Iron is a transition metal whose physicochemical properties make it the focus of vital biologic processes in virtually all living organisms. Among numerous roles, iron is essential for oxygen transport, cellular respiration, and DNA synthesis. Paradoxically, the same characteristics that biochemistry exploits make iron a potentially lethal substance. In the presence of oxygen, ferrous iron (Fe2+) will catalyze the production of toxic hydroxyl radicals from hydrogen peroxide. In addition, Fe3+ is virtually insoluble at physiologic pH. To protect tissues from deleterious effects of Fe, mammalian physiology has evolved specialized mechanisms for extracellular, intercellular, and intracellular iron handling. Here we show that developing erythroid cells, which are taking up vast amounts of Fe, deliver the metal directly from transferrin-containing endosomes to mitochondria (the site of heme biosynthesis), bypassing the oxygen-rich cytosol. Besides describing a new means of intracellular transport, our finding is important for developing therapies for patients with iron loading disorders.
Introduction
Although its requirement for life in almost all known organisms has been recognized for decades, some of the most fundamental cell biologic processes of iron (Fe) still elude modern science. Mammalian physiology demands a constant source of bioavailible Fe, which is a functional component of hemoproteins, iron-sulfur cluster containing proteins, and other iron proteins. However, in its reduced form (Fe2+), iron catalyzes the production of toxic hydroxyl radicals through Fenton chemistry, while the ferric version (Fe3+) is virtually insoluble at physiologic pH.1,–3 Nevertheless, the adult human body contains approximately 4 g of Fe, more than 80% of which is in hemoglobin (Hb).4 Under normal conditions, around 2 million red blood cells (RBCs) are produced per second. Hence, erythropoiesis requires approximately 25 mg of iron, daily, all of which is delivered via transferrin (Tf). The plasma contains approximately 3 μM diferric Tf, the Fe of which is concentrated in maturing erythroid tissue to the equivalent of 20 mM iron, in the form of Hb. This exceptionally rapid utilization of the potentially toxic metal requires stringent regulation mechanisms that permit efficient production of hemoglobin, while protecting developing red blood cells and other tissues from iron's harmful properties.5
Virtually every tissue acquires its iron by receptor mediated endocytosis of Tf (for review, see Richardson and Ponka6 and Hentze et al7 ): Diferric Tf binds to its cognate receptor on the cell surface; this binding is succeeded by internalization of the receptor-ligand complex. The release of Fe from Tf is achieved within the endosome by a lowering of the vesicular pH through the activity of the v-ATPase proton pump. After its liberation from Tf in the acidified endosome, Fe must be reduced (possibly by the recently identified Steap38 ) before it is transported across the vesicular membrane by the divalent metal transporter (DMT1).9,–11
The immediate fate of iron, after having been exported from the endosome, is largely unknown. Through some mechanism, the metal must move from the vesicular exporter, past both mitochondrial membranes, to the matrix, where the enzyme that inserts Fe2+ into the protoporphyrin IX (PPIX) ring, ferrochelatase (FC), resides.7,12,13 So far, the only possible player in the movement of iron within mitochondria is a mitochondrial inner membrane protein that was first identified as an organellar iron import protein in yeast (Mrs3/4)14 and later confirmed in mice (mitoferrin).15 Interestingly, inhibitors of heme biosynthesis (isonicotinic acid hydrazide, succinylacetone)16,,,–20 or mutation of a heme biosynthetic enzyme, 5-aminolevulinic acid synthase (ie, patients with X-linked sideroblastic anemia)21,22 cause Fe to accumulate in the mitochondria of Hb producing cells (yielding ring sideroblasts in the latter example), demonstrating that iron delivered to these cells is specifically targeted to the mitochondria.
The accepted model for iron transfer from the transferrin-containing endosome proposes that endocytosed iron is exported to the cytosol and complexes with some low molecular weight carrier, to form what is generally known as the labile iron pool (LIP).23,24 However, this much sought after iron complex has never been identified. Furthermore, ferrous iron, the substrate of DMT1, would be considerably toxic in the oxygen-rich cytosol of the developing erythroid cell.25 Importantly, previous results from our laboratory, which demonstrate that there is virtually no chelatable iron in reticulocytes that are not metabolically active, are in conflict with the generally accepted characterization of the LIP.26 In the present study, we use reticulocytes, which are acquiring vast amounts of iron for Hb synthesis, to examine the delivery of iron from the endocytic vesicle to the mitochondria.
Materials and methods
Materials
Unless stated otherwise, all chemicals were acquired from Sigma-Aldrich (Oakville, ON). All 37°C incubations with reticulocytes were performed using minimum essential media (MEM) supplemented with 1% bovine serum albumin (BSA), 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and 10 mM sodium bicarbonate, pH 7.4 (henceforth referred to as “incubation medium”), unless otherwise noted. 59Fe2-Tf and 59Fe-salicylaldehyde isonicotinoyl hydrazone (59Fe-SIH2) were made from 59Fe-chloride (Perkin Elmer, Woodbridge, ON) and labeling apo-Tf, as previously described.27–29 Radioactivity measurements were performed using a Cobra II gamma counter (Packard Instruments, Meriden, CT). Confluent dishes of Huh7 cells (hepatocyte-like cell line) were generously provided by Dr Kostas Pantopoulos (McGill University, Montréal, Canada). B-[(2,2′-bipyridin-4-yl)aminocarbonyl]benzyl ester (RDA) was a generous gift of Dr R. Sustmann (University of Duisburg-Essen, Germany).
Transient lysis–resealing of reticulocytes
Reticulocytes were extracted via cardiac puncture, from CD-1 female mice that had been treated for 3 consecutive days with 50 mg/kg phenylhydrazine (administered intraperitoneally) and allowed to recuperate for 2 or 3 days (ie, mice were bled on day 6 or 7 from first injection). This method allowed us to harvest blood that contained 30%–50% reticulocytes (verified by new methylene blue staining); we refer to these samples as “reticulocytes.” The cells were washed in phosphate buffered saline (PBS) and resuspended in a minimal volume of PBS containing 1 mM of a membrane-impermeant form of desferrioxamine (HES-DFO; Biomedical Frontiers, Inc., Minneapolis, MN) or fluorescein isothiocyanate-dextran conjugate (10 000 kDa; FITC-dextran) where indicated. Lysis and resealing were performed according to the method of Scott et al.30 Briefly, lysis was accomplished by loading the cells into dialysis tubing (approximately 500 μL/15 cm of 11 mm diameter, 3500 molecular weight cut-off tubing) and dialyzing against 5 mM potassium phosphate buffer (pH 7.4) for 20 minutes at 4°C. The tubes were immediately placed in an isotonic solution (5 mM potassium phosphate buffer with 0.9% NaCl and 0.1% glucose), which was prewarmed to 37°C, and incubated for 30 minutes at that temperature. After this resealing step, the cells were washed in ice cold PBS, until the supernatant became clear.
Iron uptake and incorporation into heme
For all incubation steps, a 10% to 20% hematocrit of the reticulocyte suspension was used. For resealed cells: Washed, resealed cells were incubated for 60 minutes at 37°C in incubation medium. Because we have observed that some HES-DFO is present within intracellular vesicles following resealing, this incubation serves to remove HES-DFO, via exocytosis, that may have accumulated within vesicles. These cells were subsequently treated with 1 μM 59Fe2-Tf or 59Fe-SIH2 for 2.5 hours at 37°C. After washing the cells in ice-cold PBS, the heme and nonheme 59Fe were separated by an acid lysis/trichloroacetic acid precipitation method that was previously described.16,26
For experiments with nonresealed reticulocytes, washed cells were incubated for 60 minutes at 4°C in the presence of 1 μM 59Fe2-Tf. A cohort of 59Fe-labeled endosomes were generated in these cells by adding bafilomycin A1 (60 nM; inhibitor of v-ATPase responsible for endosomal acidification26,31 ) for the last 30 minutes of this incubation and then warming the cells to 37°C for 30 minutes. Unbound 59Fe2-Tf and bafilomycin were removed by washing the cells in PBS and surface-bound radioactivity was removed by a 120 minutes treatment at 4°C with 10 μM 56Fe2-Tf. The cells were next treated with the indicated concentrations of N-(6-aminohexyl)-5-chloro-1-naphthalene-sulfonamide (W-7; specific calmodulin inhibitor32,33 ) for 30 minutes at 4°C. Incorporation of 59Fe into heme was monitored by incubating the samples at 37°C for the indicated time intervals, washing in PBS, and determining the heme and nonheme radioactivity as mentioned above. Treatment with W-7 for microscopy experiments was done similarly (ie, 30 minutes at 4°C and left in the media during fluorescent Tf uptake).
For non-Tf Fe uptake experiments: 59Fe-ascorbate (59FeAsc) was generated by combining 59FeCl3 with a sodium ascorbate solution to yield a molar ratio of 1:44 (iron to ascorbate). 59FeAsc or 59Fe-Tf2 was then immediately added to the reticulocytes to reach the indicated final concentrations and the cells were incubated for 30 minutes. The cells were then washed in ice cold PBS and treated for 30 minutes with 1 mg/mL pronase to remove any radioactivity bound to proteins at the cell surface.
Confocal microscopy
Washed reticulocytes were resuspended in incubation medium and treated with 500 nM MitoTracker CMXRos (Molecular Probes, Eugene, OR) for 20 minutes at 37°C. Excess probe was removed by washing the samples with ice-cold PBS. The samples were resuspended in Iscove modified Dulbecco media (IMDM) and transferred to a glass coverslip bottom culture dish (MatTek, Ashland, MA) and placed on the stage of a Zeiss LSM 510 meta (Carl Zeiss, Toronto, ON) confocal inverted microscope that had been outfitted with a heated stage (37°C) and a Plan-Apochromat 100x oil objective with a numerical aperture of 1.4. Before image acquisition, 500 μL of prewarmed (37°C) Alexa Green 488 transferrin (AG-Tf; 500 nM; Invitrogen, Carlsbad, CA) was added. A multichannel protocol was used to scan the sample once for each fluorochrome every 50 milliseconds, yielding videomicrographs with a framerate of 20/sec. Vesicular movement was tracked using MetaMorph™ Version 7 (Universal Imaging, Molecular Devices, Sunnyvale, CA).
For plasma membrane labeling, reticulocytes or Huh7 cells, in IMDM supplemented with 10% fetal calf serum, were incubated with 2.5 μg/mL or 0.5 μg/mL, respectively, N-(3-triethylammoniumpropyl)-4-(4-(dibutylamino)styryl) pyridinium dibromide (FM 1–43; Molecular Probes) at room temperature for 10 minutes in coverslip bottom dishes and subjected to confocal microscopy, using a 63x objective (NA 1.4). Where indicated, 500 nM Alexa Green 488-Tf was added at the same time as the plasma membrane label.
Mitochondrial iron/wide-field microscopy
Loading of mitochondria with rhodamine B-[(2,2′-bipyridin-4-yl)aminocarbonyl]benzyl ester (RDA; Fe-quenchable fluorophore that localizes to mitochondria) was performed as previously described for the chemically similar reagent, RPA (rhodamine B 4-[(1,10-phenanthrolin-5-yl)aminocarbonyl]benzyl ester).34 Briefly, the reticulocytes were incubated at 37°C for 25 minutes in 300 nM RDA, washed with PBS, and reincubated at 37°C for 20 minutes. Cells were washed in PBS, resuspended in IMDM, and loaded onto coverslip bottom culture dishes.
Live, wide-field imaging was done at 100X (1.45 NA) on an Olympus IX81 TIRF (total internal reflection fluorescence) microscope (Olympus, Center Valley, CA). Cells were kept at 30°C (Weather Station, Optical Analysis Corp., Nashua, NH). The 488 nm (200 mW Argon) or 543 nm (1 mW Helium-Neon) laser lines were turned so they passed through the sample giving wide-field excitation using a z488/543rpc dichroic and a 488/543 dual emission filter (Chroma Technology Corp., Rockingham, VT). The lasers were coupled to the microscope by a fiber optic cable, and controlled with an AOTF (acousto-optic tunable filter; Prairie Technologies, Madison, WI). Images were collected simultaneously using 2 Cascade 512B cameras (Photometrics, Tuscon, AZ) and a dualcam (Optical Insights, Tucson, AZ) equipped with an EGFP/RFP filter set. MetaMorph software (Molecular Devices, Sunnyvale, CA) controlled the instrument.
Electron microscopy
Reticulocytes were incubated with 1 μM HRP-Tf (Jackson ImmunoResearch Laboratories, West Grove, PA) at 37°C for 10 minutes, washed 3 times in ice-cold PBS, and fixed with 2.5% glutaraldehyde. HRP was developed by suspending the fixed cells in a 1 mg/mL solution of diaminobenzidine (DAB) in PBS, adding H2O2 (BioShop, Burlington, ON) to 0.01%, and incubating at room temperature for 30 minutes. Developing was halted by washing the cells with PBS, following which the cells were resuspended in 2.5% glutaraldehyde and processed as previously described for EM: osmication, dehydration, infiltration with epon, and ultra-thin sectioning.35
Data analysis
Events of endosome/mitochondria contact were identified by visual examination of the videomicrographs. The area for intensity measurements for the mitochondria of interest was determined from either the average or maximum projection of a number of images calculated using MetaMorph software. The intensity in identified regions of interest was calculated over time for 52 separate endosome/mitochondria contacts and more than 40 control mitochondria that did not show endosome contact. The data were averaged and smoothed in Sigma Plot software (SPSS Inc., Chicago, IL) using the negative exponential filter set at 0.10. The plot shown in Figure 6E is the average of all the curves measured; error bars represent standard error.
All of the biochemical results presented are representative of at least 4 replicate experiments. Except for Figure 6E, error bars represent standard deviations.
Results
Iron from transferrin-containing endosomes can bypass the cytosol
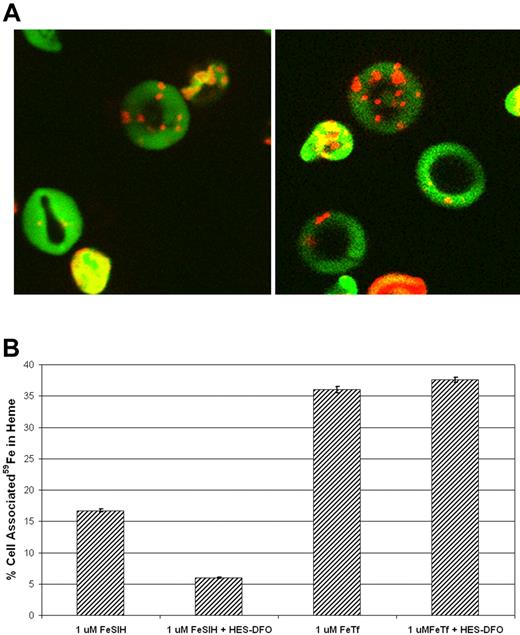
We have exploited a technique, previously used only with RBCs, to load reticulocytes' cytosol with the membrane impermeant chelator, HES-DFO (see “Materials and methods”). To verify that the reticulocyte membrane is as amenable to transient hypotonic lysis and resealing as that of the erythrocyte, we loaded blood cells, harvested from phenylhydrazine treated mice, with fluorescein isothiocyanate-dextran. To differentiate between RBCs and reticulocytes, the cells were subsequently labeled with MitoTracker Red CMXRos. As depicted in Figure 1A, reticulocytes took up a significant amount of the fluorescent conjugate, while retaining sound morphology.
Cytoplasmic HES-DFO does not block 59Fe2-Tf incorporation into heme. Reticulocytes were exposed to transient lysis and resealing, as described in Experimental Procedures. (A) Cells were resealed in the presence of FITC-dextran (green), treated with MitoTracker CMXRos (red), and evaluated by confocal microscopy. (B) Cells were resealed in the absence or presence of 1 mM HES-DFO and then treated with either 1 μM 59Fe-SIH2 or 1 μM 59Fe2-Tf for 2.5 hour at 37°C. Heme and nonheme fractions were separated, measured by gamma counting, and the percentage of cell-associated 59Fe in heme was plotted. Error bars represent standard deviations (n = 3).
Cytoplasmic HES-DFO does not block 59Fe2-Tf incorporation into heme. Reticulocytes were exposed to transient lysis and resealing, as described in Experimental Procedures. (A) Cells were resealed in the presence of FITC-dextran (green), treated with MitoTracker CMXRos (red), and evaluated by confocal microscopy. (B) Cells were resealed in the absence or presence of 1 mM HES-DFO and then treated with either 1 μM 59Fe-SIH2 or 1 μM 59Fe2-Tf for 2.5 hour at 37°C. Heme and nonheme fractions were separated, measured by gamma counting, and the percentage of cell-associated 59Fe in heme was plotted. Error bars represent standard deviations (n = 3).
After confirming that reticulocytes can be resealed with high molecular weight compounds, we loaded these cells with HES-DFO. Reticulocytes with cytoplasmic HES-DFO incorporated an equivalent amount of radioiron, from 59Fe2-Tf into heme compared with controls (Figure 1B), indicating that Tf borne 59Fe cannot be intercepted by the chelator. To confirm that the chelator was taken up by the cells and that it is available to bind cytosolic Fe, the resealed cells were incubated in the presence of 59Fe-SIH2, a membrane permeable complex that is able to provide Fe for heme biosynthesis.29,36 Indeed, the incorporation of 59Fe into heme, in cells containing HES-DFO, was compromised when we used this form of iron. Together, these data indicate that Fe derived from Tf-containing vesicles can reach ferrochelatase, on the mitochondrial inner membrane, without entering a chelatable, cytoplasmic pool.
Inhibition of vesicular motility blocks Fe incorporation into heme
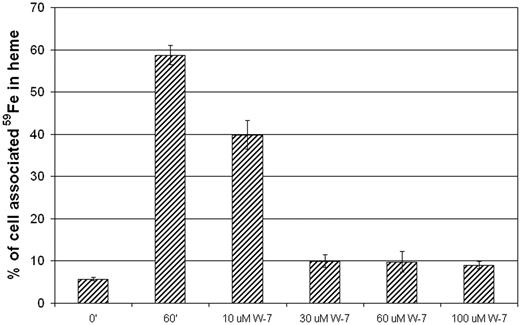
Using bafilomycin, an inhibitor of the v-ATPase proton pump, we created a cohort of 59Fe2-Tf laden vesicles within reticulocytes. Because iron will not be released from Tf until the vesicular pH is lower than 5.5, when bafilomycin-treated reticulocytes were incubated in the presence of 59Fe2-Tf, the iron entered the endosomal compartment but remained therein. After washing out the inhibitor, the radioiron was available for heme biosynthesis (Figure 2, 60′). Confocal microscopy of reticulocytes treated with this inhibitor and subsequently incubated with Alexa Green 488-Tf (AG-Tf) yielded videomicrogaphs qualitatively identical to those acquired in the absence of bafilomycin (“Transferrin-containing vesicles contact mitochondria” section below).
Endosomal motility is required for 59Fe availability. The endosomal compartment of reticulocytes was loaded with 59Fe2-Tf by treating the cells sequentially with bafilomycin (60 nM; 30 minutes at 37°C) and 59Fe2-Tf (1 μM; 30 minutes at 37°C). Where indicated, increasing concentrations of W-7 were included in the reincubation step (60 minutes at 37°C). The incorporation of 59Fe into heme is shown as the percentage of total cell-associated 59Fe. 0′, before reincubation; 60′, 60 minutes reincubation (in the absence of W-7). Error bars represent standard deviations (n = 3).
Endosomal motility is required for 59Fe availability. The endosomal compartment of reticulocytes was loaded with 59Fe2-Tf by treating the cells sequentially with bafilomycin (60 nM; 30 minutes at 37°C) and 59Fe2-Tf (1 μM; 30 minutes at 37°C). Where indicated, increasing concentrations of W-7 were included in the reincubation step (60 minutes at 37°C). The incorporation of 59Fe into heme is shown as the percentage of total cell-associated 59Fe. 0′, before reincubation; 60′, 60 minutes reincubation (in the absence of W-7). Error bars represent standard deviations (n = 3).
When added to reticulocytes before addition of AG-Tf, the specific calmodulin inhibitor, W-7, completely blocked endocytosis (Video S1). If we added the W-7 after a brief 37°C incubation in the presence of AG-Tf, the already formed vesicles are completely immobilized (Video S2). To investigate the effect of inhibition of vesicular movement on the delivery of iron to FC, we first treated reticulocytes with bafilomycin, warmed the cells in the presence of 59Fe2-Tf, and washed the inhibitor and unbound 59Fe2-Tf from the cells. The samples remained on ice for 2 hours in 10 μM 56Fe2-Tf to displace any surface-bound radioiron transferrin. As mentioned above, when these reticulocytes were warmed to 37°C, the 59Fe from the loaded vesicles was incorporated into heme; however, when W-7 was added to the cells prior to (and during) warming, utilization of the vesicular 59Fe was blocked (Figure 2). To ensure that W-7 does not directly inhibit heme biosynthesis, we loaded reticulocyte mitochondria with 59Fe, using succinylacetone as previously described,18,20 washed out the inhibitor, treated the cells with W-7, and incubated the cells at 37°C for an hour. The cells treated with W-7 did not show any significant decrease in Fe incorporation into heme compared with untreated controls (Figure S1A, available on the Blood website; see the Supplementary Materials link at the top of the online article). In an additional control experiment, the presence of 30 μM W-7 elicited only a small inhibition in the incorporation of 59Fe into heme when it is supplied as 59Fe-SIH2, a form that bypasses the Tf receptor pathway (Figure S1B). Together, these data indicate that free, vesicular iron is not simply exported from the vesicles; movement of the organelles is required for proper targeting of the metal to mitochondria.
Cytoplasmic, “free” Fe2 + is used inefficiently
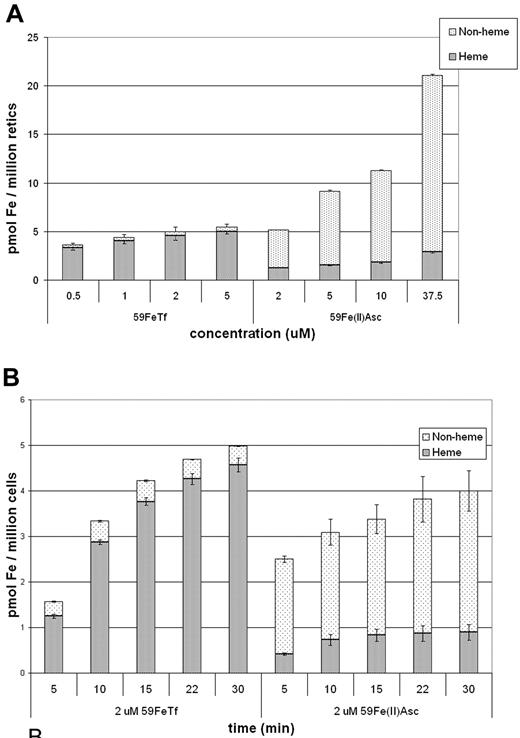
According to our proposed model,12,20,26 further tested in the current study, efficient transfer of iron to mitochondria occurs through a transient interaction between the endosome, which contains a high concentration of iron, and the mitochondrion. Therefore, we predicted that the utilization of “free” ferrous iron in the cytosol for heme synthesis would be comparatively inefficient. Because DMT1 resides at the cell surface before endocytosis37 and is also functional at pH 7.4,38 it was possible for us to artificially create a “LIP” in reticulocytes by incubating them in the presence of 59Fe(II)-ascorbate. Somewhat surprisingly, at pH 7.4, uptake of the ferrous ascorbate was more efficient than that of Fe2Tf (Figure 3), at the higher concentrations, indicating that DMT1 is not limiting in Fe2Tf iron uptake. Importantly, even though the cellular uptake was over 2-fold greater (on a per cell basis), the proportional utilization of the intracellular 59Fe was much lower when 59Fe(II)-ascorbate was used compared with 59Fe2Tf; at 2 μM for both forms, uptake was approximately equivalent, yet the percentage of Fe in heme for the Tf sample was approximately 92%, while that of the ferrous ascorbate sample was only approximately 24%. Unexpectedly, the concentration dependence experiments (Figure 3A) demonstrate a saturation of Tf iron uptake (in the low micromolar range), while relatively high levels of Fe-ascorbate did not saturate the non-Tf uptake system. We postulate that this reflects an accumulation of a nonheme form of iron within the reticulocytes. Furthermore, the time dependence of iron incorporation into heme that is normally observed when using 59Fe-Tf as the iron source was lost when we used 59Fe(II)-ascorbate (Figure 3B). These findings stress the key role of the Tf-Tf receptor pathway in the highly efficient use of Fe for heme synthesis.
Utilization of 59Fe from Tf is more efficient than from 59Fe-ascorbate. (A) Concentration dependence: Cells were incubated for 30 minutes with the indicated concentrations of either 59Fe2-Tf or 59Fe(II)-ascorbate and the heme and nonheme fractions measured and displayed as pmol Fe per million cells. (B) Time dependence: Cells were incubated in 2 μM 59Fe2-Tf or 59Fe(II)-ascorbate for the indicated times and analyzed as in (A). Error bars represent standard deviations (n = 3).
Utilization of 59Fe from Tf is more efficient than from 59Fe-ascorbate. (A) Concentration dependence: Cells were incubated for 30 minutes with the indicated concentrations of either 59Fe2-Tf or 59Fe(II)-ascorbate and the heme and nonheme fractions measured and displayed as pmol Fe per million cells. (B) Time dependence: Cells were incubated in 2 μM 59Fe2-Tf or 59Fe(II)-ascorbate for the indicated times and analyzed as in (A). Error bars represent standard deviations (n = 3).
Transferrin-containing vesicles contact mitochondria
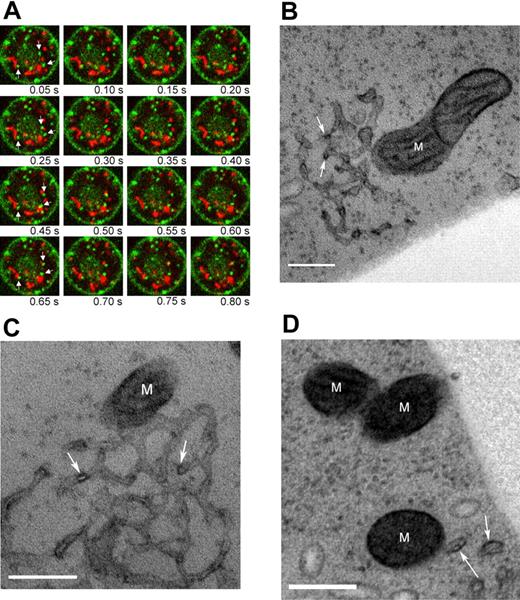
To visualize the movement of endosomal vesicles with respect to mitochondria, we used confocal microscopy to examine cells that were doubly fluorescence labeled. Reticulocytes with MitoTracker Red CMXRos-stained mitochondria were incubated on a heated (37°C), confocal microscope stage in 500 nM AG-Tf. Images were acquired at 20 frames per second, allowing real time evaluation of the X-Y position of transferrin-containing endosomes; all videomicrographs were recorded within 20 minutes of the addition of the cells to the Tf solution. Binding and internalization of the AG-Tf occurred immediately. At least 3 populations of organelles containing fluorescent green label can be distinguished (Figure 4A; Video S3): (1) small, extremely motile, (2) large, moderately motile, (3) large, mostly immobile vesicles. The former 2 species can be seen to transiently abut one or more mitochondria within the 20 second video capture. The interaction between the larger organelles and the mitochondria are usually less transient that that of the smaller vesicles. In addition, we quantitatively tracked the most motile vesicles in the X-Y plane and found that their average velocity was 2.77 (± 0.43 μm/s). These videos clearly demonstrate that the Fe2-Tf containing vesicles penetrate considerably the reticulocyte cytosol and repeatedly get very close to or touch mitochondria.
Transferrin-containing vesicles move to mitochondria. (A) Confocal micrograph time series of a representative reticulocyte with labeled mitochondria (red) and holo-transferrin (green). Panels proceed from left to right with a 50 ms interval. Arrows in the leftmost panel of each row highlight some of the vesicles that appear to interact with mitochondria over the course of the videomicrograph. Cell diameter is approximately 8 μm. The corresponding videomicrograph is shown in Video S3. (B-D) TEM images of reticulocytes labeled with HRP-Tf. M, mitochondrion; arrows indicate HRP-Tf. Scale bar is 200 nm.
Transferrin-containing vesicles move to mitochondria. (A) Confocal micrograph time series of a representative reticulocyte with labeled mitochondria (red) and holo-transferrin (green). Panels proceed from left to right with a 50 ms interval. Arrows in the leftmost panel of each row highlight some of the vesicles that appear to interact with mitochondria over the course of the videomicrograph. Cell diameter is approximately 8 μm. The corresponding videomicrograph is shown in Video S3. (B-D) TEM images of reticulocytes labeled with HRP-Tf. M, mitochondrion; arrows indicate HRP-Tf. Scale bar is 200 nm.
Using TEM, we verified that the Tf-containing endosomes actually touch mitochondria in these cells. The vesicles, identified by staining with HRP-Tf, appeared either as part of an endosomal network (Figure, 4B-C) or solitary vesicles (Figure 4D). Web-like endosomes similar to the ones we have documented here were previously observed by Stoorvogel et al39 and it is likely that the individual structures we observe are simply cross-sections through the network. Importantly, both these structures appeared to be intimately associated with mitochondria (Figure 4B-D) in numerous sections. The developing of HRP-labeled compartments with DAB treatment allowed us to visualize the compartments that contained the Tf. In cells where we did not apply the conjugated protein, we were unable to visualize the endosomal networks. We speculate that osmication alone was insufficient to label these organelles in reticulocytes, since the high concentration of hemoglobin blunted the contrast profile of our samples.
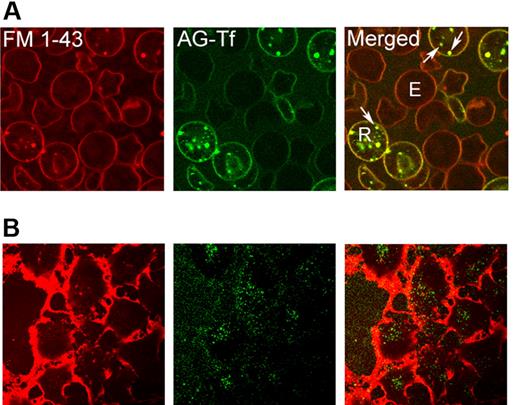
In an attempt to investigate whether Tf-containing endosomes specifically, or more frequently, “contacted” mitochondria compared with vesicles with some other cargo in our live imaging system, we labeled reticulocytes with both AG-Tf and FM 1-43, a fluorescent probe that targets membrane phospholipids. Interestingly, when these dual-labeled reticulocytes were incubated in the presence of full media (containing 10% fetal calf serum), all of the endocytotic vesicles contained transferrin (Figure 5A). These data suggest that the vast majority of the reticulocyte's endocytic machinery is occupied by Tf, which was present in all of the observed vesicles, colocalized with the FM 1-43. As a positive control, we labeled Huh7 (hepatocyte cell line) similarly and show endocytosis of membrane phospholipid into vesicles devoid of fluorescent transferrin (Figure 5B). In addition, we were unable to observe any uptake of fluorescent insulin in reticulocytes, while it was readily endocytosed in Huh7 cells (data not shown).
In reticulocytes, all plasma membrane-derived, intracellular structures contain transferrin. Cells were visualized by confocal microscopy. (A) Reticulocytes were treated with FM 1-43 and subsequently incubated at 37°C in the presence of AG-Tf. R, reticulocyte; E, erythrocyte; arrows, collocalized FM 1-43 and Tf. Scale bar is 5 μm. (B) Huh7 cells were treated with FM 1-43 and then with AG-Tf, as in panel A. To visualize the internalized dye, it was required to increase the photomultiplier tube gain to levels that elicit saturation of the dense, membrane signal surrounding these thin, adherent cells. Arrows indicate FM 1-43 vesicles that do not contain Tf. Scale bar is 10 μm.
In reticulocytes, all plasma membrane-derived, intracellular structures contain transferrin. Cells were visualized by confocal microscopy. (A) Reticulocytes were treated with FM 1-43 and subsequently incubated at 37°C in the presence of AG-Tf. R, reticulocyte; E, erythrocyte; arrows, collocalized FM 1-43 and Tf. Scale bar is 5 μm. (B) Huh7 cells were treated with FM 1-43 and then with AG-Tf, as in panel A. To visualize the internalized dye, it was required to increase the photomultiplier tube gain to levels that elicit saturation of the dense, membrane signal surrounding these thin, adherent cells. Arrows indicate FM 1-43 vesicles that do not contain Tf. Scale bar is 10 μm.
Endosome-mitochondrion interaction increases chelatable mitochondrial iron
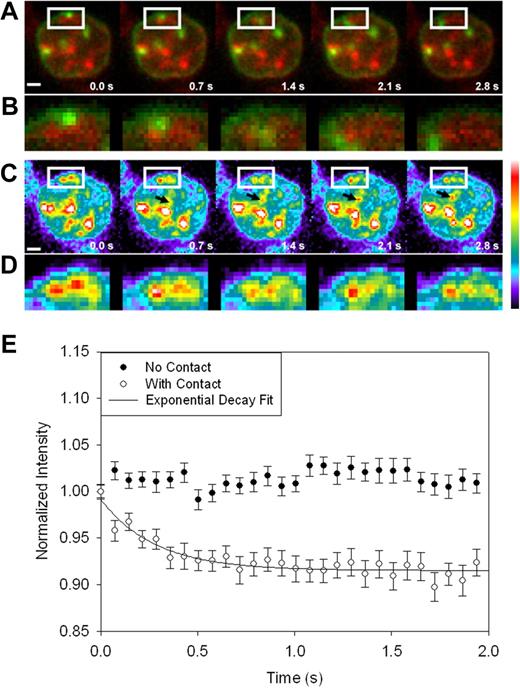
To investigate whether the observed interorganellar contacts were functional interactions, we loaded reticulocytes' mitochondria with a fluorescent metallosensor and monitored its intensity during endocytosis of diferric transferrin. Rhodamine B-[(2,2′-bipyridin-4-yl)aminocarbonyl]benzyl ester (RDA) has been shown to accumulate in mitochondria, in a membrane potential-dependent manner, and to be quenched by iron.40 Live imaging was performed using AG-Tf to specifically label the endosomes (as above) with wide-field illumination using expanded beams from the 488 nm (Ar) and 543 nm (He-Ne) lasers directed straight through the objective. A rapid quenching of the mitochondrial signal was consistently observed when endosomes appeared to contact mitochondria (Figure 6; Video S4). The relationship between endosomal proximity and RDA quenching was evaluated by determining the red fluorescent signal intensity within a region of interest that included a mitochondrion before and immediately after an endosome “contacts” the mitochondrion during the time series. Note that, while mitochondria which exhibit these contact events (Figure 6A, white box, enlarged in Figure 6D) show a decrease in average fluorescence intensity (Figure 6C, and white box enlarged in Figure 6D), the intensities of surrounding mitochondria (indicated by the arrow in Figure 6C) sometimes increase slightly over the time course. Analysis of “contacted” mitochondria (n = 52) demonstrates an average 10% decrease in fluorescence intensity of the iron-sensitive dye, with an exponential decay half time of 0.28 (± 0.05 s; r2 = 0.88; Figure 6E). In contrast, the average intensity of RDA within mitochondria that do not appear to interact with endosomes remains quite steady over time.
Endosome-mitochondria proximity increases chelatable, mitochondrial iron. Alexa Green 488 transferrin (green) and RPA (red) were imaged simultaneously with a dualcam system and 2 Cascade 512B cameras. (A) The white box shows a region where a transferrin containing endosome appears to contact a mitochondria, enlarged in (B). (C) Pseudo color images of the red signal to more clearly illustrate the decrease in intensity of the signal following contact with the iron containing endosome. An “uncontacted” mitochondrion whose intensity does not decrease with time is denoted by black arrows. (D) Enlarged images of the region outlined by the white box in C. Images are an average of 10 image frames, corrected for background intensity and were smoothed with a 2 × 2 pixel low pass filter. Scale bar is 1 μm. (E) Plot of the intensity versus time for mitochondria that either made contact with endosomes (open circles) or control mitochondria that did not contact endosomes (closed circles). Error bars represent standard error of the mean. Data were collected and smoothed as described in “Materials and methods; Data analysis”.
Endosome-mitochondria proximity increases chelatable, mitochondrial iron. Alexa Green 488 transferrin (green) and RPA (red) were imaged simultaneously with a dualcam system and 2 Cascade 512B cameras. (A) The white box shows a region where a transferrin containing endosome appears to contact a mitochondria, enlarged in (B). (C) Pseudo color images of the red signal to more clearly illustrate the decrease in intensity of the signal following contact with the iron containing endosome. An “uncontacted” mitochondrion whose intensity does not decrease with time is denoted by black arrows. (D) Enlarged images of the region outlined by the white box in C. Images are an average of 10 image frames, corrected for background intensity and were smoothed with a 2 × 2 pixel low pass filter. Scale bar is 1 μm. (E) Plot of the intensity versus time for mitochondria that either made contact with endosomes (open circles) or control mitochondria that did not contact endosomes (closed circles). Error bars represent standard error of the mean. Data were collected and smoothed as described in “Materials and methods; Data analysis”.
Discussion
A previously proposed, and conventionally accepted, component of the iron uptake system is a low-molecular-weight, cytoplasmic-iron-binding moiety. This intermediate would act as a chaperone, mediating transfer from the endosomal vesicle to the mitochondrion, the site of heme biosynthesis. The idea of a LIP was first conceived in 1946 by Greenberg and Wintrobe41 as well as by Ross,42 in the same year, both of whom used data from radiotracer experiments to determine that there was a “metabolic pool” of body iron that could be used for erythropoiesis. This concept was modified by Jacobs, who speculated that a labile, low-molecular weight Fe intermediate was present in both erythroid and nonerythroid cells.23,24 In spite of this, more than 40 years after the conception of the LIP, the nature of this chelatable cellular Fe has still not been revealed. The only supportive evidence for the presence of a LIP are from in vitro experiments showing that membrane permeable chelators are able to bind nonheme iron from cultured cells, including reticulocytes. However, the intracellular source of this Fe is, at best, vaguely identifiable in these studies17,28,43 ; it is just as likely that chelators strip Fe from organelles or membrane components as from the cytoplasm. Hence, the so-called LIP has been defined by the amount of iron that the experimentor's chelator was able to bind. In the present study, we overcame this ambiguity of using permeant chelators by resealing reticulocytes with HES-DFO and thus show that vesicular iron is not transported into the cytosol.
Using electron microscopy, we demonstrate that transferrin-containing endosomes come into contact with mitochondria, while from fluorescence microscopy data we show that this contact results in an increase in chelatable mitochondrial iron. These transient contacts are very likely to be dependent on the activity of both molecular motors and docking complexes. The recent discovery of the genetic mutation in the hemoglobin deficit (hbd) mouse,44,45 whose reticulocytes demonstrate reduced transferrin cycling,46,47 further supports the notion that endosomal motility is necessary because the yeast homologue of the gene in question, Sec15l1, is known to be involved in vesicular docking. We expect that myosin Vb is a major contributor to this movement, based on recent findings by Provance et al.48 Previously, one could envisage a mechanism whereby the vesicle would only have to form in order to allow the acidification of the microenvironment surrounding the Fe2-Tf complex and the reduction of the metal to Fe2+. This former model would require little or no movement of the formed vesicle because the free and reduced iron would then exit the organelle and enter the “LIP.” Considering our results, the most attractive model for transfer of iron from endosomes to mitochondria is one in which a transient, yet intimate, relation between the 2 organelles facilitates a direct transfer of the metal, circumventing any free, cytosolic intermediary. Due to the distinct properties of the endosomal and mitochondrial membranes and the fact that we do not observe a leakage of fluorescent transferrin to the mitochondria, it is doubtful that these 2 compartments actually fuse, rather we propose that a relaying of Fe occurs from endosomal protein to mitochondrial protein.
The presence of a strong iron chelator in the cytosol of the reticulocytes had no effect on the incorporation of 59Fe2-Tf into heme. It may be argued that there could still exist a cytoplasmic chaperone that tightly binds vesicle-derived Fe and shuttles it to the mitochondria, thus shielding it from chelation by HES-DFO. This is unlikely, however, because when the Fe was delivered as Fe-SIH2, its incorporation into heme was effectively blocked by the cytosolic HES-DFO. One would expect that if a “labile” moiety that shuttled iron from the vesicle existed and could bind iron more efficiently than HES-DFO (DFO has an affinity for iron of 10−33), it would be able likewise to sequester Fe from the Fe-SIH2 chelate. In addition, Martinez-Medellin and Schulman27 observed that, when rabbit reticulocytes were treated with 59Fe-Tf, radioiron was apparent only in either hemoglobin or a stromal-bound fraction. Furthermore, the recent study by Schranzhofer et al49 showed a decrease in the sensitivity of the cytosolic iron regulatory proteins to Tf-derived iron in erythroid progenitors, as the cells differentiate. Together, these results are consistent with our model of iron delivery in Hb-synthesizing cells whereby the metal does not enter the cytosol, but is rapidly shuttled from endosome to the site of heme synthesis. It is important to note that our experiments were limited to reticulocytes and, therefore, we cannot ignore the possibility that this pathway is unique to hemoglobin-synthesizing cells. This is not an unlikely prospect given that there have been profound differences, with regards to iron metabolism, demonstrated in such cells.12
After the more than 20 years that have elapsed since Morgan and Appleton's seminal discovery of Tf internalization50 evolved into an unequivocally proven endocytosis model,51,,,–55 the postendosomal path of iron remains totally enigmatic; the only model that has ever emerged is the one invoking an inscrutable “LIP” that allegedly serves as an intermediary between endosomes and mitochondria. The results reported here have challenged this concept by describing a mechanism of intracellular Fe trafficking whereby Tf-containing endosomes cede their iron atoms directly to mitochondria. To the best of our knowledge, this is the first evidence of interorganellar interaction having the functional consequence of facilitating the transport of a micronutrient. Importantly, this may be a paradigm for Fe trafficking in cells in general, because there is vast literature showing movement of Tf-TfR-containing endosomes to various intracellular structures, such as Golgi complexes, endoplasmic reticulum and perinuclear structures (reviewed in Kühn et al,56 Ponka and Lok,57 and Maxfield and McGraw58 ). Unfortunately, virtually all of these studies use Tf as a marker, not considering the actual function of the protein. Because the only known functions of Tf are the transport and delivery of Fe through the circulation and to cells, the movement of Tf-containing endosomes toward various intracellular structures is probably related to iron delivery to these organelles.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
P.P. is grateful to the Marine Biological Laboratory (Woods Hole, MA), B. Speck, Quantomix (Rehovot, Israel), and Universal Imaging (Sunnyvale, CA) for assistance and support. The authors thank Biomedical Frontiers, Inc. for their generous gift of HES-DFO, Drs. R. Sustmann and U. Rauen for their generosity and assistance with the RDA, and Jim Seams, S. Graf, E. Corson, Dr H. Valli, and J. Mui for technical support. The authors are also grateful to Drs. R. Lill, P. Macpherson, H. McBride, R. Johnstone, M. Vidal, P. Stahl, and P. Tupper for their advice, and Dr E. Cook for his assistance with the data analysis.
The research of P.P. was funded in part by a grant from the Canadian Institutes of Health Research (CIHR). The research of O.S. was funded by the National Institutes of Health (NHLBI). A.S. was supported by a studentship from the CIHR.
Authorship
Contribution: A.D.S., A.-S.Z., O.S., and P.P. conceived the experiments. A.D.S. conducted them and wrote the paper with conceptual and editorial assistance from P.P., O.S., and C.B., who also analyzed the data. O.S and P.P. contributed equally to the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prem Ponka, Lady Davis Institute for Medical Research, 3755 Cote Ste.-Catherine Rd., Montréal, QC H3T 1E2, CANADA, email. prem.ponka@mcgill.ca

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal