules of DCs through RANKL
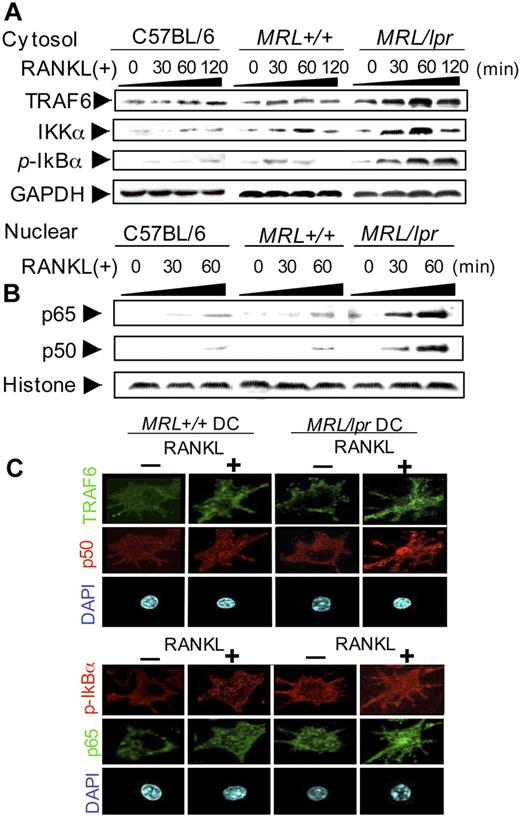
It is well known that the RANK/RANKL pathway includes a lot of signaling molecules.24,25 Among them, NF-κB plays a key role for DC maturation and activation.26 NF-κB activation through RANK/RANKL occurs by nuclear translocation following inducible phosphorylation of inhibitory IκB by IKK complex.27,28 Therefore, IKKα expression and phosphorylation of IκB (p-IκB) in RANKL-stimulated BMDCs from B6, MRL+/+, and MRL/lpr mice were analyzed by Western blotting. In addition, the expression of TRAF6 downstream from RANK in RANKL-stimulated BMDCs from MRL/lpr and control mice was determined.
Increasing expression of TRAF6 after stimulation with RANKL was detected in BMDCs from MRL+/+ mice. In MRL/lpr mice, a much higher expression of TRAF6 in the BMDCs was observed in contrast to that of the control mice. In addition, the expression of IKKα of MRL/lpr DCs was up-regulated relative to those of B6 and MRL+/+ DCs (Figure 3A). Notably, the prominent phosphorylation of IκB of MRL/lpr DCs was detected compared with that of control DCs parallel with the increased expressions of TRAF6 and IKKα (Figure 3A). Furthermore, nuclear translocation of NF-κB subunits (p65 and p50) of RANKL-stimulated MRL/lpr DCs had increased remarkably compared with that of control DCs (Figure 3B). To define the movements of RANKL-mediated signaling molecules of MRL/lpr DCs, the expression of each protein was confirmed by confocal microscopic analysis. Consistent with the results of Western blot analysis using the cytoplasmic and nuclear proteins, the increased nuclear translocation of NF-κB and the up-regulated TRAF6 and p-IκB in the cytoplasm from RANKL-stimulated MRL/lpr DCs were observed in contrast to those from control DCs (Figure 3C). These findings suggest that the Fas molecule might regulate NF-κB activation of DCs through RANKL signaling.
RANKL signaling molecules in MRL/lpr DCs. (A) The expressions of TRAF6, IKKα, and phospho-IκB (pIκB) of RANKL-stimulated BMDCs from C57BL/6, MRL+/+, and MRL/lpr mice were detected using the cytoplasmic extracts by Western blot analysis. The BMDCs were stimulated with RANKL (100 ng/mL) from 0 to 120 minutes. GAPDH was used as a housekeeping protein. (B) Nuclear translocation of NF-κB (p65 and p50) of RANKL-stimulated BMDCs from C57BL/6, MRL+/+, and MRL/lpr mice was detected by immunoblot. Histone was used as a housekeeping protein of the nuclear extracts. (C) Increased TRAF6 and p-IκB in the cytoplasm, and accelerated nuclear translocation of p50 and p65 in the nucleus of RANKL-stimulated MRL/lpr DCs were detected by confocal microscopic analysis. BMDCs from MRL/lpr and MRL+/+ mice were stimulated for 48 hours with or without RANKL, fixed in 3% PFA on a glass slide, and stained with anti-p65, anti-p50, TRAF6, and p-IκBα followed by Alexa Fluor 488–labeled (green) or Alexa Fluor 568–labeled (red) anti-mouse or anti-rabbit IgG as the second antibodies. The nuclei were stained with DAPI. Original magnification, × 630. All data are representative of 3 to 5 independent experiments.
RANKL signaling molecules in MRL/lpr DCs. (A) The expressions of TRAF6, IKKα, and phospho-IκB (pIκB) of RANKL-stimulated BMDCs from C57BL/6, MRL+/+, and MRL/lpr mice were detected using the cytoplasmic extracts by Western blot analysis. The BMDCs were stimulated with RANKL (100 ng/mL) from 0 to 120 minutes. GAPDH was used as a housekeeping protein. (B) Nuclear translocation of NF-κB (p65 and p50) of RANKL-stimulated BMDCs from C57BL/6, MRL+/+, and MRL/lpr mice was detected by immunoblot. Histone was used as a housekeeping protein of the nuclear extracts. (C) Increased TRAF6 and p-IκB in the cytoplasm, and accelerated nuclear translocation of p50 and p65 in the nucleus of RANKL-stimulated MRL/lpr DCs were detected by confocal microscopic analysis. BMDCs from MRL/lpr and MRL+/+ mice were stimulated for 48 hours with or without RANKL, fixed in 3% PFA on a glass slide, and stained with anti-p65, anti-p50, TRAF6, and p-IκBα followed by Alexa Fluor 488–labeled (green) or Alexa Fluor 568–labeled (red) anti-mouse or anti-rabbit IgG as the second antibodies. The nuclei were stained with DAPI. Original magnification, × 630. All data are representative of 3 to 5 independent experiments.
Survival signaling of MRL/lpr DCs
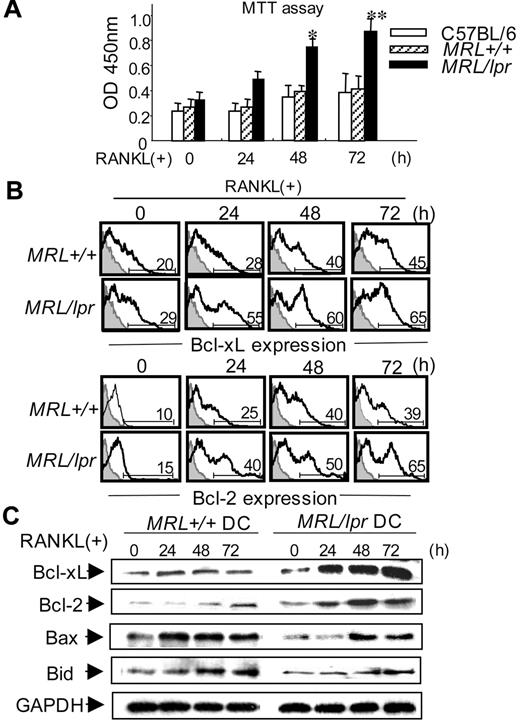
To determine the growth of RANKL-stimulated BMDCs from MRL/lpr mice, the cell growth was analyzed by MTT assay. At 48 and 72 hours after RANKL stimulation, significantly increased growth of MRL/lpr DCs was observed compared with those of B6 and MRL+/+ DCs (Figure 4A).
Antiapoptotic signaling through RANKL in MRL/lpr DCs. (A) BMDCs were stimulated with 100 ng/mL mouse recombinant RANKL from 0 to 72 hours, and the cell growth was analyzed by MTT assay. Data are means ± SD of triplicate samples and are representative of 4 independent experiments. *P > .05; **P > .01, MRL/lpr versus control mice. (B) BMDCs from MRL/lpr and control mice were stimulated with RANKL (100 ng/mL) from 0 to 72 hours, and the intracellular expressions of Bcl-xL and Bcl-2 were analyzed by flow cytometry. Numbers above horizontal lines indicate percent Bcl-xL+ or Bcl-2+ cells. Results are representative of 3 independent experiments. (C) BMDCs from MRL+/+ and MRL/lpr mice were treated with RANKL (100 ng/mL) from 0 to 72 hours. Bcl-xL, Bcl-2, Bax, and Bid proteins were detected by Western blot analysis. GAPDH was used as a control for loading. Data are representative of 3 independent experiments.
Antiapoptotic signaling through RANKL in MRL/lpr DCs. (A) BMDCs were stimulated with 100 ng/mL mouse recombinant RANKL from 0 to 72 hours, and the cell growth was analyzed by MTT assay. Data are means ± SD of triplicate samples and are representative of 4 independent experiments. *P > .05; **P > .01, MRL/lpr versus control mice. (B) BMDCs from MRL/lpr and control mice were stimulated with RANKL (100 ng/mL) from 0 to 72 hours, and the intracellular expressions of Bcl-xL and Bcl-2 were analyzed by flow cytometry. Numbers above horizontal lines indicate percent Bcl-xL+ or Bcl-2+ cells. Results are representative of 3 independent experiments. (C) BMDCs from MRL+/+ and MRL/lpr mice were treated with RANKL (100 ng/mL) from 0 to 72 hours. Bcl-xL, Bcl-2, Bax, and Bid proteins were detected by Western blot analysis. GAPDH was used as a control for loading. Data are representative of 3 independent experiments.
We next analyzed survival signaling molecules, Bcl-xL and Bcl-2, that are antiapoptotic molecules among the Bcl-2 family by flow cytometry. Enhanced expressions of both intracellular Bcl-xL and Bcl-2 in RANKL-stimulated MRL/lpr DCs were observed at 24 and 72 hours after the stimulation in contrast to that in MRL+/+ DCs (Figure 4B). Moreover, prominent amounts of both Bcl-xL and Bcl-2 in RANKL-stimulated MRL/lpr DCs were detected compared with those in MRL+/+ DCs using Western blot analysis (Figure 4C). By contrast, the expressions of Bax and Bid, apoptosis signaling molecules, in RANKL-stimulated MRL/lpr DCs were lower than those of MRL+/+ DCs (Figure 4C). These data suggest that RANKL signal leads to enhancing the survival of Fas-deficient DCs through up-regulation of antiapoptotic molecules.
RANKL signaling and Fas-mediated apoptosis of DCs
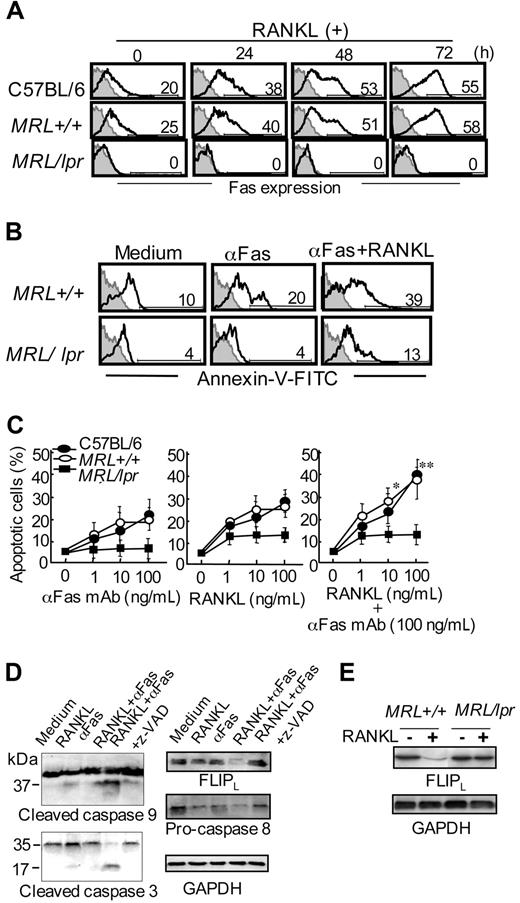
To examine the association of RANKL signaling with Fas-mediated apoptosis in DCs, Fas expression on BMDCs stimulated with RANKL was analyzed. A time-dependent increase in Fas expression on RANKL-stimulated BMDCs from normal B6 and MRL+/+ mice, but not MRL/lpr mice, was observed (Figure 5A). We found an enhanced apoptosis of DCs when stimulated with both anti-Fas mAb and RANKL, whereas the apoptosis induced by RANKL or anti-Fas mAb was slightly increased (Figure 5B-C). Moreover, to clarify the molecular pathway of enhanced Fas-mediated apoptosis of DCs by RANKL, the movement of caspases downstream from Fas to undergo apoptosis was analyzed using MRL+/+ DCs by Western blotting. Although a small amount of cleaved caspase-3 and caspase-9 of RANKL-stimulated MRL+/+ DCs was observed, we detected an increased active form of caspase-3 and caspase-9 in the MRL+/+ DCs stimulated with both anti-Fas and RANKL (Figure 5D). The activation of these caspases was clearly inhibited by the addition of caspase inhibitor (z-VAD; Figure 5D). Furthermore, the expression of FLIPL, an inhibitory molecule against Fas-mediated apoptosis,29,30 was significantly decreased by both RANKL and anti-Fas mAb parallel with the movement of the caspases (Figure 5D). It has been reported that FLIPL is constitutively expressed in DCs to play an inhibitory role for Fas-mediated apoptosis of DCs.12,13,31 In addition, the expression of pro–caspase-8 was decreased by the stimulation of RANKL or anti-Fas mAb, and the expression was even lower with stimulation by both RANKL and anti-Fas mAb (Figure 5D). For MRL/lpr DCs, the expression of FLIPL was not reduced with RANKL stimulation (Figure 5E). These results strongly suggest that RANKL signaling may contribute to Fas-mediated apoptosis of DCs by controlling the expression of FLIPL.
Fas signaling through RANKL in DCs. (A) BMDCs from normal B6, MRL+/+, and MRL/lpr mice were cultured with RANKL from 0 to 72 hours. Fas expression on the DCs was detected by flow cytometry. Numbers above horizontal lines indicate percentage of Fas+ cells. Results are representative of 3 independent experiments. (B) BMDCs from MRL+/+ and MRL/lpr mice were incubated with anti-Fas mAb, or anti-Fas mAb and RANKL, for 48 hours. Apoptotic cells were detected by flow cytometric analysis. Numbers above horizontal lines indicate percentage of Annexin-V+ cells. Results are representative of 3 independent experiments. (C) Apoptotic cells stimulated with anti-Fas mAb (1-100 ng/mL), RANKL (1-100 ng/mL), or RANKL and anti-Fas mAb (100 ng/mL) for 48 hours. B6 (●), MRL+/+ (○), and MRL/lpr (■) mice are shown. Data are means ± SD of 3 independent experiments. *P > .05; **P > .01. (D) BMDCs (5 × 104 cells) were cultured treated with RANKL (100 ng/mL) and anti-Fas mAb (100 ng/mL) in the presence or absence of inhibitor z-VAD for 48 hours. The expressions of caspase-9, caspase-3, caspase-8, and FLIPL of the stimulated BMDCs from control mice were analyzed by Western blot. GAPDH was used as a control for loading. Results are representative of 3 independent experiments. (E) FLIPL expression of RANKL-stimulated BMDCs from MRL+/+ and MRL/lpr mice was detected by immunoblot. GAPDH was used as a control for loading. Results are representative of 3 independent experiments.
Fas signaling through RANKL in DCs. (A) BMDCs from normal B6, MRL+/+, and MRL/lpr mice were cultured with RANKL from 0 to 72 hours. Fas expression on the DCs was detected by flow cytometry. Numbers above horizontal lines indicate percentage of Fas+ cells. Results are representative of 3 independent experiments. (B) BMDCs from MRL+/+ and MRL/lpr mice were incubated with anti-Fas mAb, or anti-Fas mAb and RANKL, for 48 hours. Apoptotic cells were detected by flow cytometric analysis. Numbers above horizontal lines indicate percentage of Annexin-V+ cells. Results are representative of 3 independent experiments. (C) Apoptotic cells stimulated with anti-Fas mAb (1-100 ng/mL), RANKL (1-100 ng/mL), or RANKL and anti-Fas mAb (100 ng/mL) for 48 hours. B6 (●), MRL+/+ (○), and MRL/lpr (■) mice are shown. Data are means ± SD of 3 independent experiments. *P > .05; **P > .01. (D) BMDCs (5 × 104 cells) were cultured treated with RANKL (100 ng/mL) and anti-Fas mAb (100 ng/mL) in the presence or absence of inhibitor z-VAD for 48 hours. The expressions of caspase-9, caspase-3, caspase-8, and FLIPL of the stimulated BMDCs from control mice were analyzed by Western blot. GAPDH was used as a control for loading. Results are representative of 3 independent experiments. (E) FLIPL expression of RANKL-stimulated BMDCs from MRL+/+ and MRL/lpr mice was detected by immunoblot. GAPDH was used as a control for loading. Results are representative of 3 independent experiments.
Effects of Fas siRNA on the function of DCs
To further confirm the enhanced antiapoptotic signal of RANKL-stimulated DCs by Fas deficiency in the MRL/lpr mice, we used siRNA of Fas to evaluate the apoptotic signaling and activation of normal BMDCs from control mice.
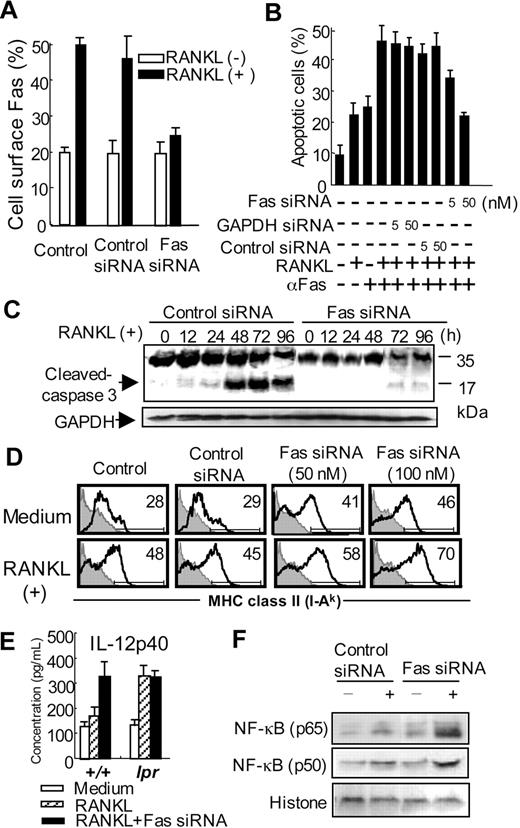
When BMDCs transfected with the siRNA of Fas were stimulated with RANKL for 48 hours, RANKL-induced Fas expression was decreased to the unstimulated level (Figure 6A). We next examined the inhibitory effect of Fas siRNA on RANKL/Fas-induced apoptosis of BMDCs. As expected, apoptosis of RANKL- and anti-Fas mAb-stimulated BMDCs was clearly inhibited by Fas siRNA, but not GAPDH siRNA or an irrelevant control (Figure 6B). Moreover, to clarify apoptosis signal of RANKL/Fas-mediated apoptosis of DCs, cleavage of caspase-3 was examined using Fas siRNA. A cleaved caspase-3 of BMDCs transfected with control siRNA was detected at 48 and 72 hours after RANKL stimulation, but no active form of caspase-3 of BMDCs transfected with Fas siRNA was observed (Figure 6C).
Effects of Fas siRNA on the functions of DCs. (A) Fas expression on Fas siRNA-transfected BMDCs from control mice was analyzed after the stimulation of RANKL. GAPDH siRNA or irrelevant oligonucleotides was used as control. Data are means ± SD of triplicate samples and are representative of 3 independent experiments. (B) Inhibitory effect of Fas siRNA on Fas and RANKL-induced apoptosis of BMDCs from control mice was observed. Apoptotic cells were detected by flow cytometric analysis. Data are means ± SD and representative of 3 independent experiments. (C) Fas siRNA-transfected BMDCs were stimulated with RANKL from 0 to 96 hours. Caspase-3 expression was analyzed by immunoblot. GAPDH was used as a control for loading. Data are representative of 3 independent experiments. (D) MHC class II expression on the Fas siRNA-transfected BMDCs stimulated with RANKL was detected by flow cytometric analysis. Numbers above horizontal lines indicate percentage of MHC class II+ cells. Data are representative of 3 independent experiments. (E) Fas siRNA-transfected BMDCs from MRL+/+ and MRL/lpr mice were stimulated with RANKL, and the secretion of IL-12 from the DCs was detected by ELISA. Data are means ± SD of triplicate samples and are representative of 3 independent experiments. (F) Nuclear translocation of NF-κB subunits (p65 and p50) was detected using the nuclear extracts of Fas siRNA-transfected BMDCs from control mice by Western blot analysis. Histone was used as a control for loading. Data are representative of 3 independent experiments.
Effects of Fas siRNA on the functions of DCs. (A) Fas expression on Fas siRNA-transfected BMDCs from control mice was analyzed after the stimulation of RANKL. GAPDH siRNA or irrelevant oligonucleotides was used as control. Data are means ± SD of triplicate samples and are representative of 3 independent experiments. (B) Inhibitory effect of Fas siRNA on Fas and RANKL-induced apoptosis of BMDCs from control mice was observed. Apoptotic cells were detected by flow cytometric analysis. Data are means ± SD and representative of 3 independent experiments. (C) Fas siRNA-transfected BMDCs were stimulated with RANKL from 0 to 96 hours. Caspase-3 expression was analyzed by immunoblot. GAPDH was used as a control for loading. Data are representative of 3 independent experiments. (D) MHC class II expression on the Fas siRNA-transfected BMDCs stimulated with RANKL was detected by flow cytometric analysis. Numbers above horizontal lines indicate percentage of MHC class II+ cells. Data are representative of 3 independent experiments. (E) Fas siRNA-transfected BMDCs from MRL+/+ and MRL/lpr mice were stimulated with RANKL, and the secretion of IL-12 from the DCs was detected by ELISA. Data are means ± SD of triplicate samples and are representative of 3 independent experiments. (F) Nuclear translocation of NF-κB subunits (p65 and p50) was detected using the nuclear extracts of Fas siRNA-transfected BMDCs from control mice by Western blot analysis. Histone was used as a control for loading. Data are representative of 3 independent experiments.
In addition, to confirm the role of Fas signal for the RANKL-induced activation of DCs, MHC class II expression, as 1 of some activation markers on RANKL-stimulated BMDCs treated with the siRNA, was analyzed by flow cytometry. RANKL-induced expression of MHC class II was still more enhanced by the transfection with Fas siRNA, but not control siRNA (Figure 6D). IL-12 secretion from RANKL-stimulated BMDCs of MRL+/+ mice was elevated by the treatment with Fas siRNA, indicating that Fas deficiency positively controls RANKL signaling as observed in the DCs from MRL/lpr mice (Figure 6E). Moreover, the accelerated nuclear translocation of NF-κB in MRL/lpr DCs was observed in RANKL-stimulated BMDCs treated with Fas siRNA (Figure 6F). These findings indicate that RANKL-stimulated DCs from normal mice may be maintained by Fas-mediated apoptosis through caspase and NF-κB cascade.
Inhibitory effect of RANK siRNA on Fas-mediated apoptosis of DCs
To clarify the direct association between RANK/RANKL and Fas signaling of DCs, Fas-mediated apoptosis of RANK-deficient DCs was analyzed using the siRNA of RANK as shown in the protocol (Figure S1A, available on the Blood website; see the Supplemental Figures link at the top of the online article). The knockdown of RANK by the siRNA in BMDCs was confirmed by analyzing the protein expression of RANK on flow cytometry and Western blotting as shown in Figure S1B-C. Then, Fas expression and apoptosis of RANK-deficient DCs from MRL+/+ and MRL/lpr mice was analyzed as shown in Figure S1D-E. Expectedly, RANKL-induced Fas expression was decreased to the level of unstimulation in RANK-decifient DCs from MRL+/+ mice (Figure S1D). Furthermore, the Fas- and RANKL-induced apoptosis was reduced to the level of anti-Fas stimulation (Figure S1E).
Influence of activated MRL/lpr DCs on arthritis
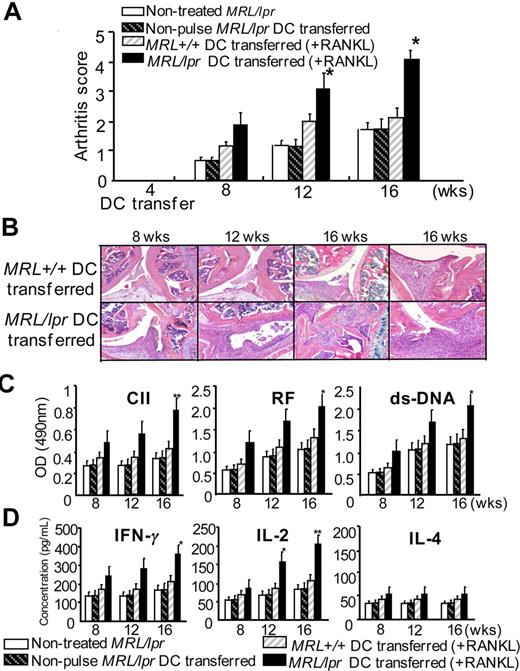
It is still unclear whether the activated and increased DCs from MRL/lpr mice influence autoimmune lesions such as arthritis in the mice. To determine whether the activated DCs from MRL/lpr mice may influence autoimmune lesions in vivo, the adoptive transfer of BMDCs stimulated with RANKL and CII into MRL/lpr mice at 4 weeks was performed to analyze arthritis lesions of the recipient mice. The histologic score of arthritis in the mice transferred with RANKL- and CII-stimulated MRL/lpr DCs was significantly higher at 12 and 16 weeks of age than those of the mice transferred with nonstimulated MRL/lpr DCs, RANKL- and CII-stimulated MRL+/+ DCs, or the untreated mice (Figure 7A). Histologic findings of the recipient mice transferred with RANKL- and CII-stimulated MRL/lpr DCs showed severe subsynovial inflammation, synovial hyperplasia, pannus formation, cartilage erosion, and bone destruction (Figure 7B). In contrast, the histology of the mice transferred with RANKL- and CII-stimulated MRL+/+ DCs was mild, similar to untreated MRL/lpr mice (Figure 7B). Anti-CII Ab and RF of sera from the recipient mice transferred with RANKL- and CII-stimulated MRL/lpr DCs were significantly increased (Figure 7C). Moreover, increased secretions of IFN-r and IL-2, not IL-4 from T cells stimulated with anti-CD3 mAb of the recipient transferred with RANKL- and CII-stimulated MRL/lpr DCs, were detected compared with those of the control recipients (Figure 7D). These results indicate that the RANKL-stimulated DCs might play an important role in the acceleration of autoimmune arthritis in MRL/lpr mice through T helper 1 (Th1) response.
Effects of the RANKL-stimulated MRL/lpr DCs transfer on autoimmune arthritis. BMDCs pulsed with CII and stimulated with RANKL were transferred into MRL/lpr recipients (4 weeks of age). The recipient MRL/lpr mice were analyzed at 8 to 16 weeks of age. (A) Histologic score of autoimmune arthritis in MRL/lpr mice transferred with RANKL-stimulated MRL/lpr DCs was evaluated compared with control mice until 16 weeks of age. Data are means ± SD of 5 to 7 mice in each group. *P > .05; **P > .01, MRL+/+ DCs versus MRL/lpr DCs transferred. (B) Histologic analysis of autoimmune lesions from MRL/lpr mice transferred with RANKL-stimulated MRL/lpr DCs and MRL+/+ DCs at 8, 12, and 16 weeks of age was performed. The sections of joints from the recipients were stained with HE. Data are representative of 5 to 7 mice in each group. (C) RF, anti-CII antibodies, and anti-dsDNA antibodies of sera from the recipients at 16 weeks of age were detected by ELISA. (D) The secretions of IFNγ, IL-2, and IL-4 in the supernatants from ILN T cells stimulated with anti-CD3 mAb were detected by ELISA. Data are means ± SD of triplicate samples and are representative of 5 to 7 mice in each group. *P > .05; **P > .01, MRL+/+ DCs versus MRL/lpr DCs transferred.
Effects of the RANKL-stimulated MRL/lpr DCs transfer on autoimmune arthritis. BMDCs pulsed with CII and stimulated with RANKL were transferred into MRL/lpr recipients (4 weeks of age). The recipient MRL/lpr mice were analyzed at 8 to 16 weeks of age. (A) Histologic score of autoimmune arthritis in MRL/lpr mice transferred with RANKL-stimulated MRL/lpr DCs was evaluated compared with control mice until 16 weeks of age. Data are means ± SD of 5 to 7 mice in each group. *P > .05; **P > .01, MRL+/+ DCs versus MRL/lpr DCs transferred. (B) Histologic analysis of autoimmune lesions from MRL/lpr mice transferred with RANKL-stimulated MRL/lpr DCs and MRL+/+ DCs at 8, 12, and 16 weeks of age was performed. The sections of joints from the recipients were stained with HE. Data are representative of 5 to 7 mice in each group. (C) RF, anti-CII antibodies, and anti-dsDNA antibodies of sera from the recipients at 16 weeks of age were detected by ELISA. (D) The secretions of IFNγ, IL-2, and IL-4 in the supernatants from ILN T cells stimulated with anti-CD3 mAb were detected by ELISA. Data are means ± SD of triplicate samples and are representative of 5 to 7 mice in each group. *P > .05; **P > .01, MRL+/+ DCs versus MRL/lpr DCs transferred.
Discussion
Studies in the past several years have established an important role of DCs in controlling many immune responses.1,2 However, the mechanism of maintenance of peripheral DCs has been unclear. The present study indicates that the maintenance of DCs, including cell growth, activation, and apoptosis in the periphery, is regulated by a crosstalk between RANKL and Fas signaling, and that the RANKL/Fas system in DCs may play a key role for the maintenance of peripheral tolerance.
DCs are heterogeneous in terms of phenotype, localization, and function. Several DC subsets have been described based on surface expressions, such as CD11c, B220, CD11b, CD4, and CD8α.3,4 It has been reported that plasmacytoid DCs induce Th2 cell differentiation in response to certain stimuli, and that myeloid DCs favor a Th1 response.6 Among myeloid DCs, 4 subsets are known: CD11b+CD8α+, CD11b+CD4+, CD11b+CD4−, and CD11blowCD8α−CD4−.3,5,6 The function of each subset has been obscure. In this study, increased CD11c+CD11b+CD8α+ myeloid DCs from MRL/lpr mice were observed, implying that the maintenance of Th1 DCs in the periphery may occur through Fas signaling, and that Th1 DCs from MRL/lpr mice might influence immune disorders such as autoimmune lesions and lymphadenopathy in the mice. It has been described that defective T-cell function of MRL/lpr mice plays a key role for pathogenesis of the immune disorder.10,32,33 Our data demonstrate that dysfunction of DCs from MRL/lpr mice accelerates autoimmunity mediated by T and B cells.
It has been reported that the Fas-mediated apoptosis of DCs is resistant due to a constitutive FLIP that can block Fas-induced apoptosis, whereas Fas is expressed on the surface of DCs.13,31 Upon Fas activation, a set of effector molecules is recruited to the receptor leading to form a signaling complex. Initially, FADD binds to Fas, recruits caspases, and activates a cascade of apoptosis. The present study indicates that Fas-mediated apoptosis of DCs can be induced by the addition of activated RANK/RANKL signaling, suggesting that there may be a crosstalk between the Fas and RANK pathway for DC maintenance in the periphery. In addition, our results indicate that the expression of FLIPL of DCs is controlled by RANKL and Fas signaling, leading to NF-κB activation. This is consistent with the recent report that cFLIP controls NF-κB activation and maintenance in lymphocytes and DCs.34 The molecular mechanism may be mediated by activation-induced cell death (AICD), which is well known to act as a system to maintain the peripheral T cells through Fas-mediated apoptosis.35,–37 Activated or autoreactive T cells are considered to delete by using AICD. Our data suggest that “AICD” of DCs may be mediated by the Fas and RANK pathway.
Bcl-2 family proteins, including Bcl-2 and Bcl-xL, are considered to be critical for DC survival, and to be regulated by NF-κB.7,38 In this study, Bcl-xL and Bcl-2 expressions of MRL/lpr DCs were considerably up-regulated by RANKL stimulation, leading to hyperproliferation of DCs. In addition, NF-κB activation of MRL/lpr DCs induced by RANKL was accelerated, suggesting that the Fas signaling pathway might influence NF-κB activation via RANKL signaling in DCs. The NF-κB family has emerged as a key transducer of inflammatory signals to DC maturation and activation, and is well known to be activated by various stimuli such as RANK/RANKL, CD40, and toll-like receptors (TLRs).14,39,40 Accelerated signaling pathways, including IKKα, IκB, and NF-κB downstream from TRAF6, was observed in MRL/lpr DCs stimulated by RANKL. Therefore, RANKL signaling leading to NF-κB activation through TRAF6 in DCs may be regulated by Fas-induced signaling.
DCs are crucial for the pathogenesis of autoimmune disease because of their potent antigen-presenting activity and unique ability to activate naive T cells.41 DCs have also been described to prime autoreactive T cells and induce the local inflammation of the synovial membrane in arthritis.42 It was reported that the transfer of collagen-pulsed BMDCs into congenic DBA/1 mice results in inflammatory arthritis.43 In addition, antigen-pulsed DCs have been shown to induce disease in experimental autoimmune encephalomyelitis, a murine model of multiple sclerosis.44 Our study showed that transfer of RANKL-stimulated MRL/lpr DCs pulsed with collagen into MRL/lpr mice clearly accelerates arthritis in the recipients, suggesting that the activated DCs stimulated by RANKL may accelerate autoreactivity of T and B cells to enhance the productions of pathogenic cytokines and autoantibidies in MRL/lpr mice. As for autoimmune arthritis of MRL/lpr mice, the increased DCs, especially myeloid DCs in the periphery, may play the key role for the pathogenesis. It was previously reported that an imbalance favoring development of DCs from myeloid-committed progenitors predisposes to autoimmune lesion in nonobese diabetic (NOD) mice.45 Therefore, analyzing the differentiation and function of myeloid DCs may lead to understanding the pathogenic mechanism of autoimmune disease.
Taken together, the new finding in the present study is that the maintenance of DCs, including cell growth, activation, and apoptosis in the periphery, is regulated by crosstalk between RANKL and Fas signaling, and that the RANKL/Fas signaling in DCs may play a crucial role in the maintenance of immune tolerance.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ai Nagaoka, Hiroyo Amo, and Satoko Yoshida for technical assistance.
This work was supported by the Ministry of Education, Science, and Culture of Japan (Grants-in-Aid for Scientific Research nos. 17109016 and 17689049).
Authorship
Contribution: T.I. performed all experiments and wrote the manuscript; N.I. codesigned experiments, coperformed confocal microscopic analysis, and wrote the manuscript; K.M. codesigned experiments and wrote the manuscript; M.K. coperformed ELISA with T.I.; R.A. performed Western blot analysis; Y.H. unified the project and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yoshio Hayashi, Department of Oral Molecular Pathology, Institute of Health Biosciences, The University of Tokushima Graduate School, 3–18-15 Kuramotocho, Tokushima 770-8504, Japan; e-mail: hayashi@dent.tokushima-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal