Tumor cell–associated tissue factor (TF) is a powerful determinant of metastatic potential. TF may increase metastasis by supporting thrombin-mediated proteolysis, through intracellular signaling events mediated by the TF cytoplasmic domain, through TF/fVIIa/fXa–mediated activation of protease-activated receptors, or through a combination of these processes. To better define the relationship between tumor cell-associated TF and circulating hemostatic factors in malignancy, we generated a set of C57Bl/6-derived tumor lines genetically lacking TF, expressing wild-type murine TF, or expressing a mutant TF lacking the cytoplasmic domain. Comparison of the metastatic potential of these cells in immunocompetent mice with genetic deficits in prothrombin, platelet function, or fibrinogen revealed that TF supports metastasis through mechanisms independent of the cytoplasmic domain, but dependent on each of these distal hemostatic factors. TF was neither required for primary tumor growth nor necessary for initial localization of embolized tumor cells within the lungs. Rather, tumor cell fate studies indicated TF supports metastasis by increasing the survival of micrometastases. One mechanism linking TF to metastasis is through a fibrin(ogen)-dependent and platelet-dependent restriction in natural killer cell–mediated clearance of micrometastases. However, TF also supported the early success of micrometastases through an additional mechanism independent of natural killer cells, but coupled to circulating prothrombin.
Introduction
Tissue factor (TF) is the membrane-associated glycoprotein receptor for coagulation factors VIIa and X that serves as the primary physiologic initiator of blood coagulation. In addition to supporting proteolytic events that ultimately lead to local thrombin generation, TF is also proposed to directly contribute to intracellular signaling events through the TF cytoplasmic domain and TF/fVIIa/fXa–mediated activation of PAR-1 and PAR-2.1,,–4 A significant body of evidence has accumulated linking tumor cell-associated procoagulant function to cancer biology. Multiple clinical studies have shown a correlation between TF expression by tumor cells and advanced disease stage and poor outcome.5,,,–9 Furthermore, experimental data generated using animal models of tumor metastasis strongly favor the view that TF expression by malignant cells supports metastatic success.10,,,,–15 Similarly, thrombin-mediated proteolysis,16,,,,–21 fibrin(ogen),22,23 and PAR-mediated platelet activation24 also appear to be significant determinants of metastatic potential. Both platelets and fibrinogen were shown to support metastatic potential by limiting the capacity of natural killer (NK) cells to clear newly established micrometastatic foci.25,26 However, hemostatic factors are likely to influence tumor dissemination through multiple mechanisms and the precise pathways coupling TF to malignancy remain to be defined
The tandem importance of tumor cell–associated TF and circulating coagulation system components in malignancy is consistent with the hypothesis that TF supports metastasis by providing cancer cells a means of directing proteolytic events leading to local thrombin generation and the formation of tumor cell–associated microthrombi. However, an intriguing alternate possibility is that TF supports tumor cell dissemination by mechanism(s) uncoupled from “traditional” thrombin generation and subsequent thrombus formation. In this regard, significant attention has focused on potential intracellular signaling events coupled to the cytoplasmic portion of TF. This interest was driven in part by early studies indicating that tumor cells expressing a mutant form of TF lacking the cytoplasmic domain were far less metastatic than tumor cells expressing full-length TF.11,12,14 However, interpretation of these early studies was made more complex by the use of nonmurine tumor lines in xenograft assays in mice, the use of tumor cells expressing human TF or human TF derivatives in a setting where all other factors were of murine origin, and the requisite use of immunocompromised mice. Nevertheless, many studies have provided provocative evidence for an important linkage between TF-mediated signaling events and several key cellular processes capable of influencing metastasis, including cytoskeletal organization,27 cell adhesion/migration,28,–30 apoptosis,31,32 and angiogenesis.33,34 An important role for the TF cytoplasmic domain in cellular signaling is also supported by more recent studies of transgenic mice constitutively expressing a TF derivative lacking the cytoplasmic tail (TFΔCT mice).35,36 These animals display normal developmental and reproductive success,35,36 indicating that signaling events dependent on the TF cytoplasmic domain are not required for animal viability. However, TFΔCT mice appear to exhibit an exuberant angiogenic response in the context of several experimental challenges, including tumor angiogenesis.1 Detailed analyses of TFΔCT mice suggest that altered angiogenesis observed in these animals is caused by loss of intracellular TF-mediated events coupled to the regulation of PAR-2 signaling in endothelial cells.1
Taken together, the available data suggest that tumor cell-associated TF supports tumor dissemination through mechanisms linked to TF-mediated thrombin generation, TF-mediated intracellular signaling, or both. To better define the relationship between tumor cell-associated TF and circulating hemostatic factors in establishing metastatic potential, we used TF−/− embryonic fibroblasts to generate a set of C57Bl/6-derived fibrosarcoma cell lines that were genetically incapable of TF expression (TFO), and derivative lines that expressed either wild-type murine tissue factor (TFWT), or a truncation mutant of murine TF lacking the cytoplasmic domain (TFΔTail). Detailed studies of the growth and metastasis of these cells in immunocompetent C57Bl/6 mice with and without genetic deficits in prothrombin, fibrinogen, and platelet function revealed that tumor cell–derived TF was not essential for primary tumor growth or angiogenesis. However, TF expression was critical for tumor cell metastasis in a manner dependent on all 3 circulating hemostatic factors tested, but entirely independent of the TF cytoplasmic domain. Furthermore, these studies reveal that TF and soluble hemostatic factors cooperate to support the metastatic potential of circulating tumor cells through a combination of NK cell–dependent and NK cell–independent mechanisms.
Materials and methods
Transgenic mice
Gene-targeted mice deficient in tissue factor,37 Gαq,38 fibrinogen,39 and NK cell function40,41 were described previously and genotyped using established polymerase chain reaction (PCR) protocols. Transgenic mice expressing low levels (≈7% of normal) of prothrombin (referred to as fIILow mice) were originally established by introducing a human fII transgene into mice homozygous for a prothrombin-null allele (ie, fII−/−/hfIITg+ mice).42 All mice were backcrossed 6 to 7 generations into a C57BL/6 background except Gαq-deficient mice, which were backcrossed 4 generations. Cohorts of Gαq-deficient and fIILow mice were paired with wild-type littermate controls. Fibrinogen-deficient (Fib−/−) mice were paired with Fib+ /− littermate controls. The study protocols were approved by the Cincinnati Children's Hospital Research Foundation Institutional Animal Care and Use Committee in accordance with the guidelines of the National Institutes of Health.
Generation of TF-deficient tumor cells
Embryonic fibroblast cultures were prepared from an E10.5 TF−/− embryo harvested from a C57Bl/6-inbred TF+ /− female crossed with a C57Bl/6-inbred TF+ /− male. The cultures were repeatedly passaged at low density until the cells became senescent. After several additional weeks in culture, proliferative colonies that had escaped senescence emerged that were then cloned and confirmed to be TF−/− by PCR. An immortalized isolate was transfected with a previously described Ha-Ras expression vector [pDCR-Ha-Ras(G12V)] 43 using FuGene 6 transfection reagent (Roche Diagnostics, Basel, Switzerland) and stable transfectants selected with 800 μg/mL G418 (Sigma, St Louis, MO). Twenty morphologically transformed clones were collected and screened for tumorigenicity by subcutaneous injection in immunocompetent C57Bl/6 mice, 6 of which formed rapidly growing tumors. One of these TF−/− tumor cell isolates (hereafter referred to as Ha-Ras+ /TF−/−) was selected as a parental clone for subsequent genetic manipulation of TF expression and tumor biology studies.
PCR amplification and cloning of murine TF cDNA
A cDNA carrying the entire murine tissue factor coding sequence was generated by PCR amplification using a mouse cDNA library as a source of template and the TF forward primer 5′-AGCCCTTGGACATGGCGATCCTCGTG-3′ and the reverse primer 5′-TTAGCGCTTCAGCCTTTCCTCTATGCCAAG-3′ corresponding to the nucleotides 96 to 121 and 981 to 1010, respectively, in the cDNA numbering system of Hartzell et al44 and molecularly cloned into the mammalian expression vector pCDNA3.1 + /Hygro (Invitrogen, San Diego, CA; Figure 1). A derivative of this expression vector was generated by PCR-based mutagenesis encoding a truncated form of murine TF lacking the majority of the cytoplasmic tail. The encoded mutant protein retained the full transmembrane domain and 2 amino acids of the cytoplasmic domain (Cys275 and Lys276 in the Hartzell et al44 number system) to support membrane anchoring. The sequence of the mutagenic reverse primer used was 5′-GGCTCGAGCTACTTGCACAGAGATATGGACAGGAGGATGAT-3′, where the nucleotide substitutions resulting in a stop codon are shown in bold and a simultaneously introduced XhoI site is shown in italics. The nucleotide sequences of both cDNAs were confirmed by direct sequence analysis (data not shown; see Figure 1 for additional details).
Generation and characterization of novel C57BL/6-derived TF cell lines. (A) Schematic representation of the wild-type TF expression vector used to generate TFWT cells. The TF expression vector was generated by cloning a PCR-amplified murine TF cDNA into the multiple cloning site of the plasmid pcDNA3.1 + /Hygro (Invitrogen) containing both a CMV promoter and a polyadenylation signal derived from the bovine growth hormone gene (BGH pA). P1 and P2 indicate the relative positions of primer sequences used to generate a TF cDNA cassette encoding a truncated form of the protein lacking the cytoplasmic tail (TFΔTail). (B) The mutagenic P2 primer changed the CGC codon at position 934-936 in the Hartzell et al44 numbering system to a termination codon (TAG) and introduced a downstream XhoI site to assist in molecular cloning (nucleotide substitutions denoted by *). The PCR product generated by these primers was digested with PpuMI and XhoI and ligated into a PpuMI/XhoI cut pcDNA3.1 + /Hygro/TF vector. (C) Diagnostic fluorescence-activated cell sorting analysis of murine TF expression on the surface of tumor cell lines using the ICON recombinant fusion protein containing murine fVII linked to the Fc region of IgG and a phycoerythin-conjugated Fc antibody. Note that a comparable pattern of TF expression was observed in TFWT (green) and TFΔTail (red) cell populations based on fluorescence distribution and mean fluorescence intensity (59 and 61 arbitrary fluorescence units, respectively). Control analyses shown for comparison are TFO cells processed in parallel for fluorescence-activated cell sorting analysis using ICON protein and phycoerythin-conjugated Fc antibody (gray) and TFWT cells processed using the phycoerythin-conjugated Fc antibody, but in the absence of ICON protein (blue).
Generation and characterization of novel C57BL/6-derived TF cell lines. (A) Schematic representation of the wild-type TF expression vector used to generate TFWT cells. The TF expression vector was generated by cloning a PCR-amplified murine TF cDNA into the multiple cloning site of the plasmid pcDNA3.1 + /Hygro (Invitrogen) containing both a CMV promoter and a polyadenylation signal derived from the bovine growth hormone gene (BGH pA). P1 and P2 indicate the relative positions of primer sequences used to generate a TF cDNA cassette encoding a truncated form of the protein lacking the cytoplasmic tail (TFΔTail). (B) The mutagenic P2 primer changed the CGC codon at position 934-936 in the Hartzell et al44 numbering system to a termination codon (TAG) and introduced a downstream XhoI site to assist in molecular cloning (nucleotide substitutions denoted by *). The PCR product generated by these primers was digested with PpuMI and XhoI and ligated into a PpuMI/XhoI cut pcDNA3.1 + /Hygro/TF vector. (C) Diagnostic fluorescence-activated cell sorting analysis of murine TF expression on the surface of tumor cell lines using the ICON recombinant fusion protein containing murine fVII linked to the Fc region of IgG and a phycoerythin-conjugated Fc antibody. Note that a comparable pattern of TF expression was observed in TFWT (green) and TFΔTail (red) cell populations based on fluorescence distribution and mean fluorescence intensity (59 and 61 arbitrary fluorescence units, respectively). Control analyses shown for comparison are TFO cells processed in parallel for fluorescence-activated cell sorting analysis using ICON protein and phycoerythin-conjugated Fc antibody (gray) and TFWT cells processed using the phycoerythin-conjugated Fc antibody, but in the absence of ICON protein (blue).
Cell transfection and fluorescence-activated cell sorting
Ha-Ras+ /TF−/− cells were transfected with the empty mammalian expression vector pCDNA3.1 + /Hygro (Invitrogen) or derivatives encoding either full-length murine TF or the truncation mutant lacking the cytoplasmic domain using FuGene 6 transfection reagent. Stable transfectants were selected with 200 μg/mL hygromycin B (Atlantic Biologicals, Lawrenceville, GA). Transfectants with restored TF expression were selected for cell populations with similar TF expression levels by fluorescence-activated cell sorting separation using a recombinant fusion protein comprising murine fVII joined to the Fc portion of human IgG (previously termed “ICON”45 ), and a phycoerythrin-conjugated goat antihuman Fc antibody (Biomeda, Foster City, CA) as previously described.45
Tumor cell inoculation into mice and histologic evaluation
Tumor cells were grown in complete Dulbecco modified Eagle medium (DMEM) with Hygromycin B. For in vivo experiments, the cells were harvested by brief exposure to trypsin/EDTA, washed, and resuspended in ice-cold phosphate-buffered saline. Experimental metastasis assays and subcutaneous tumor growth studies were performed as previously described.22,23,26 For histologic analyses, fixed tumor tissue was embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H and E). Vessel immunostaining was performed using a rat antimouse CD31 monoclonal antibody (clone #MEC 13.1; BD Pharmigen, San Diego, CA), a biotinylated rabbit antirat (mouse absorbed) polyclonal antibody (Vector Laboratories, Burlingame, CA), and the Vector Elite ABC kit (Vector Laboratories).
Fate analyses of tumor cells and immunologic depletion of NK cells
For analyses of tumor cell fate, tumor cells were labeled with 0.5 μCi/mL of [125I] -iododeoxyuridine (Amersham, Piscataway, NJ) (2000 Ci/mmol) as previously described.22,26 The cells were harvested and intravenously injected into mice as described. Organs were harvested at specified time points and washed in 70% ethanol for 3 days to remove free isotope. Radiolabel levels in the input inoculum and mouse organs were measured with a Cobra gamma counter (Packard, Meridien, CT). The inoculi were routinely tested for the percentage of radiolabel incorporated into macromolecules by TCA precipitation and consistently found to be 99% or more (data not shown). NK cells were immunologically depleted using a rabbit, antimouse asialo GM1 polyclonal antibody (Cedarlane, Burlington, NC) following an established protocol.26 Control mice were treated with an equivalent amount of nonimmune rabbit IgG (Cedarlane).
Results
Tumor cell-associated tissue factor expression is a crucial determinant of metastatic potential
To better define the relationship between tumor cell–associated TF and circulating hemostatic system components in determining metastatic potential, we generated novel Ha-Ras–transformed fibrosarcoma cells from C57Bl/6-derived TF−/− embryonic fibroblasts (see Methods for details). These cells were transfected with expression vectors encoding wild-type murine tissue factor (TFWT), a truncated murine TF lacking the cytoplasmic domain (TFΔTail), or empty vector (TFO) (Figure 1 and “Materials and methods”). Transfectants were not recloned, but the TF-expressing cells (TFWT and TFΔTail) were sorted by fluorescence-activated cell sorting for cell populations with comparable TF expression using a protein conjugate of murine fVII and the Fc portion of human IgG (see “Materials and methods”).45 Diagnostic fluorescence-activated cell sorting analyses of TFWT and TFΔTail cells revealed that these cells maintained high and comparable levels of TF expression even after extended periods in culture. As expected, TFO cells exhibited no detectable TF expression (Figure 1C).
The fact that the TFWT, TFΔTail, and TFO tumor cells were C57Bl/6-derived permitted a direct analysis of the contribution of TF to tumor growth and metastatic potential in immunocompetent C57Bl/6 mice. To determine the importance of tumor cell-associated TF expression as well as the specific role of TF cytoplasmic domain-mediated signaling events in tumor growth, we inoculated the dorsal subcutis of histocompatible C57Bl/6 mice with TFWT, TFΔTail, or TFO tumor cells. All mice developed palpable tumors within 5 days of injection with 100% penetrance and these tumors grew at similar rates based on serial calipation over an 18-day observation period, regardless of tumor cell genotype. (data not shown). Tumor mass measured at the time of euthanization was indistinguishable in mice challenged with TFWT, TFΔTail, and TFO tumor cells in 3 independent experiments (Figure 2A). Primary tumors (n = 4) established with TFWT, TFΔTail, and TFO tumor cells were arbitrarily selected for microscopic analyses and found to be histologically indistinguishable (Figure 2C,2D show representative views). The tumors were densely packed with anaplastic-appearing cells with occasional small areas of necrosis. Tumor vasculature was analyzed by immunohistochemical staining of tumor sections using anti-CD31 antibodies. Consistent with the comparable growth rates of TFWT, TFΔTail, and TFO cells, tumor genotype had no appreciable qualitative affect on tumor vasculature (Figure 2E,2F, and data not shown) or vessel density (Figure 2B).
Tumor cell-associated TF expression is not required for tumor growth or tumor angiogenesis. (A) Weight of tumors formed 18 days after injection of 5 × 105 TFWT, TFΔTail, or TFO tumor cells into the dorsal subcutis of wild-type C57Bl/6 mice (n = 9 for each tumor cell genotype). Note that there is no significant difference in tumor mass, regardless of the presence, absence, or form of tumor cell–associated TF. (B) Comparative analysis of vascular density in subcutaneous tumors harvested 18 days after injection of TFWT, TFΔTail, or TFO tumor cells. Tumor tissue sections (n = 3-4 per tumor cell genotype) were stained with an anti-CD31 antibody and vessels were enumerated within 10 200× fields. Note that no significant difference in vessel density was observed in any pair-wise comparison of cohorts. The error bars in (A) and (B) indicate the SEM. (C-F) Microscopic appearance of tumor sections prepared from primary tumors established with TFWT, TFΔTail, and TFO tumor cells. Representative TFWT (C and E) and TFO (D and F) tumors sections processed for hematoxylin/eosin staining (C and D) or processed for immunohistochemical detection of the endothelial cell marker, CD31 (brown precipitate) (E and F). The scale bars indicate 50 μm (C and D) or 100 μm (E and F).
Tumor cell-associated TF expression is not required for tumor growth or tumor angiogenesis. (A) Weight of tumors formed 18 days after injection of 5 × 105 TFWT, TFΔTail, or TFO tumor cells into the dorsal subcutis of wild-type C57Bl/6 mice (n = 9 for each tumor cell genotype). Note that there is no significant difference in tumor mass, regardless of the presence, absence, or form of tumor cell–associated TF. (B) Comparative analysis of vascular density in subcutaneous tumors harvested 18 days after injection of TFWT, TFΔTail, or TFO tumor cells. Tumor tissue sections (n = 3-4 per tumor cell genotype) were stained with an anti-CD31 antibody and vessels were enumerated within 10 200× fields. Note that no significant difference in vessel density was observed in any pair-wise comparison of cohorts. The error bars in (A) and (B) indicate the SEM. (C-F) Microscopic appearance of tumor sections prepared from primary tumors established with TFWT, TFΔTail, and TFO tumor cells. Representative TFWT (C and E) and TFO (D and F) tumors sections processed for hematoxylin/eosin staining (C and D) or processed for immunohistochemical detection of the endothelial cell marker, CD31 (brown precipitate) (E and F). The scale bars indicate 50 μm (C and D) or 100 μm (E and F).
The tumor cells generated in these studies were found to have little or no capacity for spontaneous metastasis to the lung or other organs from primary cutaneous tumors in excess of 500 mg. However, based on earlier studies of circulating hemostatic factors and metastasis, our primary interest was to analyze the impact of tumor cell-associated TF on metastasis in later phases, after tumor cells have gained access to the circulation. To definitively establish the importance of tumor cell-associated TF in experimental metastasis assays, single cell suspensions of TFWT, TFΔTail, and TFO tumor cells were each intravenously injected into histocompatible and immunocompetent (C57Bl/6) wild-type mice and pulmonary metastasis evaluated after 21 days. Mice injected with TFWT cells consistently had large numbers of metastatic foci within each lung lobe, whereas mice injected with TFO cells rarely exhibited even one pulmonary metastasis (Figure 3). A similar near absence of pulmonary metastases was observed in wild-type mice injected with the Ha-Ras+ /TF−/− cells that were the parental line used to generate the TFWT, TFΔTail, and TFO tumor lines (data not shown). Overt metastases were never observed in other organs (eg, liver, spleen and kidney) regardless of TF status. The phenomenal increase in metastatic potential conferred by TF was not coupled to intracellular signaling events dependent on the TF cytoplasmic domain. Cells expressing TF lacking the cytoplasmic domain (TFΔTail) exhibited the same robust metastatic potential as TFWT cells (Figure 3). Furthermore, the metastatic foci formed by both TFWT and TFΔTail cells were qualitatively similar in both size and appearance. The rare metastases observed in lungs harvested from mice injected with TFO cells were also qualitatively similar to those formed by TF-expressing cells, suggesting that tumor cell–associated TF was more important in the initial establishment of metastases rather than the subsequent growth of tumor foci. This view is consistent with the indistinguishable growth observed in cutaneous tumors established with TFWT, TFΔTail, and TFO tumor cells. These experiments were repeated 3 times with similar outcomes.
TF is a crucial determinant of metastatic potential but not the TF cytoplasmic domain. (A) Quantitative analysis of experimental pulmonary metastases formed 21 days after intravenous injection of 4 × 105 TFWT, TFΔTail, or TFO tumor cells into immunocompetent C57Bl/6 mice. The lungs were harvested and fixed in Bouin's solution to better highlight metastatic foci. Each point indicates the total number of pulmonary foci present on all lung lobes from an individual mouse and the horizontal bars represent median values within each cohort. Note that tumor cells carrying TF exhibited markedly greater metastatic potential than TFO cells (*P < .001, Mann-Whitney U test), but the elimination of the TF cytoplasmic domain had no significant affect on metastasis. (B) Typical appearance of metastatic foci present on individual lung lobes 21 days after intravenous injection of 4 × 105 cells. Note that the metastatic foci formed by TFΔTail cells were indistinguishable from TFWT foci. However, lungs harvested from mice injected with TFO cells were effectively clear of metastases.
TF is a crucial determinant of metastatic potential but not the TF cytoplasmic domain. (A) Quantitative analysis of experimental pulmonary metastases formed 21 days after intravenous injection of 4 × 105 TFWT, TFΔTail, or TFO tumor cells into immunocompetent C57Bl/6 mice. The lungs were harvested and fixed in Bouin's solution to better highlight metastatic foci. Each point indicates the total number of pulmonary foci present on all lung lobes from an individual mouse and the horizontal bars represent median values within each cohort. Note that tumor cells carrying TF exhibited markedly greater metastatic potential than TFO cells (*P < .001, Mann-Whitney U test), but the elimination of the TF cytoplasmic domain had no significant affect on metastasis. (B) Typical appearance of metastatic foci present on individual lung lobes 21 days after intravenous injection of 4 × 105 cells. Note that the metastatic foci formed by TFΔTail cells were indistinguishable from TFWT foci. However, lungs harvested from mice injected with TFO cells were effectively clear of metastases.
Tumor cell–associated tissue factor supports metastasis via interplay with circulating hemostatic system components
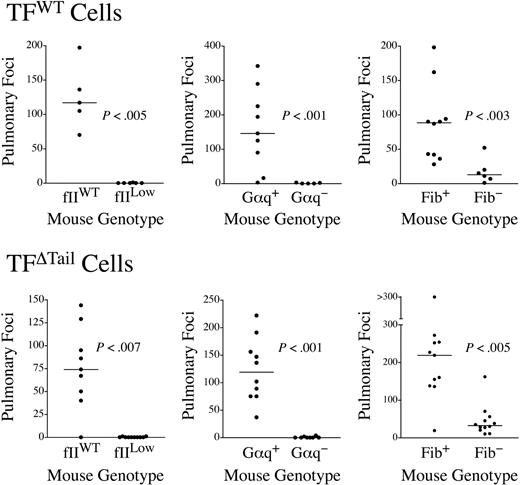
The extracellular domain of tumor cell–associated TF could support metastasis by several mechanisms apart from its traditional role in regulating the coagulation cascade, including TF/fVIIa/Xa-mediated activation of PARs 1 and 246,47 and TF/fVIIa interactions with matrix-associated TFPI-1.48 To determine whether the increase in metastatic potential conferred by TF expression is mechanistically coupled to distal hemostatic system components, we used previously described mice with a severe defect in platelet function secondary to genetic elimination of the G protein, Gαq,38 fibrinogen-deficient (Fib−) mice39 and mice with a severe quantitative deficit in prothrombin (fIILow).42 Defects in each of these circulating hemostatic system components significantly diminished the metastatic potential of TFWT cells (Figure 4). In fact, the majority of lungs harvested from fIILow mice challenged with TF-expressing tumor cells developed no pulmonary metastases, effectively phenocopying our results with TFO tumor cells in wild-type mice (Figure 4). Therefore, the metastatic phenotype conferred by tumor cell-associated TF appears to depend on distal hemostatic factors, including prothrombin.
The metastatic potential of TF-expressing tumor cells is strongly dependent on circulating hemostatic system components. Mice with genetic deficits in prothrombin (fIILow), platelet function (Gαq−), or fibrinogen (Fib−) were intravenously injected along with appropriate littermate controls with either 4 × 105 TFWT or TFΔTail cells and pulmonary foci enumerated 21 days later. The data shown represent the total number of surface pulmonary foci for each mouse injected. The horizontal bars represent median values. All P values were generated using the Mann Whitney U test.
The metastatic potential of TF-expressing tumor cells is strongly dependent on circulating hemostatic system components. Mice with genetic deficits in prothrombin (fIILow), platelet function (Gαq−), or fibrinogen (Fib−) were intravenously injected along with appropriate littermate controls with either 4 × 105 TFWT or TFΔTail cells and pulmonary foci enumerated 21 days later. The data shown represent the total number of surface pulmonary foci for each mouse injected. The horizontal bars represent median values. All P values were generated using the Mann Whitney U test.
Previous studies have suggested that the TF cytoplasmic domain interacts with components of the cytoskeleton,27 and these events could, in turn, influence TF localization in the cell membrane,49 interactions with other cellular ligands, and signal transduction events. The TF cytoplasmic domain could also be a determinant of extracellular proteolysis in vivo, including TF/fVIIa-mediated PAR-2 activation1 and TF/VIIa/Xa-mediated proteolysis leading to thrombin generation. To firmly establish whether the high metastatic potential conferred by tumor cell-associated TF lacking the cytoplasmic domain was dependent on distal coagulation factors, parallel experiments were performed with the TFΔTail tumor cells. Like TFWT tumor cells, the metastatic potential of TFΔTail tumor cells was dramatically diminished in fIILow, Gαq−, and Fib− mice relative to control animals (Figure 4). Thus, TF-driven metastasis is coupled to distal hemostatic factors regardless of the presence or absence of the cytoplasmic domain.
Although fewer in number, the metastatic lesions observed in mice with defects in circulating hemostatic system components were comparable in size to those observed in control mice. To more formally examine the role of prothrombin in the growth of established tumors, TFWT cells were subcutaneously injected into 7 fIILow and 6 wild-type mice in parallel. Palpable tumors were evident in both genotypes within 5 days of inoculation and grew at similar rates based on calipation (data not shown). Tumors harvested 18 days after inoculation from control mice reached an average mass of 557.8 mg (range, 115.3 mg-835.1 mg) and tumors harvested from fIILow mice reached an average mass of 672 mg (range, 410.4 mg-917.8 mg) (not significantly different, P < .6 with Mann Whitney U test). This experiment was repeated twice with similar results. Similarly, neither the loss of platelet function (ie, Gαq deficiency) nor fibrin(ogen) had any affect on the growth rate of TF-expressing tumor cells grown in the dorsal subcutis, consistent with previous reports23,26 (data not shown).
Tumor cell–associated TF supports metastasis by a mechanism linked to NK cell function as well as an NK cell–independent mechanism
We have previously shown that distal hemostatic system components (ie, platelets and fibrinogen) individually support metastatic potential by impairing tumor cell clearance by NK cells.26 While TF may influence tumor biology through multiple mechanisms, our leading hypothesis was that tumor cell–associated TF also supports metastasis by providing functional protection from NK cell-mediated lysis. To test this theory, we used previously described Gz-Ly49A transgenic mice known to have a severe defect in NK cell function40,41 (referred to here as NK− mice). These animals were crossed with fibrinogen-deficient mice and cohorts of the following 4 genotypes were intravenously injected with TFWT cells: Fib+ /NK+, Fib−/NK+, Fib+/NK−, and Fib−/NK− mice. Additional cohorts of Fib+/NK+ and Fib+/NK− mice were injected in parallel with TFO tumor cells. Surface pulmonary metastases were enumerated 21 days after injection. Consistent with previous findings,26 fibrinogen deficiency significantly diminished the metastatic potential of TFWT tumor cells in mice with intact NK cell function, but fibrinogen ceased to be a determinant of the metastatic potential of these cells in mice with a genetically imposed deficit in NK cell function (Figure 5A). Similar analyses of the metastatic potential of TFΔTail cells in mice with single and combined deficits in fibrinogen and NK cells mirrored the findings with TFWT cells (data not shown), indicating that loss of NK cell function did not reveal a contribution of the TF cytoplasmic domain to metastasis. A markedly different experimental outcome was observed in comparative analyses of TFWT and TFO cells in mice with and without NK cell function. Here, TFO tumor cells followed the anticipated pattern of being far less metastatic than TFWT tumor cells in mice with intact NK function, but TF remained an important determinant of metastatic potential even in the context where NK cell function was genetically removed (Figure 5B). These experiments were repeated twice with similar results. Taken together, these results indicate that tumor cell-associated TF provides tumor cells a fibrinogen-dependent means of escaping NK cell lysis. However, TF expression supports metastasis by at least one additional mechanism independent of NK cell function.
Hemostatic factors support metastasis through both an NK cell-dependent mechanism and at least one additional NK cell-independent mechanism. (A) Quantitative analyses of surface pulmonary foci formed 21 days after intravenous injection of 2 × 105 TFWT cells into mice with single and combined genetic deficits in fibrinogen (Fib−) and natural killer cells (NK−). Note that fibrinogen is a significant determinant of metastatic potential in mice with NK cell function, but not in mice lacking NK function. (B) Pulmonary foci in mice with and without NK cells challenged with either TFWT or TFO tumor cells. Note that tumor cell–associated TF remains a significant determinant of metastatic potential even in mice lacking NK cell function. (C) Quantitative analysis of [125I] -radiolabeled TFWT (black bars) or TFO (white bars) tumor cells in the lungs of control (n = 8 for TFWT and TFO), fIILow (n = 4), and Fib− mice (n = 7) 30 minutes after injection of 1 × 105 cells. Note that the majority of tumor cells injected become localized to the lung soon after injection regardless of the presence or absence of tumor cell–associated TF or circulating prothrombin and fibrinogen. (D) Residual [125I] -radiolabeled TFWT cells present in the lungs of mice with single and combined genetic deficits in NK cells and fibrinogen 24 hours after initial tumor cell injection. Note that fibrinogen is a significant determinant of early TFWT cell survival in the lungs of mice with NK cells (Fib+, n = 9; Fib−, n = 6), but fibrinogen is not a determinant of early tumor cell survival in mice with a genetic defect in NK cell function (Fib+, n = 11; Fib−, n = 7). (E) Comparative analysis of the early survival (24 hours after inoculation) of [125I] -radiolabeled TFWT (black bars) and TFO cells (white bars) in the lungs of mice with NK cells (TFWT, n = 9; TFO, n = 6) and without NK cells (TFWT, n = 11; TFO, n = 6). Note that TF was an important determinant of residual tumor cell survival in the lungs of mice with and without NK cell function. All P values were generated with the Mann Whitney U test. The horizontal bars in (A) and (B) indicate median values. The error bars in (C) through (E) indicate SEM.
Hemostatic factors support metastasis through both an NK cell-dependent mechanism and at least one additional NK cell-independent mechanism. (A) Quantitative analyses of surface pulmonary foci formed 21 days after intravenous injection of 2 × 105 TFWT cells into mice with single and combined genetic deficits in fibrinogen (Fib−) and natural killer cells (NK−). Note that fibrinogen is a significant determinant of metastatic potential in mice with NK cell function, but not in mice lacking NK function. (B) Pulmonary foci in mice with and without NK cells challenged with either TFWT or TFO tumor cells. Note that tumor cell–associated TF remains a significant determinant of metastatic potential even in mice lacking NK cell function. (C) Quantitative analysis of [125I] -radiolabeled TFWT (black bars) or TFO (white bars) tumor cells in the lungs of control (n = 8 for TFWT and TFO), fIILow (n = 4), and Fib− mice (n = 7) 30 minutes after injection of 1 × 105 cells. Note that the majority of tumor cells injected become localized to the lung soon after injection regardless of the presence or absence of tumor cell–associated TF or circulating prothrombin and fibrinogen. (D) Residual [125I] -radiolabeled TFWT cells present in the lungs of mice with single and combined genetic deficits in NK cells and fibrinogen 24 hours after initial tumor cell injection. Note that fibrinogen is a significant determinant of early TFWT cell survival in the lungs of mice with NK cells (Fib+, n = 9; Fib−, n = 6), but fibrinogen is not a determinant of early tumor cell survival in mice with a genetic defect in NK cell function (Fib+, n = 11; Fib−, n = 7). (E) Comparative analysis of the early survival (24 hours after inoculation) of [125I] -radiolabeled TFWT (black bars) and TFO cells (white bars) in the lungs of mice with NK cells (TFWT, n = 9; TFO, n = 6) and without NK cells (TFWT, n = 11; TFO, n = 6). Note that TF was an important determinant of residual tumor cell survival in the lungs of mice with and without NK cell function. All P values were generated with the Mann Whitney U test. The horizontal bars in (A) and (B) indicate median values. The error bars in (C) through (E) indicate SEM.
One relatively trivial mechanism that might account for a TF-dependent, but NK cell–independent, increase in metastasis is that tumor cell-associated TF alone, or in concert with circulating hemostatic factors, increases the initial localization of circulating tumor cells within the pulmonary vasculature. To explore this concept, we tracked the fate of [125I] -radiolabeled TFWT and TFO cells after intravenous injection into control, prothrombin-deficient, and fibrinogen-deficient mice (≈ 50 000 cpm/mouse). The animals were killed 30 minutes after inoculation and the lungs, heart, spleen, kidney, and a portion of liver and blood were harvested for analysis of residual radiolabel. Approximately 80%-90% of the initially injected tumor cells were present in the lungs at 30 minutes, regardless of tumor cell or mouse genotype (Figure 5C). Very little radiolabel (> 2%) was present in the blood, liver, heart, spleen, or kidney, regardless of tumor cell or mouse genotype (data not shown). Thus, neither tumor cell-associated TF nor distal hemostatic system components are significant factors in the initial localization/adhesion of circulating tumor cells within the pulmonary vasculature.
These tumor cell fate studies were extended to explore whether tumor cell–associated TF and/or fibrinogen supported the stable adhesion/survival of tumor cells in the lungs. [125I] -radiolabeled TFO or TFWT tumor cells were injected into cohorts of mice with single and combined deficits in fibrinogen and NK cells, and the residual radiolabeled tumor cells in the lungs were measured after 24 hours. As two complementary approaches to eliminate NK cells, we analyzed mice with a genetic defect in NK cells (NK−) and mice immunologically depleted of NK cells with an antiasialo GM1 antibody.26 A substantial fraction of the TFWT cells initially injected remained present in the lungs of control (NK-sufficient) mice at the 24-hour time point (≈ 25%), and this was diminished approximately 5-fold in animals lacking fibrinogen (Figures 5D,6A). The elimination of tumor cell–associated TF resulted in an even more dramatic reduction in early tumor cell survival. Here, less than 1% of the TFO cells that were initially present in the lungs remained present in control mice at the 24-hour time point, and essentially none was detectable in the other organs evaluated (Figures 5E,6C, and data not shown). The genetic or immunologic elimination of NK cell function improved the early survival of both TFWT and TFO cells, further indicating that these cells are NK cell sensitive in vivo. Consistent with our findings with macroscopically apparent pulmonary metastases 3 weeks after injection, fibrinogen was effectively neutralized as a determinant of the early survival of TFWT cells if NK cell function was genetically or immunologically eliminated (Figures 5D,6A). Consistent with previously published reports using other tumor cell lines,25,26 studies in Gαq-deficient mice showed that platelet function was also effectively neutralized as a major determinant of the early survival of TFWT cells if NK cells were depleted with an antiasialo GM1 antibody (Figure 6B). In contrast, early fate studies comparing radiolabeled TFO and TFWT cells showed that tumor cell-associated TF remained a significant determinant of early tumor cell survival, even in the absence of NK cells (Figures 5E,6C). These studies indicate that both the NK cell–dependent and NK cell–independent mechanisms coupling tumor cell–associated TF to metastatic potential act very early after the entrance of tumor cells into the circulation to alter the likelihood of tumor cell survival. To determine whether the NK cell–independent mechanism linking tumor cell–associated TF expression to early micrometastatic success is coupled to prothrombin, we compared the early survival of [125I] -radiolabeled TFWT cells in cohorts of control and fIILow mice immunologically depleted of NK cells. Genetic diminution of circulating prothrombin profoundly reduced 24-hour tumor cell survival in the lungs (Figure 6D). More significantly, like tumor cell–associated TF, prothrombin remained a significant determinant of tumor cell survival even in mice lacking NK cells. A strong inference from these findings is that the NK cell–independent mechanism whereby tumor cell procoagulant supports early micrometastatic success is at least partially dependent on circulating prothrombin and, presumably, thrombin generation.
Immunologic depletion of NK cells establishes NK cell–independent mechanism coupling both TF and prothrombin to metastatic success. (A) Comparative analysis of residual [125I] -radiolabeled TFWT cells present in the lungs of cohorts of fibrinogen deficient (Fib−) and control (Fib+) mice pretreated with either an anti-NK Ig (antiasialo GM1) known to deplete NK cells (n = 11 for each genotype) or nonimmune IgG (n = 12 for each genotype) harvested 24 hours after intravenous injection of 1 × 105 tumor cells. Note that immunologic depletion of NK cells, like genetic depletion of NK cells, eliminates fibrin(ogen) as a determinant of the early survival of TFWT cells. (B) Quantitative analysis of residual [125I] -radiolabeled TFWT cells (n = 5-6 for each group) in the lungs of Gαq+ and Gαq− mice. Note that platelet function, like fibrinogen, ceases to be a determinant of the early survival of TFWT cells in mice immunologically depleted of NK cells. (C) Comparative analysis of residual [125I] -radiolabeled TFWT (black bars) or TFO cells (white bars) cells (n = 6-7 for each group) in the lungs 24 hours after injection of 105 tumor cells. Note that tumor cell-associated TF remains a significant determinant of early tumor cell survival even in mice immunologically depleted of NK cells. (D) Quantitative analysis of residual [125I] -radiolabeled TFWT cells (n = 6-7 for each group) in the lungs of fIILow and control mice. Note that circulating prothrombin levels, like tumor cell TF expression, remain a significant determinant of early tumor cell survival even after the immunologic depletion of NK cells. All P values were generated with the Mann Whitney U test. The error bars represent SEM.
Immunologic depletion of NK cells establishes NK cell–independent mechanism coupling both TF and prothrombin to metastatic success. (A) Comparative analysis of residual [125I] -radiolabeled TFWT cells present in the lungs of cohorts of fibrinogen deficient (Fib−) and control (Fib+) mice pretreated with either an anti-NK Ig (antiasialo GM1) known to deplete NK cells (n = 11 for each genotype) or nonimmune IgG (n = 12 for each genotype) harvested 24 hours after intravenous injection of 1 × 105 tumor cells. Note that immunologic depletion of NK cells, like genetic depletion of NK cells, eliminates fibrin(ogen) as a determinant of the early survival of TFWT cells. (B) Quantitative analysis of residual [125I] -radiolabeled TFWT cells (n = 5-6 for each group) in the lungs of Gαq+ and Gαq− mice. Note that platelet function, like fibrinogen, ceases to be a determinant of the early survival of TFWT cells in mice immunologically depleted of NK cells. (C) Comparative analysis of residual [125I] -radiolabeled TFWT (black bars) or TFO cells (white bars) cells (n = 6-7 for each group) in the lungs 24 hours after injection of 105 tumor cells. Note that tumor cell-associated TF remains a significant determinant of early tumor cell survival even in mice immunologically depleted of NK cells. (D) Quantitative analysis of residual [125I] -radiolabeled TFWT cells (n = 6-7 for each group) in the lungs of fIILow and control mice. Note that circulating prothrombin levels, like tumor cell TF expression, remain a significant determinant of early tumor cell survival even after the immunologic depletion of NK cells. All P values were generated with the Mann Whitney U test. The error bars represent SEM.
Discussion
These studies demonstrate that tumor cell-associated TF is a crucial determinant of metastatic potential in immunocompetent animals carrying the full spectrum of circulating hemostatic factors. Tumor cells devoid of TF (TFO) were far less metastatic than TF-expressing cells when tested in standard C57Bl/6 mice. A simplifying factor in interpreting these findings was that a complete lack of tumor cell-associated TF was genetically assured in TFO tumor cells, regardless of the local or systemic regulatory factors encountered by the tumor cells in vivo. While TF was found to be a major determinant of metastatic success, tumor cell-associated TF was not essential to the formation of a supportive tumor stroma or primary tumor growth. More detailed comparative analyses of the metastatic potential of TFWT and TFΔTail tumor cells in mice with deficits in key coagulation proteins indicated that tumor cell TF cooperates with circulating hemostatic factors, including distal components of the coagulation cascade, to support metastatic disease. Furthermore, tumor cell–associated TF supports the early survival of micrometastases by a mechanism coupled to fibrinogen-dependent inhibition of NK cell function. However, TF was found to support pulmonary metastasis through at least one additional NK cell–independent mechanism that remains to be fully defined but is prothrombin-dependent, unrelated to initial tumor cell localization within the lungs, and affects early tumor cell survival.
In addition to the well-recognized role of TF in initiating thrombin generation, TF is thought to participate in intracellular signaling events through the TF cytoplasmic domain.1,,–4 The potential influence of the TF cytoplasmic domain on tumor biology has been of keen interest, and several previous studies reported that tumor cell xenografts expressing truncated forms of human TF lacking the cytoplasmic domain were less metastatic than cells expressing full-length TF.11,12,14 However, the present studies unambiguously showed that tumor cells expressing a truncated form of murine TF lacking the cytoplasmic domain retained the same tumor growth properties and metastatic potential as tumor cells expressing wild-type murine TF. These data show that any intracellular signaling events or extracellular proteolytic processes that depend on the presence of the TF cytoplasmic tail are dispensable for the malignant phenotype. Several experimental differences between earlier studies and the present work may account for these findings. Whereas other studies have used nonnative (eg, human or hamster) tumor cells expressing nonnative (ie, human) TF in xenograft assays with immunocompromised mice,11,12,14 the present studies focus on mouse tumor cells, mouse TF, and histocompatible mice. This raises the possibility that functional alterations imposed by pairing human TF with nonnative ligands (ie, mouse fVIIa and fX), nonnative protease substrates, derangements in the immune system, or both, might introduce an added significance to the cytoplasmic domain in supporting metastasis. An alternate possibility is that the precise site of TF cytoplasmic domain truncation may have had distinct functional consequences with regard to metastatic potential. The conserved membrane-proximal cysteine that is palmitoylated50 in wild-type TF (Cys275 in the murine TF numbering system used by Hartzell et al44 ) and the immediately adjacent Lys276 residue was retained in the TFΔTail tumor cells used here. The general functional integrity of this TF truncation mutant is underscored by both the functional capacity to engage recombinant murine fVII and the previously reported developmental success and normal hematologic profile of TFΔCT knock-in mice engineered with the same stop codon.35 Another factor that might account for the variable importance of the TF cytoplasmic domain in tumor metastasis is that signaling pathways or proteolytic properties linked to the TF cytoplasmic domain may be tumor cell–dependent. This scenario could be easily imagined based on any cellular differences in the repertoire of intracellular proteins that engage or phosphorylate TF. Finally, it is conceivable that the Ha-Ras transformation used to generate the tumor cells used in the current studies could create an experimental context where intracellular signaling events linked to the TF cytoplasmic domain are either effectively eliminated or uncoupled from the metastatic phenotype. Whatever the explanation, the present studies firmly establish that the TF cytoplasmic tail is not strictly required for TF-driven tumor metastasis.
A link between TF expression and tumor angiogenesis has been suggested by several studies.15,33,51,52 The comparable growth and vasculature observed here in tumors established with TFO, TFΔTail and TFWT tumor cells indicates that tumor cell–associated TF is dispensable for tumor stroma formation and tumor cell proliferation in vivo.12,53 However, it should be emphasized that these studies do not exclude an important role for TF expression on nonmalignant, stromal cells in tumor growth and angiogenesis. The concept that TF expression by stromal cells may be relevant to tumor angiogenesis is supported by recent studies of knock-in mice expressing a truncated form of TF.1 Loss of the TF cytoplasmic tail in nontumor cells was shown to result in more rapid tumor growth and increased tumor angiogenesis.1 Selective elimination of TF from tumor cells, endothelial cells, inflammatory cells, and other supporting cells may better establish the importance of nontumor cell TF in tumor angiogenesis and growth. Furthermore, complementary analyses using tumor cells capable of spontaneous metastasis could reveal novel roles for TF expression in earlier stages of the metastatic process, which cannot be defined using experimental metastasis assays.
One inference of our comparative studies of TFO, TFWT, and TFΔTail cells is that the extracellular domain of TF constitutes the primary determinant of metastatic potential. The general importance of the extracellular domain further implies that the interaction of TF with its protease ligands fVIIa and fX represents a central facet of the malignant phenotype. This concept is supported by previous findings that either blocking TF interaction with fVIIa13,–15 and/or inhibition of fXa activity54 diminishes metastatic potential. However, multiple potential mechanisms/pathways can be envisioned whereby tumor cell–associated TF/fVIIa/fXa supports metastasis. First, based on the capacity of TF/fVIIa/fXa to activate PAR-1 and PAR-2,46,47 metastatic potential may be controlled by thrombin-independent PAR signaling pathways. Second, given that TF/fVIIa can support cell adhesion to matrix-immobilized TFPI-1,48 TF/fVIIa may support adhesion or migration through a thrombin-independent mechanism. Finally, tumor cell-associated TF/fVIIa/fXa may support metastasis through local thrombin generation and thrombin-mediated proteolysis, including platelet and endothelial PAR activation and fibrin deposition.22,–24,26 Our studies showing that tumor cell metastasis is exquisitely dependent on the combined availability of tumor cell–associated TF and circulating prothrombin suggest that any contribution of TF to metastasis outside of thrombin generation is either small or superseded by an overarching requirement for thrombin-mediated proteolysis. Given that genetic defects in key thrombin targets (ie, platelets and fibrinogen) also significantly diminished the metastatic potential of TF-expressing cells, the simplest interpretation of all the available data is that TF directs metastatic potential in large part through thrombin generation and ultimately thrombin-mediated proteolysis. Of course, TF/fVIIa/fXa-mediated cleavage of substrates other than thrombin, including PAR-1 and PAR-2, may be of significant importance to metastatic potential in other experimental contexts. The level of PAR-1 and PAR-2 expression on the tumor cells used in the present studies is currently unknown. PARs expression could conceivably influence the relative importance of TF-mediated thrombin-dependent and thrombin-independent events capable of supporting metastasis. More detailed analyses using tumor cells with investigator-defined alterations in TF, PAR-1, and PAR-2 expression would be invaluable in further defining the role of TF-mediated PAR signaling in metastasis.
A previously recognized mechanism by which both fibrinogen and platelets contribute to metastatic success is by limiting NK cell-mediated clearance of tumor cell emboli.25,26 The present studies using tumor cells whose metastatic potential is acutely TF-dependent underscore that fibrin(ogen)-dependent and platelet-dependent evasion of NK cell-mediated clearance is one major advantage conferred to tumor cells by TF expression. However, detailed studies of TF-null and TF-expressing cells in mice with and without NK cells revealed the presence of at least one additional NK cell-independent mechanism whereby TF supports metastasis. One theory consistent with the available data is that TF-initiated thrombin generation and subsequent tumor cell-autonomous PAR signaling improves metastatic properties. In this regard, there are multiple compelling reports showing that tumor cells treated with thrombin or PAR-activating peptides in vitro before intravenous injection display increased metastatic potential.19,20,32 A viable alternative theory consistent with the individual importance of platelet PAR-424 and fibrinogen22,23 in supporting malignancy in mice is that thrombin activation of a combination of substrates (eg, fXI, fVIII, fV, fibrinogen, fXIII, and platelet-associated and endothelial cell–associated PARs) improves the mechanical stability of tumor cell emboli. Here, the combined activation of platelets, endothelial cells, and fibrin deposition might increase resistance to shear forces in the bloodstream that might either disrupt or dissociate tumor cells that initially succeeded in localizing to the pulmonary vasculature. Under this theory, loss of TF or circulating prothrombin would result in a fundamental reduction in tumor cell mechanical stability along with increased susceptibility to NK cell–mediated elimination. Real-time microscopic evaluation of individual tumor cell emboli and NK cells within the lung vasculature, together with studies of tumor cells with genetically defined alterations in TF and PARs, will be important to more fully appreciate the contribution and interplay of these cell–associated factors in determining tumor cell fate. However, it is clear that therapeutic strategies designed to impede tumor cell–associated thrombin generation could be highly effective in blocking the progression of metastatic disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Angela Drew and Dr Mingfu Zhou for their expert assistance with the immunohistochemistry.
This work was supported in part by grants HL074363 (J.S.P.) and HL71555 (J.L.D.) from the National Institutes of Health and the Swebilius Translational Cancer Research Award (Z.H.) from the C.G. Swebilius Foundation/Yale Cancer Center.
National Institutes of Health
Authorship
Contribution: J.S.P. designed the research, performed research, analyzed data, and wrote the manuscript. K.E.T, J.V.M, C.M.L, K.W.K, and K.A.B collected data, performed research, and were vital to the generation of gene-targeted mice. M.J.F. provided expert assistance. Z.H. provided a key reagent and valuable guidance. J.L.D. designed the research and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: J. L. Degen, Children's Hospital Research Foundation, Children's Hospital Medical Center, CHRF Room 2042, 3333 Burnet Ave., Cincinnati, OH 45229-3039; e-mail: degenjl@cchmc.org.




![Figure 5. Hemostatic factors support metastasis through both an NK cell-dependent mechanism and at least one additional NK cell-independent mechanism. (A) Quantitative analyses of surface pulmonary foci formed 21 days after intravenous injection of 2 × 105 TFWT cells into mice with single and combined genetic deficits in fibrinogen (Fib−) and natural killer cells (NK−). Note that fibrinogen is a significant determinant of metastatic potential in mice with NK cell function, but not in mice lacking NK function. (B) Pulmonary foci in mice with and without NK cells challenged with either TFWT or TFO tumor cells. Note that tumor cell–associated TF remains a significant determinant of metastatic potential even in mice lacking NK cell function. (C) Quantitative analysis of [125I] -radiolabeled TFWT (black bars) or TFO (white bars) tumor cells in the lungs of control (n = 8 for TFWT and TFO), fIILow (n = 4), and Fib− mice (n = 7) 30 minutes after injection of 1 × 105 cells. Note that the majority of tumor cells injected become localized to the lung soon after injection regardless of the presence or absence of tumor cell–associated TF or circulating prothrombin and fibrinogen. (D) Residual [125I] -radiolabeled TFWT cells present in the lungs of mice with single and combined genetic deficits in NK cells and fibrinogen 24 hours after initial tumor cell injection. Note that fibrinogen is a significant determinant of early TFWT cell survival in the lungs of mice with NK cells (Fib+, n = 9; Fib−, n = 6), but fibrinogen is not a determinant of early tumor cell survival in mice with a genetic defect in NK cell function (Fib+, n = 11; Fib−, n = 7). (E) Comparative analysis of the early survival (24 hours after inoculation) of [125I] -radiolabeled TFWT (black bars) and TFO cells (white bars) in the lungs of mice with NK cells (TFWT, n = 9; TFO, n = 6) and without NK cells (TFWT, n = 11; TFO, n = 6). Note that TF was an important determinant of residual tumor cell survival in the lungs of mice with and without NK cell function. All P values were generated with the Mann Whitney U test. The horizontal bars in (A) and (B) indicate median values. The error bars in (C) through (E) indicate SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/1/10.1182_blood-2007-01-065995/2/m_zh80130703260005.jpeg?Expires=1764983921&Signature=vXVT4y5Tm84Zxm5mcJz4tG9GU6zyo6SaKICkGZoLYOrVPcxeYOv~94myK1z9O0sPEb9I24PAtzR636NWcDHLWj46vgNuEt0qoKl0jeofo~XmLYo8GwAIi3PKu3Q790gUw~QzZj77HiC~bXR8y1BiE4fol91rTDIsk-gY12oEOySpgXJIPOR8twCyVWuWuuYuRftsxfH7NU62HyT8AdByd0vnwXOf2zhwvjLVAqg1n5a9kGflHBZGUZwcE9BUSujMHcCeb~JimouD8FQj5TB4wWtTqwQxZ81WCJurmGBadrpuX~1EgD6~q1vORH3hoBfTbYdMLCOT1K5aI5vs7HnYdg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Immunologic depletion of NK cells establishes NK cell–independent mechanism coupling both TF and prothrombin to metastatic success. (A) Comparative analysis of residual [125I] -radiolabeled TFWT cells present in the lungs of cohorts of fibrinogen deficient (Fib−) and control (Fib+) mice pretreated with either an anti-NK Ig (antiasialo GM1) known to deplete NK cells (n = 11 for each genotype) or nonimmune IgG (n = 12 for each genotype) harvested 24 hours after intravenous injection of 1 × 105 tumor cells. Note that immunologic depletion of NK cells, like genetic depletion of NK cells, eliminates fibrin(ogen) as a determinant of the early survival of TFWT cells. (B) Quantitative analysis of residual [125I] -radiolabeled TFWT cells (n = 5-6 for each group) in the lungs of Gαq+ and Gαq− mice. Note that platelet function, like fibrinogen, ceases to be a determinant of the early survival of TFWT cells in mice immunologically depleted of NK cells. (C) Comparative analysis of residual [125I] -radiolabeled TFWT (black bars) or TFO cells (white bars) cells (n = 6-7 for each group) in the lungs 24 hours after injection of 105 tumor cells. Note that tumor cell-associated TF remains a significant determinant of early tumor cell survival even in mice immunologically depleted of NK cells. (D) Quantitative analysis of residual [125I] -radiolabeled TFWT cells (n = 6-7 for each group) in the lungs of fIILow and control mice. Note that circulating prothrombin levels, like tumor cell TF expression, remain a significant determinant of early tumor cell survival even after the immunologic depletion of NK cells. All P values were generated with the Mann Whitney U test. The error bars represent SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/1/10.1182_blood-2007-01-065995/2/m_zh80130703260006.jpeg?Expires=1764983921&Signature=Pt9G37I8tcmu0Iu9Vk6NXNOGMiSSFV2c4001jiidrVDZnfJk5C9yQiMhtKuHWTfGA0SyO~2CTf6OQJHM1D8IIKEL1SI0gEtxVjP3fDJIHjq1mg~irvMUtUfolqB7p8cyL2pdDA0aEq9TCaaYa6~SpvU9DneAdiqfTHQEpow3bZPQcdGCilxekiG82XIaq~3-QIKwjJyGSrt4f7R5lOYrHoHQ168KCvoSImVM-QQ0qdUydLen8kEKthH7kImTNivhr81OlrVdvVVeH~VOoPpV~O7QiSSmxP1ExrKhWUbJW5~rnGGvOM9VTwE-9JVLdSUfsDvFEE1nCM-adwZLiqwdzQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal