Gab2 is an important adapter molecule for cytokine signaling. Despite its major role in signaling by receptors associated with hematopoiesis, the role of Gab2 in hematopoiesis has not been addressed. We report that despite normal numbers of peripheral blood cells, bone marrow cells, and c-Kit+Lin−Sca-1+ (KLS) cells, Gab2-deficient hematopoietic cells are deficient in cytokine responsiveness. Significant reductions in the number of colony-forming units in culture (CFU-C) in the presence of limiting cytokine concentrations were observed, and these defects could be completely corrected by retroviral complementation. In earlier hematopoiesis, Gab2-deficient KLS cells isolated in vitro responded poorly to hematopoietic growth factors, resulting in an up to 11-fold reduction in response to a cocktail of stem cell factor, flt3 ligand, and thrombopoietin. Gab2-deficient c-Kit+Lin− cells also demonstrate impaired activation of extracellular signal-regulated kinase (ERK) and S6 in response to IL-3, which supports defects in activating the phosphatidylinositol-3 kinase (PI-3K) and mitogen-associated protein kinase (MAPK) signaling cascades. Associated with the early defects in cytokine response, competitive transplantation of Gab2−/− bone marrow cells resulted in defective long-term multilineage repopulation. Therefore, we demonstrate that Gab2 adapter function is intrinsically required for hematopoietic cell response to early-acting cytokines, resulting in defective hematopoiesis in Gab2-deficient mice.
Introduction
One of the most prominent motifs in signaling molecules is the Src homology-2 (SH2) domain found in JAKs, signal transducers and activators of transcription (STATs), Grb2, p85, Shc, and others. These SH2 domains are able to bind and “dock” with the phosphorylated tyrosine residues that are common in signal transduction pathways. Multiple protein-binding motifs are present in many of the adapter molecules, leading to multimeric complexes that may also include CrkL, PLC, SHIP, and SHP-2. The Grb2-associated binding protein (Gab) family of adapter proteins (Gab1, Gab2, Gab3) include a family of scaffolding/docking/adapter molecules involved in multiple signaling pathways, including the phosphatidylinositol-3 kinase (PI-3K) and mitogen-associated protein kinase (MAPK) pathways, and include multiple protein-binding sites.1,–3 These proteins are tyrosine phosphorylated following cytokine stimulation and are able to interact with a large number of partners. The mechanisms that confer specificity in directing which interactions occur in any particular cell type upon cytokine stimulation remain to be determined. Gab1 deficiency results in embryonic lethality, and conditional deletion of Gab1 shows a role for Gab1 in promoting extracellular signal-regulated kinase (ERK) activation in hepatic function.4,5 Gab1 acts as an adapter protein to link gp130 signaling to the ERK pathway.6 In contrast, Gab3 knockout mice do not show major phenotypes.2
Gab2 is tyrosine phosphorylated by several early-acting cytokine receptors such as flt3, c-Kit, IL-3R, and c-Mpl, and contains proline-rich and pleckstrin homology (PH) domains that promote binding to signaling molecules.1,7,8 This cytokine activation profile is very similar to STAT5. Gab2 activates the PI-3K and MAPK pathways and can regulate hematopoietic cell migration functions.9 Gab proteins also contain a large number of consensus serine/threonine sequences, suggesting possible phosphorylation as a secondary mode of regulation, similar to STATs. Interestingly, phosphorylation of Gab2 on serine 623 by MAPK regulates its association with SHP-2 and results in decreased STAT5 activation.10
Gab2−/− mice are viable but lack allergic response,11 and it has been reported that their bone marrow (BM) is osteopetrotic due to decreased osteoclast differentiation via RANK-induced progenitor differentiation.12 Gab2 deficiency has also been shown to alter mast cell development13 in a manner similar to STAT5-deficient mice.14 In addition to a role in normal development, Gab2 is increasingly being described as associated with mammary cancer and hematologic malignancies. It is important for epidermal growth factor (EGF) signaling and breast cancer cell proliferation.15,16 Gab2 has also been described as a key intracellular intermediate for leukemic transformation mediated by BCR-ABL,17 and Gab2 plays an important role in the expansion of Friend virus-infected erythroid progenitor cells.18 Additional roles for Gab2 in leukemic PI-3K signaling are emerging. It is known that PI-3K activation is important for BCR-ABL–induced leukemias,19 and that both STAT520 and Gab217 play important roles. Furthermore, enhanced sensitivity of chronic myeloid leukemia (CML) to antiproliferative drugs can be achieved by combined inhibition of STAT5 and Gab2 expression.21
Given the important roles for Gab2 in normal and oncogenic cytokine signaling, we thus set out to define its role in hematopoiesis. Here, we report that Gab2−/− mouse BM has significant defects that are consistent with a major cell-intrinsic role in potentiating responses to early-acting cytokines.
Materials and methods
Mice
Gab2−/− mice were obtained from Toshio Hirano (Osaka University, Osaka, Japan). All mice used in the experiments were generated and maintained by heterozygote crosses and genotyping. Although Gab2−/− mice are fertile, this breeding strategy was found to be the most efficient with mice younger than 4 months of age and supplied littermate wild-type mice as controls. The C57BL/6 (CD45.2) mice and the congenic strains B6.SJL-PtprcaPep3b/BoyJ (CD45.1) were obtained from the Jackson Laboratory (Bar Harbor, ME) and housed in a specific pathogen-free environment. All mouse studies were approved by the institutional animal care and use committee at Case Western Reserve University.
Western blot analysis
Hematology and CFU assays
Peripheral blood was obtained from the retro-orbital sinus following puncture using microcapillary tubes. Smaller microcapillary tubes were spun in a stat-spin microcentrifuge for reading hematocrits manually. For white blood cell counts, cells were diluted in isotonic diluent and analyzed using a Coulter counter Z2 (Beckman Coulter, Fullerton, CA). BM was harvested from both hind limbs (tibias and femurs) of either Gab2−/− or littermate wild-type mice. BM cells were flushed into phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS; HyClone, Logan, UT), and total nucleated cell counts were performed with the use of a hemacytometer. BM cells were plated in methylcellulose medium (Stem Cell Technologies, Vancouver, BC, Canada) in the presence of growth factor. Growth factor combinations included the following cocktails: 10 ng/mL recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF) alone for assaying myeloid colonies, or a cocktail of 20 ng/mL murine IL-3, 50 ng/mL recombinant human IL-6, 50 ng/mL recombinant murine stem cell factor (SCF), and 3 U/mL recombinant human erythropoietin (EPO) for assaying total myeloid and erythroid colonies. On day 7 of culture, BM colonies of more than 50 cells were counted. All hematopoietic cytokines were from R&D Systems (Minneapolis, MN).
Flow cytometric analysis and cell sorting
Flow cytometry was used to enumerate hematopoietic subpopulations and to analyze signal transduction. For cell-sorting experiments of c-Kit+Lin−Sca-1+ (KLS) cell populations, BM cells were harvested in PBS/2% FBS (Hyclone). After erythrocyte lysis, cells were purified by lineage depletion using magnetically labeled lineage antibody microbeads and a magnet according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). Lineage-depleted cells were labeled with phycoerythrin (PE)-conjugated antibodies to lineage markers that included Ly-6G (Gr-1), CD11b (Mac-1), CD45R/B220, CD4 (L3T4), CD8 (Ly2), and Ter119/Ly76. The cells also were stained with antibodies to fluorescein isothiocyanate (FITC)-conjugated Ly-6A/E (Sca-1) and allophycocyanin (APC)-conjugated CD117 (c-Kit). Cells negative for lineage markers were gated, and Sca-1 and CD117 double-positive cells were sorted. Additional antibodies were used to define short-term hematopoietic stem cells (HSCs; KLS Flk2+), long-term HSCs (KLS Flk2neg), or additional progenitor populations as described.23,24
Intracellular flow cytometric analysis of phosphorylated signaling proteins
BM cells were harvested from femurs and tibias into IMDM containing 1% BSA (Sigma, St Louis, MO). Erythrocytes were lysed, and mononuclear cells were incubated in the harvesting medium at 37°C for 4 to 6 hours. The cells were stimulated with 10 ng/mL murine IL-3 (R&D Systems). For the in vitro inhibitor experiments, BM cells were harvested as described in “Hematology and CFU assays.” Cells were split, and those subject to inhibition were incubated for 1 hour with 50 μM LY294002 or 50 μM PD98059 (Cell Signaling Technology, Danvers, MA) prior to stimulation with 10 ng/mL IL-3 for the indicated times. Control samples were incubated with DMSO vehicle (0.1%). Following stimulation, cells were fixed with paraformaldehyde at a final concentration of 2% (16% ampules; Electron Microscopy Sciences, Hatfield, PA) at room temperature for 10 minutes. The cells were then washed twice with PBS, permeabilized with ice-cold 95% methanol while vortexing, placed on ice for 20 minutes, and then stored at −20°C at a minimum of overnight and up to 2 weeks. Upon removal from storage, the samples were washed 2 times with PBS and incubated for 1 hour in Hanks balanced salt solution containing 4% FBS (fluorescence-activated cell sorting [FACS] buffer; Hyclone). The cells were pelleted and then incubated with anti-CD16/CD32 antibody (eBiosciences, San Diego, CA) on ice for 15 minutes. Primary antibodies (phosphorylated [p] S6 and pERK; Cell Signaling Technology) were added at optimized concentrations in FACS buffer and incubated at room temperature for 30 minutes. Samples were washed once with FACS buffer. FITC-conjugated secondary Ab and pSTAT5-Alexa 647 (BD Biosciences) were added at optimized concentrations and incubated in the dark at room temperature for 30 minutes. Samples were washed once with PBS and analyzed on a Becton Dickinson LSR II (150 000 events per sample; Becton Dickinson, San Jose, CA). Data were collected using the DIVA software (Becton Dickinson) and analyzed using FlowJo (Tree Star, Ashland, OR).
In vitro cytokine growth response assay using sorted KLS cells
Sorted KLS cells (500 cells per well/5 wells per experiment) were put into liquid suspension culture in the presence of cytokine cocktails to stimulate proliferation for 6 days as previously described.25 The cytokine combinations included 50 ng/mL murine SCF, 50 ng/mL murine flt3 ligand (FL), 50 ng/mL murine thrombopoietin (TPO), 20 ng/mL murine IL-3, and 50 ng/mL human IL-6.
Retroviral complementation
BM was harvested from both hind limbs (tibias and femurs) of either Gab2−/− or littermate wild-type mice. BM cells were flushed into PBS containing 2% FBS (Hyclone) and counted using a hemacytometer. After erythrocyte lysis, cells were cultured in liquid suspension culture containing the cytokine combination of IL-3 (20 ng/mL), IL-6 (50 ng/mL), and SCF (50 ng/mL). After 2 days of culture, BM cells were collected and cocultured with irradiated (15 Gy [1500 rad], 137Cs source) producer cell lines in presence of polybrene (6 μg/mL). BM cells were harvested 2 days later from irradiated producer cell lines, and culture was then continued for 2 days in cytokine liquid culture. Typical initial transduction efficiencies ranged from 53% to 83%. The green fluorescent protein (GFP)-positive cells were sorted by flow cytometry and seeded in methylcellulose medium. After 7 days of culture, the colony-forming units in culture (CFU-C) number was counted, and colonies with less than 50 cells were scored.
Competitive repopulation assays
Competitive repopulation assays were conducted as previously described.22 Briefly, BM cells were harvested from both hind limbs of donor mice and mixed with competitor CD45.1 cells at a donor equivalent ratio of 1:1. The mixed BM cells were injected into lethally irradiated recipient CD45.1 mice (11 Gy [1100 rad], 137Cs source). The level of long-term engraftment was determined in recipient mice 16 weeks after transplantation following bleeding from the retro-orbital sinus. Peripheral blood leukocytes were analyzed 8, 12, and 16 weeks after transplantation by flow cytometry for CD45.2+ cells and multiple lineages on a BD LSR (BD Biosciences) as previously described. BM cells were harvested from primary recipients at times up to 4 months after transplantation and injected via the lateral tail vein into lethally irradiated secondary recipients (11 Gy [1100 rad]). The recipient mice expressed CD45.1, and engraftment was determined by flow cytometry.
Statistical analyses
P values were calculated by a 2-tailed t test using InStat for Windows version 1.5 (University of Reading, United Kingdom) or Microsoft Excel (Redmond, WA).
Results
Gab2−/− mice have normal hematopoietic cell numbers but reduced BM CFU-C that can be corrected by retroviral complementation
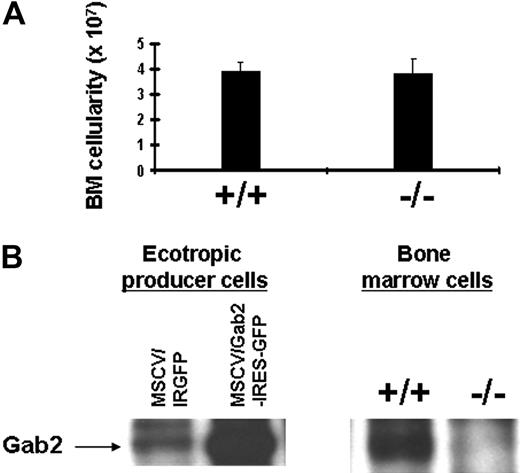
To determine the role of Gab2 in hematopoiesis, we obtained Gab2−/− mice that have been described previously.13,26 We backcrossed these mice 9 generations to C57BL/6 to achieve a consistent background and to facilitate BM transplantation experiments using congenic recipients. Gab2−/− mice on the C57BL/6 background were found to be viable and presented no major phenotypes that altered survival at any age. To test whether Gab2−/− deficiency would alter the BM cellularity, the absolute number of BM cells was compared between wild-type and Gab2−/− mice (Figure 1A). The absolute number of total BM cells in wild-type and Gab2−/− mice were not significantly different. Furthermore, mice as old as 7 months showed normal BM cellularity (data not shown). To demonstrate that Gab2−/− mice lack Gab2 expression, we performed immunoprecipitation-Western blot (IP/WB) on BM cells from wild-type and Gab2−/− mice. As controls, we included ecotropic retroviral producer cells expressing Gab2 from the murine stem cell virus (MSCV) promoter, which also drives expression of an internal ribosomal entry sequence (IRES) and GFP (Figure 1B). For the controls, we observed overexpression of retroviral Gab2 relative to the endogenous Gab2. For the BM samples, Gab2 expression was not detected in mice containing the targeted Gab2 locus.
Gab2−/− mouse BM cells are present in normal absolute numbers despite the absence of Gab2 expression. (A) Total BM cells were harvested from individual littermate wild-type and Gab2−/− mice. The total cellularity is shown for n = 10 wild-type and Gab2−/− mice. No significant difference was observed (P = .7). (B) BM lysates from wild-type and Gab2−/− mice were immunoprecipitated with anti-Gab2 antibody and then subjected to Western blot analysis with anti-Gab2 antibody. The antibody used for these studies was provided by Toshio Hirano. IP/WB included positive control ecotropic producer cells expressing Gab2 from the MSCV-Gab2-IRGFP vector. The negative control ecotropic producer cells contained only the MSCV-IRGFP empty vector. Note that the producer cells (3T3-based) expressed some endogenous Gab2; this was increased with the vector containing Gab2. Error bars represent the mean plus or minus a standard deviation.
Gab2−/− mouse BM cells are present in normal absolute numbers despite the absence of Gab2 expression. (A) Total BM cells were harvested from individual littermate wild-type and Gab2−/− mice. The total cellularity is shown for n = 10 wild-type and Gab2−/− mice. No significant difference was observed (P = .7). (B) BM lysates from wild-type and Gab2−/− mice were immunoprecipitated with anti-Gab2 antibody and then subjected to Western blot analysis with anti-Gab2 antibody. The antibody used for these studies was provided by Toshio Hirano. IP/WB included positive control ecotropic producer cells expressing Gab2 from the MSCV-Gab2-IRGFP vector. The negative control ecotropic producer cells contained only the MSCV-IRGFP empty vector. Note that the producer cells (3T3-based) expressed some endogenous Gab2; this was increased with the vector containing Gab2. Error bars represent the mean plus or minus a standard deviation.
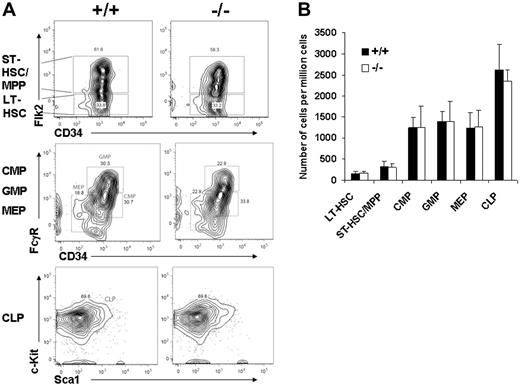
We next measured peripheral blood counts in Gab2−/− mice and wild-type littermates. No differences were observed in red blood cell or white blood cell counts, and the results are shown in Table 1. Within the white blood cell population, no difference in absolute lymphocyte, neutrophil, or monocyte count was observed. Since the total blood counts and BM counts were normal, we next determined whether the absolute number of primitive KLS Flk2neg cells in the BM was changed. Representative flow cytometry analyses are shown for gating on the KLS fraction first, followed by Flk2 and CD34 (Figure 2A). Further analyses of the common myeloid progenitor (CMP), common lymphoid progenitor (CLP), granulocyte-macrophage progenitor (GMP), and megakaryocyte-erythroid progenitor (MEP) showed no differences between wild-type and Gab2−/− BM. The absolute number of cells within each fraction also was not changed (Figure 2B).
Steady-state peripheral blood hematology
| Hematologic parameter . | +/+ . | −/− . |
|---|---|---|
| White blood cell, per μL | 10 000 ± 2 800 | 9 000 ± 2 500 |
| Absolute lymphocyte count, per μL | 8 000 ± 1 600 | 7 000 ± 1 900 |
| Absolute neutrophil count, per μL | 1 500 ± 300 | 1 400 ± 400 |
| Absolute monocyte count, per μL | 600 ± 100 | 700 ± 200 |
| Red blood cell, × 103/μL | 3 000 ± 100 | 3 000 ± 400 |
| Hematocrit, % | 52 ± 3 | 51±7 |
| Hematologic parameter . | +/+ . | −/− . |
|---|---|---|
| White blood cell, per μL | 10 000 ± 2 800 | 9 000 ± 2 500 |
| Absolute lymphocyte count, per μL | 8 000 ± 1 600 | 7 000 ± 1 900 |
| Absolute neutrophil count, per μL | 1 500 ± 300 | 1 400 ± 400 |
| Absolute monocyte count, per μL | 600 ± 100 | 700 ± 200 |
| Red blood cell, × 103/μL | 3 000 ± 100 | 3 000 ± 400 |
| Hematocrit, % | 52 ± 3 | 51±7 |
Sample sizes consisted of 6 mice per group, analyzed between 6 to 8 weeks of age. Red and white blood cell counts were determined using a Coulter counter. Hematocrits were obtained by manually scoring microcapillary tubes following centrifugation. Differential counts were determined by manually scoring blood smears. No significant differences were observed between wild-type and Gab2−/− mice for any hematologic parameter. All values except hematocrit were rounded to the nearest 100.
Wild-type and Gab2−/− BM have the same absolute number of primitive hematopoietic stem/progenitor cells. (A) BM cells were blocked with normal mouse serum; stained with lineage antibody cocktails, including Gr-1, Mac-1, B220, Ter119, CD4, and CD8; and combined with combinations of Sca-1, c-Kit, Flk2 (flt3), CD34, and additional markers as described in “Materials and methods.” Shown are representative profiles for lineage-negative BM cells analyzed for the indicated sets of markers defining short-term HSC/multipotent progenitor (ST-HSC/MPP) cells, long-term HSCs (LT-HSCs), granulocyte-macrophage progenitor (GMP) cells, megakaryocyte-erythroid progenitor (MEP) cells, and the common myeloid progenitor (CMP) and common lymphoid progenitor (CLP) cells. The values in the wild-type and Gab2−/− panels represent the percentage of the total nucleated cells staining with the associated markers. (B) A total of 6 independent wild-type and 6 independent Gab2−/− mice were used to analyze the populations described above. The values represent the number of cells per 106 viable adult BM cells obtained from 2 tibias and 2 femurs per mouse. The plotted values represent the average numbers of the 6 mice per group and the standard deviation. Error bars represent the mean plus or minus a standard deviation.
Wild-type and Gab2−/− BM have the same absolute number of primitive hematopoietic stem/progenitor cells. (A) BM cells were blocked with normal mouse serum; stained with lineage antibody cocktails, including Gr-1, Mac-1, B220, Ter119, CD4, and CD8; and combined with combinations of Sca-1, c-Kit, Flk2 (flt3), CD34, and additional markers as described in “Materials and methods.” Shown are representative profiles for lineage-negative BM cells analyzed for the indicated sets of markers defining short-term HSC/multipotent progenitor (ST-HSC/MPP) cells, long-term HSCs (LT-HSCs), granulocyte-macrophage progenitor (GMP) cells, megakaryocyte-erythroid progenitor (MEP) cells, and the common myeloid progenitor (CMP) and common lymphoid progenitor (CLP) cells. The values in the wild-type and Gab2−/− panels represent the percentage of the total nucleated cells staining with the associated markers. (B) A total of 6 independent wild-type and 6 independent Gab2−/− mice were used to analyze the populations described above. The values represent the number of cells per 106 viable adult BM cells obtained from 2 tibias and 2 femurs per mouse. The plotted values represent the average numbers of the 6 mice per group and the standard deviation. Error bars represent the mean plus or minus a standard deviation.
Since no overt hematologic phenotypes were observed, we next tested whether their response to hematopoietic cytokines was altered by Gab2 deficiency. We found that Gab2 deficiency affected all subtypes of committed hematopoietic progenitor colonies examined. BM cells from wild-type and Gab2−/− mice were plated in standard methylcellulose progenitor assays in IL-3, IL-6, SCF, and EPO. As shown in Table 2, there was a marked reduction in the absolute number of total CFU-C, multipotential (CFU-granulocyte-erythrocyte-macrophage-megakaryocyte [GEMM]), myeloid (CFU-granulocyte macrophage [GM], CFU-granulocyte [G], and CFU-macrophage [M]), and erythroid (erythroid burst-forming unit [BFU-E]) progenitors generated from Gab2−/− mice compared with wild-type mice. Additional assays for CFU-preB-lymphocyte progenitors also showed a reduction (wild-type, 10 000 cells/μL ± 2900 cells/μL vs Gab2−/−, 5300 cells/μL ± 700 μL), but this did not reach statistical significance (P = .06; n = 3). Therefore, Gab2 deficiency reduces myeloid clonogenic progenitor colony-forming ability. Similar reductions in CFU-GM were observed using GM-CSF as the sole cytokine (data not shown). Collectively, these data argue that an intrinsic Gab2 hematopoietic progenitor cell defect contributes to deficiencies at the level of committed hematopoietic progenitors.
Absolute numbers of BM myeloid and erythroid progenitor subsets
| Hematopoietic colony type . | +/+ . | −/− . |
|---|---|---|
| CFU-C (total) | 111 800 ± 21 100 | 59 400 ± 19 800* |
| CFU-GEMM | 8 600 ± 1 900 | 3 800 ± 1 700* |
| CFU-GM | 60 000 ± 11 500 | 34 600 ± 9 100* |
| CFU-G | 16 900 ± 6 300 | 8 400 ± 5 600* |
| CFU-M | 11 100 ± 3 200 | 5 300 ± 3 000* |
| BFU-E | 15 400 ± 7 100 | 7 200 ± 2 600* |
| Hematopoietic colony type . | +/+ . | −/− . |
|---|---|---|
| CFU-C (total) | 111 800 ± 21 100 | 59 400 ± 19 800* |
| CFU-GEMM | 8 600 ± 1 900 | 3 800 ± 1 700* |
| CFU-GM | 60 000 ± 11 500 | 34 600 ± 9 100* |
| CFU-G | 16 900 ± 6 300 | 8 400 ± 5 600* |
| CFU-M | 11 100 ± 3 200 | 5 300 ± 3 000* |
| BFU-E | 15 400 ± 7 100 | 7 200 ± 2 600* |
All assays were performed using methylcellulose medium from Stem Cell Technologies, according to the manufacturer's instructions. Data are the absolute number of CFUs in both hind limbs of 6 mice of each genotype presented as the means plus or minus the standard deviation. Total CFU-C includes all of the subsets as determined by multiplying the progenitor frequency by the BM cellularity. All values were rounded to the nearest 100.
*P < .05 relative to wild-type (+/+).
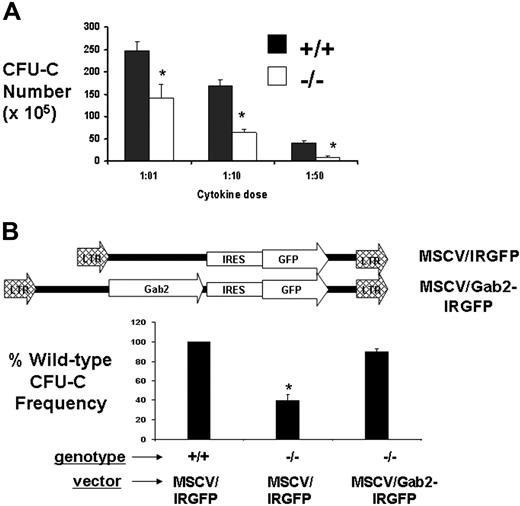
We next determined whether the defects in myeloid CFU-C were cytokine-dosage dependent and specific for the absence of Gab2. BM was harvested from wild-type and Gab2−/− mice for analysis of in vitro CFU-C activity in the presence of IL-3 (20 ng/mL), IL-6 (50 ng/mL), and SCF (50 ng/mL). These concentrations were for standard 1 × doses (indicated as 1:01). We also tested 10- or 50-fold reduced concentrations of the cocktail as indicated by 1:10 and 1:50. BM was pooled from 3 mice for each assay. Reduction in CFU-C progenitors per 105 BM cells was observed that increased from 1.8-fold to 2.6-fold to 5.1-fold, corresponding with the decreasing cytokine concentrations, respectively (Figure 3A). These reductions in BM CFU-C were observed in the absence of a decline in the total BM cellularity, suggesting that defective cytokine response in vitro reduces the frequency of CFU-C in methylcellulose medium. Retroviral complementation studies were performed using the ecotropic producer cell lines described in Figure 1. BM cells were transduced by coculture, washed from the plates, and expanded for 2 additional days before sorting or GFP expression by flow cytometry. The sorted cells were plated in methylcellulose medium and assayed for CFU-C number (Figure 3B). Strikingly, the add-back of Gab2 restored the CFU-C number to the same as that of wild-type BM. As a control, BM transduced with the empty-vector control (IRES-GFP) showed the expected reduced number of CFU-C.
Defective CFU-C activity in the absence of Gab2 is correctable by retroviral complementation. (A) Gab2−/− and littermate wild-type mice (6 to 8 weeks of age) were humanely killed, and the BM cells were harvested and plated in methylcellulose medium to assay for the CFU-C frequency in response to a cytokine cocktail of SCF, IL-3, and IL-6 in either high-dose (1:01) or 1:10 and 1:50 dilutions. Day-7 BM CFUs-C were assayed in methylcellulose medium after culture with limiting cytokine concentrations. *P > .05 relative to wild-type BM. (B) MSCV-based retroviral vectors expressing Gab2-IRES-GFP or IRES-GFP empty vector were used to test whether CFU-C activity could be restored by retroviral-mediated gene transfer. A total of 3 separate retroviral Gab2 complementation assays were done. In each experiment, 3 Gab2−/− mice and 3 littermate wild-type mice were used. BM cells were collected and transduced by coculture followed by flow cytometry sorting for GFP+ cells. Sorted GFP+ cells were plated in methylcellulose medium and then assayed for CFU-C number after 7 days. As a control, BM cells transduced with the empty vector (IRES-GFP) were included. *P = .001 relative to MSCV-Gab2-IRGFP-transduced Gab2−/− BM, indicating significant correction of the defect. Error bars represent the mean plus or minus a standard deviation.
Defective CFU-C activity in the absence of Gab2 is correctable by retroviral complementation. (A) Gab2−/− and littermate wild-type mice (6 to 8 weeks of age) were humanely killed, and the BM cells were harvested and plated in methylcellulose medium to assay for the CFU-C frequency in response to a cytokine cocktail of SCF, IL-3, and IL-6 in either high-dose (1:01) or 1:10 and 1:50 dilutions. Day-7 BM CFUs-C were assayed in methylcellulose medium after culture with limiting cytokine concentrations. *P > .05 relative to wild-type BM. (B) MSCV-based retroviral vectors expressing Gab2-IRES-GFP or IRES-GFP empty vector were used to test whether CFU-C activity could be restored by retroviral-mediated gene transfer. A total of 3 separate retroviral Gab2 complementation assays were done. In each experiment, 3 Gab2−/− mice and 3 littermate wild-type mice were used. BM cells were collected and transduced by coculture followed by flow cytometry sorting for GFP+ cells. Sorted GFP+ cells were plated in methylcellulose medium and then assayed for CFU-C number after 7 days. As a control, BM cells transduced with the empty vector (IRES-GFP) were included. *P = .001 relative to MSCV-Gab2-IRGFP-transduced Gab2−/− BM, indicating significant correction of the defect. Error bars represent the mean plus or minus a standard deviation.
Gab2-deficient early hematopoietic progenitor cells have decreased proliferation and attenuated signal transduction in response to early-acting cytokines
Next, we set out to functionally characterize the cytokine responsiveness of sorted KLS cells derived from wild-type and Gab2−/− BM. KLS cells were put into liquid suspension culture in the presence of various cytokine cocktails important for KLS cell expansion and survival. Total cell expansion in vitro over a 6-day incubation period was determined (Table 3). The cytokine combinations included IL-36S, IL-3SFT, IL-3S, IL-3F, IL-3T, and SFT, as previously described.25 As expected, IL-3 was the most potent cytokine in increasing overall cell numbers. Stimulation with early-acting growth factors such as SCF (S), FL (F), and TPO (T) revealed the greatest deficiency for Gab2-deficient KLS cells. These growth factor combinations are known to synergize with IL-3 to promote cell proliferation and survival during short-term ex vivo culture. This result demonstrates the KLS cells lacking Gab2 expression have reduced cytokine responsiveness in vitro.
In vitro cytokine-stimulated cell expansion from sorted KLS cells
| Cytokine cocktail . | WT KLS, fold expansion . | Gab2−/− KLS, fold expansion . | WT expansion/Gab2−/− expansion . |
|---|---|---|---|
| IL-36S | 831 ± 156 | 355 ± 190* | 2.3 |
| IL-3SFT | 675 ± 142 | 279 ± 122* | 2.4 |
| IL-3S | 506 ± 169 | 168 ± 110 | 3.0 |
| IL-3F | 150 ± 104 | 25 ± 19 | 6.0 |
| IL-3T | 50 ± 35 | 16 ± 14 | 3.1 |
| SFT | 157 ± 37 | 14 ± 4* | 11.1 |
| Cytokine cocktail . | WT KLS, fold expansion . | Gab2−/− KLS, fold expansion . | WT expansion/Gab2−/− expansion . |
|---|---|---|---|
| IL-36S | 831 ± 156 | 355 ± 190* | 2.3 |
| IL-3SFT | 675 ± 142 | 279 ± 122* | 2.4 |
| IL-3S | 506 ± 169 | 168 ± 110 | 3.0 |
| IL-3F | 150 ± 104 | 25 ± 19 | 6.0 |
| IL-3T | 50 ± 35 | 16 ± 14 | 3.1 |
| SFT | 157 ± 37 | 14 ± 4* | 11.1 |
IL-3 indicates murine IL-3 (20 ng/mL); 6, human IL-6 (50 ng/mL); S, murine stem cell factor (50 ng/mL); F, murine flt-3 ligand (50 ng/mL); and T, murine thrombopoietin (50 ng/mL). KLS cells were sorted by FACS and plated in 96-well plates in the presence of various cytokine cocktails. After 6 days in culture, the total nucleated cell count was determined using a hemacytometer. The fold cell expansion was calculated based on the starting number of cells seeded per well. The results are the averages plus or minus standard deviation for 3 experiments.
*P < .05 relative to wild-type KLS (WT KLS).
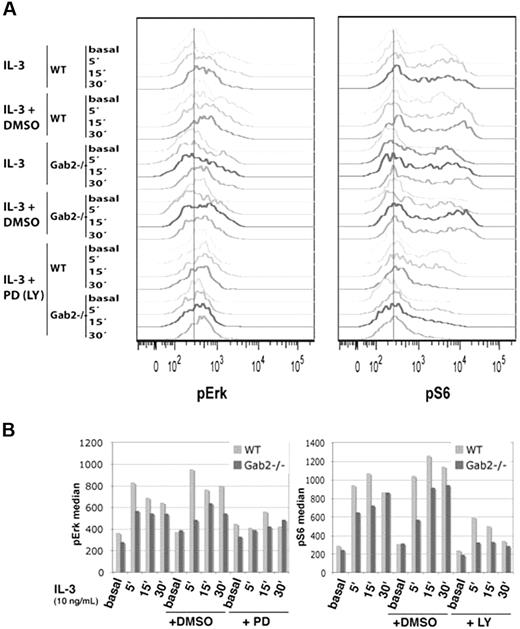
To assess the consequences of Gab2 deficiency on downstream signaling pathways that are activated by IL-3, we used intracellular flow cytometry to measure pERK and pS6 levels in c-Kit+Linlow cells, which are enriched for stem/progenitor activity (Figure 4). Cells from Gab2−/− mice demonstrated modest but consistent reductions in pERK and pS6 levels in response to IL-3. This defect in S6 activation was exacerbated in Gab2−/− cells that were incubated with 50 μM of the PI-3K inhibitor LY294002 for 1 hour before IL-3 stimulation, which supports an important role for Gab2 for optimal activation of PI-3K signaling. c-Kit+Linlow and c-Kit−Linlow cells comprise 2% to 3% and approximately 20% of total BM nucleated cells, respectively. Gating on the entire Linlow population also revealed a decrease in pERK and pS6 levels in response to IL-3 that was more pronounced than in the c-Kit+Linlow subpopulation (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). As expected, Linlow cells from wild-type and Gab2−/− mice equally phosphorylated STAT5 in response to IL-3.
BM c-Kit+Linlow cells from Gab2−/− mice are deficient in phosphorylation of S6 and ERK. Whole BM from wild-type and Gab2−/− mice was serum and cytokine starved for 4 hours and stimulated with IL-3 (10 ng/mL) for 5, 15, or 30 minutes alone or in the presence of DMSO control (0.1%), 50 μM PD98059 (for pERK measurement), or 50 μM LY294002 (for pS6 measurement). Levels of phosphorylated ERK and S6 were measured using phosphospecific flow cytometry in the c-Kit+/Lin−/low subset. Flow cytometry profiles are shown in the top panels for basal (starved and unstimulated) or IL-3 stimulated cells. Values of the median intensity of fluorescence for each phosphoprotein measured are provided below each set of flow cytometry histograms. Light-gray bars are wild-type, and dark-gray bars are Gab2−/−. Shown is 1 representative experiment from 5 total experiments.
BM c-Kit+Linlow cells from Gab2−/− mice are deficient in phosphorylation of S6 and ERK. Whole BM from wild-type and Gab2−/− mice was serum and cytokine starved for 4 hours and stimulated with IL-3 (10 ng/mL) for 5, 15, or 30 minutes alone or in the presence of DMSO control (0.1%), 50 μM PD98059 (for pERK measurement), or 50 μM LY294002 (for pS6 measurement). Levels of phosphorylated ERK and S6 were measured using phosphospecific flow cytometry in the c-Kit+/Lin−/low subset. Flow cytometry profiles are shown in the top panels for basal (starved and unstimulated) or IL-3 stimulated cells. Values of the median intensity of fluorescence for each phosphoprotein measured are provided below each set of flow cytometry histograms. Light-gray bars are wild-type, and dark-gray bars are Gab2−/−. Shown is 1 representative experiment from 5 total experiments.
Gab2 deficiency causes defective multilineage hematopoietic repopulating function
We next wanted to determine whether Gab2 deficiency affects the function of primitive hematopoietic cells in vivo. BM cells from wild-type and Gab2−/− mice were used as test cells in competitive repopulation assays (Figure 5A). For these experiments, the test cells from wild-type and Gab2−/− mice were mixed with age-matched congenic CD45.1 BM cells. The mixture was done at a 1:1 ratio and was followed by tail-vein injection into lethally irradiated CD45.1 recipients. Peripheral blood chimerism was evaluated by flow cytometry as previously described. A total of 2 independent competitive experiments were conducted, with 5 recipients per test cell group. Mean donor chimerism of mice that underwent transplantation at 8, 12, and 16 weeks following transplantation is shown in Figure 5A as the average of both competitive transplantation experiments. Gab2−/− BM had a significant competitive disadvantage, resulting in a decreased contribution (3.7-fold, 4.7-fold, and 4.3-fold at 8, 12, and 16 weeks, respectively) to peripheral blood leukocyte chimerism. To assess donor cell contribution to myeloid and lymphoid lineages in mice that underwent transplantation, multilineage analysis was conducted by detecting simultaneous expression of CD45.2 and specific lineage markers such as granulocytes (Gr-1), lymphocytes (B220 and CD4), and erythrocytes (Ter119). Donor cells from Gab2−/− BM were capable of multilineage repopulation, albeit at a consistently lower level than that of wild-type control (Figure 5B). Although steady-state hematology and stem cell content data revealed no difference between wild-type and Gab2−/− mice, competitive repopulation experiments showed decreased competitive repopulating ability in vivo, as measured by reduction in the contribution of Gab2−/− donor cells to multiple hematopoietic lineages (Figure 5C). Decreased contributions of 3.1-fold, 4.6-fold, 3.6-fold, and 5.8-fold were observed in Gr-1, Ter119, B220, and CD4 populations, respectively. These observations indicated that Gab2 defects extend back to early hematopoiesis. Furthermore, the defects were cell intrinsic, since in an experiment where wild-type BM was transplanted into wild-type or Gab2−/− hosts, no stem cell defect was observed (Figure 6).
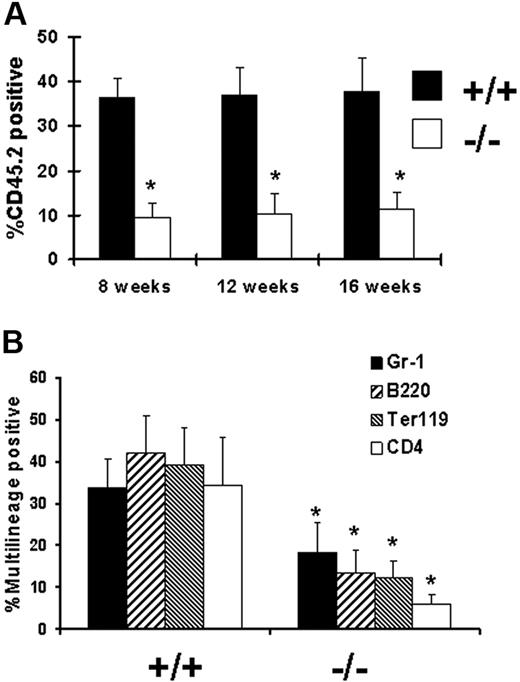
Impaired multilineage hematopoietic repopulating ability of Gab2−/− BM cells. Total BM cells (CD45.2) were collected from either Gab2−/− or wild-type mice and mixed at a 1:1 donor equivalent ratio. The BM cell mixes were then injected into lethally irradiated recipient mice (CD45.1; 11 Gy [1100 rad]). (A) Mice that underwent primary transplantation were bled from the retro-orbital venous plexus 8, 12, and 16 weeks after transplantation and analyzed for CD45.2 expression by flow cytometry; mean donor chimerism was determined. *P > .001 relative to wild-type BM. (B) To demonstrate a typical lineage analysis of the Gab2−/− engraftment, CD45.2 positive cells costaining for Gr-1, B220, Ter119, or CD4 are shown for lethally irradiated adult C57BL/6 CD45.1 recipient mice analyzed 16 weeks later. Error bars represent the average plus or minus a standard deviation. (C) The average multilineage analysis data for both experiments at 16 weeks after transplantation is shown. *P > .001 relative to wild-type BM.
Impaired multilineage hematopoietic repopulating ability of Gab2−/− BM cells. Total BM cells (CD45.2) were collected from either Gab2−/− or wild-type mice and mixed at a 1:1 donor equivalent ratio. The BM cell mixes were then injected into lethally irradiated recipient mice (CD45.1; 11 Gy [1100 rad]). (A) Mice that underwent primary transplantation were bled from the retro-orbital venous plexus 8, 12, and 16 weeks after transplantation and analyzed for CD45.2 expression by flow cytometry; mean donor chimerism was determined. *P > .001 relative to wild-type BM. (B) To demonstrate a typical lineage analysis of the Gab2−/− engraftment, CD45.2 positive cells costaining for Gr-1, B220, Ter119, or CD4 are shown for lethally irradiated adult C57BL/6 CD45.1 recipient mice analyzed 16 weeks later. Error bars represent the average plus or minus a standard deviation. (C) The average multilineage analysis data for both experiments at 16 weeks after transplantation is shown. *P > .001 relative to wild-type BM.
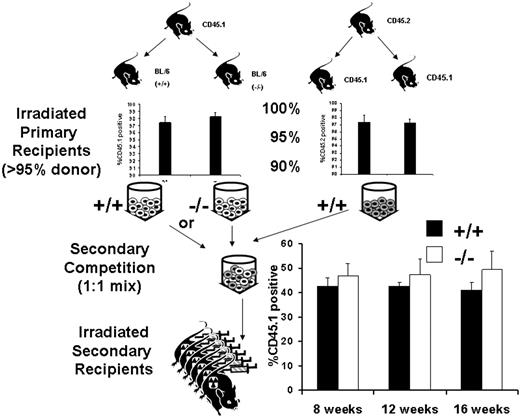
The Gab2−/− microenvironment is not defective in support of cells capable of multilineage hematopoietic repopulation. To determine whether a component of the defects observed from donor Gab2−/− BM could be due to cell-extrinsic defects in the microenvironment, we used a BM chimera approach. Wild-type CD45.1 BM cells were transplanted into lethally irradiated wild-type or Gab2−/− (CD45.2) recipients. A similar transplantation was performed with wild-type CD45.2 BM into CD45.1 recipients. Eight weeks after transplantation, the hematopoiesis was greater than 95% donor derived as indicated on the upper two graphs; the mice were killed, and the BM grafts were competed against each other (CD45.2 vs CD45.1). Shown in the lower graph are analyses of engraftment obtained 8, 12, and 16 weeks after secondary transplantation. No significant difference was observed when either wild-type or Gab2−/− mice were used as primary hosts (P < .1). Error bars represent the average plus or minus a standard deviation.
The Gab2−/− microenvironment is not defective in support of cells capable of multilineage hematopoietic repopulation. To determine whether a component of the defects observed from donor Gab2−/− BM could be due to cell-extrinsic defects in the microenvironment, we used a BM chimera approach. Wild-type CD45.1 BM cells were transplanted into lethally irradiated wild-type or Gab2−/− (CD45.2) recipients. A similar transplantation was performed with wild-type CD45.2 BM into CD45.1 recipients. Eight weeks after transplantation, the hematopoiesis was greater than 95% donor derived as indicated on the upper two graphs; the mice were killed, and the BM grafts were competed against each other (CD45.2 vs CD45.1). Shown in the lower graph are analyses of engraftment obtained 8, 12, and 16 weeks after secondary transplantation. No significant difference was observed when either wild-type or Gab2−/− mice were used as primary hosts (P < .1). Error bars represent the average plus or minus a standard deviation.
Finally, we wanted to determine whether Gab2 defects were observed in secondary hosts from the 2 sets of primary transplant recipients from Figure 5. The BM cells were transplanted into CD45.1 recipients, and donor cell engraftment was determined by FACS analysis for CD45.2. Interestingly, the ratios of Gab2−/− to wild-type cells were similar in primary and secondary recipients. Peripheral blood leukocyte chimerism was reduced 3.8-fold, 3.6-fold, and 3.4-fold relative to wild-type chimerism (Figure 7A). Multilineage analysis at 16 weeks showed an average of 1.9-fold, 3.2-fold, 3.3-fold, and 5.7-fold decreases in Gr-1, Ter119, B220, and CD4 populations, respectively (Figure 7B). Additional tertiary transplantations also showed similar results (data not shown). Collectively, these serial transplantations support the idea that Gab2−/− stem cells have normal self-renewal.
Secondary transplantation does not promote further declines in hematopoietic repopulating potential. To determine whether engraftment would decrease upon transplantation into secondary recipients, the chimeric mice that received BM cells 16 weeks earlier were killed, and the BM cells were collected and then injected into lethally irradiated CD45.1 secondary hosts. (A) The mice were bled 8 weeks, 12 weeks, and 16 weeks following secondary transplantation. Peripheral blood leukocytes were analyzed by flow cytometry for the expression of CD45.2. The results are represented as the average plus or minus a SD from the combination of 2 independent transplantation experiments with 10 recipient mice in each group. *P > .001 relative to wild-type BM. (B) Donor contribution to myeloid, lymphoid, and erythroid lineages in long-term reconstituted mice is shown. Average multilineage analysis at 16 weeks showed that the defects affected multiple hematopoietic lineages. *P > .001 relative to wild-type BM. Error bars represent the average plus or minus a standard deviation.
Secondary transplantation does not promote further declines in hematopoietic repopulating potential. To determine whether engraftment would decrease upon transplantation into secondary recipients, the chimeric mice that received BM cells 16 weeks earlier were killed, and the BM cells were collected and then injected into lethally irradiated CD45.1 secondary hosts. (A) The mice were bled 8 weeks, 12 weeks, and 16 weeks following secondary transplantation. Peripheral blood leukocytes were analyzed by flow cytometry for the expression of CD45.2. The results are represented as the average plus or minus a SD from the combination of 2 independent transplantation experiments with 10 recipient mice in each group. *P > .001 relative to wild-type BM. (B) Donor contribution to myeloid, lymphoid, and erythroid lineages in long-term reconstituted mice is shown. Average multilineage analysis at 16 weeks showed that the defects affected multiple hematopoietic lineages. *P > .001 relative to wild-type BM. Error bars represent the average plus or minus a standard deviation.
Discussion
Gab2 is as an integral cytokine signaling molecule for various early-acting cytokines. However, no direct examination of its role in hematopoiesis has been reported. Here, we describe studies to determine the role of Gab2-mediated signaling in hematopoiesis. Despite the normal appearance of the mice, normal BM cellularity, and the normal blood counts, we uncovered hematopoietic deficits in Gab2−/− mice. Reduced CFU-C activity in methylcellulose and impaired KLS cell growth in liquid culture demonstrate an intrinsic requirement for Gab2 in cytokine responsiveness. The reductions in CFU-C were also greater when the concentrations of cytokines were reduced, reflecting a general overall decreased response. The KLS defects were particularly informative with regard to early-acting cytokines that require Gab2 for growth. Notably, the defects were greatest with the less-proliferative cytokine cocktail of SCF, FL, and TPO, and with the IL-3 and FL combination. The defects in response to these cytokines suggest that Gab2 may act as a “signal relay” protein that organizes signaling complexes and amplifies growth factor-induced receptor activation in various contexts.
Remarkably, Gab2−/− mice have normal numbers of KLS Flk2neg cells during steady-state hematopoiesis, which might reflect a minimal requirement for maintenance of the early stem/progenitor cell pool. Our prior studies also observed preservation of the KLS pool in STAT5-deficient mice.22 We found that serial competitive repopulation with Gab2−/− BM was deficient relative to wild-type BM, but that defects observed in primary recipients were not augmented by secondary and tertiary transplantations. These data support a decreased functional ability of transplanted progenitor cells to respond to cytokine growth factors in the BM microenvironment rather than a reduction in either the absolute number or function of stem cells. Gab2 may function in hematopoietic cells through multiple mechanisms. A role for Gab2 in hematopoietic cell adhesion and migration has also been shown,9 although Gab2-deficient mice have not been studied in this regard. Our Gab2−/− mice were obtained from Toshio Hirano.13 However, at least 2 additional strains of Gab2−/− mice exist.11,12 One has been reported to have osteoclast differentiation defects that causes osteosclerosis.12 The possibility of a microenvironment defect was directly tested here, and the transplantation of normal wild-type BM into wild-type or Gab2−/− recipients followed by secondary competitive repopulation did not show any impact of the Gab2−/− microenvironment on engraftment. We also did not detect any abnormal bone development in these mice, even after 7 months of age (data not shown). Wada et al report reduced bone marrow cellularity with age associated with moderately increased bone mass.12 Our Gab2−/− mice had normal bone marrow cellularity at ages up to 7 months. As a possible explanation for these differences, we have noted that the Gab2−/− mice reported by Wada et al were generated by a genetic targeting strategy that retained the region coding for the PH domain, whereas our mice are completely deleted in this region.
The decreased activation of the PI-3K pathway in c-Kit+Lin− cells points toward an inefficient cytokine response in the transplantation setting. This would support a role for Gab2 in mediating an efficient and robust response to cytokines. It is important to point out that the defects observed are not as striking as those reported in p85alpha−/−p85beta+/− mice, where the PI-3K pathway was more significantly attenuated, resulting in decreased fetal liver multilineage hematopoietic repopulating ability.27 However, Gab2−/− mice have attenuated PI-3K signaling, but it is not completely suppressed. Adult Gab2−/− mice also had normal hematocrit and red blood cell count, which contrasts with the pale anemic phenotype described for more severe p85alpha−/− embryos.28 Deficiency of p110 is also very severe, and mice lacking p110α or p110β die in utero.29,30 The role of p110 has been described in B-cell development and function31,32 and in B- and T-cell receptor signaling33 as part of the PI-3K pathway.34 In contrast, Gab2 recruits negative regulators of PI-3K signaling downstream of the T-cell receptor.35,36 While we did not find evidence for a negative regulatory function in lymphocyte development from transplanted HSCs, our experiments did not explore T- or B-cell receptor signaling in detail. It is possible that Gab2 mediates a balance between positive and negative signals in hematopoiesis; this may also account for the milder phenotypes overall.
It is possible that Gab2 promotes PI-3K- and MAPK-signaling cascades through positive feedback loops in a similar manner as described for Gab1.37 Kiyatkin et al37 used a computational model validated by in vivo experiments to demonstrate that Gab1 enhances the PI-3K signaling pathway. They explain this enhancement by the Grb2-mediated membrane recruitment of Gab1, which in turn binds PI-3K, allowing its activation. PI-3K-generated PIP3 will then recruit more Gab1 to the complex through its PH domain, increasing the activation of the pathway. Subsequently, this positive feedback loop will also increase the levels of Grb2-SOS complexes and recruit SHP-2 to the membrane, which in turn dephosphorylates the Ras-GAP binding sites, making the Ras MAPK pathway more active. Our observations of reduced S6 ribosomal protein phosphorylation represents the combined suppression of PI-3K/Akt and ERK activation and fits with the hypothesis that Gab2-deficient mice have less efficient PI-3K activation but a more moderate extent of MAPK pathway attenuation. Our data strongly support a role for Gab2 as an important signal relay molecule important downstream of early-acting cytokine receptors.
ERK activation mediates proliferative effects through downstream transcription factor targets, including NF-κB, CREB, Ets-1, AP-1, and c-Myc. These transcription factors induce expression of genes important for cell-cycle progression, such as Cdks, cyclins, and growth factors, and for apoptosis prevention, such as Bcl-2. Mice lacking expression of the p44 MAPK (ERK1) show defects in maturation of CD4/CD8 thymocytes and have a defective proliferative response to T-cell receptor stimulation.38 Defects in MAPK kinase kinase 1 (MEKK1) result in defective migration and proinflammatory response of fibroblasts, epithelial cells, and embryonic stem cells.39,40 MEKK3 deficiency results in early embryonic lethality due to defective development of blood vessels and yolk sac, indicating an essential role in cardiovascular system development.41 However, the major defects in these mouse models have precluded examination of adult hematopoiesis and hematopoietic stem cell biology.
Gab2, SHP-2, and STAT5 are also believed to be critically important for leukemogenesis, and their combined inhibition leads to enhanced sensitivity of BCR-ABL-expressing chronic myeloid leukemia cells.21 Gab2 is already well defined as a potential leukemic target that is of particular relevance for activation of PI-3K signaling by BCR-ABL.17 The role of PI-3K pathway activation in leukemogenesis has been extensively studied. Negative regulation of this pathway by PTEN is critical, and its loss results in leukemic stem cell expansion42,43 and integration of multiple signals required for tumorigenesis.44 A different set of myeloproliferative disorders are characterized by aberrant Ras/MAPK activation and hypersensitivity to growth factors and overproliferation. It is now clear that Ras,45 SHP-2,46,–48 and NF149,50 play major, largely nonredundant, roles in juvenile myelomonocytic leukemia. SHP-2 is profoundly required for early hematopoietic development, and its deletion is embryonically lethal.51 Our studies indicate that Gab2 alone as a molecular target would have hematopoietic consequences, but these would still be tolerable compared with complete ablation of PI-3K or MAPK function. However, Gab2 deficiency combined with LY294002 treatment dramatically blocked pS6 activation in c-Kit+Lin− cells. This finding suggests that potential cooperativity would be expected in vivo from pharmacologic dual targeting of Gab2 and PI-3K, or possibly downstream intermediates, such as mTOR.
Since Gab2 plays a similar role as STAT5 in mast cell development,13,14 it is important to note that Gab2 deficiency is comparatively much milder than STAT5 deficiency as measured in the competitive repopulation experiments.22,25 The inability of Gab2 deficiency to affect STAT5 phosphorylation by IL-3 in primitive progenitor cells indicates that STAT5 is not tyrosine phosphorylated downstream of Gab2 in this cytokine-signaling pathway. However, the interactions among Gab2, SHP-2, and STAT5 are not well defined, and clearly the hematopoietic phenotypes are overlapping in some respects but divergent in others. Overall, these studies highlight the important cell-intrinsic defects in the cytokine response of HSCs to early-acting cytokines when Gab2 is absent, and provide new insight into cytokine signaling during hematopoiesis.
Acknowledgments
The authors thank Toshio Hirano for providing Gab2 knockout mice and anti-Gab2 antibody for these studies. We also thank Heath Bradley and Christine Couldrey for their assistance with the Gab2 knockout mouse colony and genotyping.
This work was supported by National Institutes of Health (NIH) grants R01HL073738 and R01DK059380 (K.D.B.), The Center for Stem Cell and Regenerative Medicine, and the Flow Cytometry and Radiation Resources Core Facilities of the Case Comprehensive Cancer Center (P30CA43703). W.T. is a recipient of the 2006 American Society of Clinical Oncology (ASCO) Career Development Award, and is supported by NIH grant no. K12CA076917.
National Institutes of Health
Authorship
Contribution: Y.Z., Z.W., G.L., E.D.-F., Z.K., E.H., and S.R. performed research and analyzed data; C.-K.Q., W.T., and K.S. designed research; and K.D.B. performed research, designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kevin D. Bunting, Department of Medicine, Division of Hematology/Oncology, Case Western Reserve University, WRB 2-131, Cleveland, OH 44106-7284; e-mail: kevin.bunting@case.edu.

![Figure 5. Impaired multilineage hematopoietic repopulating ability of Gab2−/− BM cells. Total BM cells (CD45.2) were collected from either Gab2−/− or wild-type mice and mixed at a 1:1 donor equivalent ratio. The BM cell mixes were then injected into lethally irradiated recipient mice (CD45.1; 11 Gy [1100 rad]). (A) Mice that underwent primary transplantation were bled from the retro-orbital venous plexus 8, 12, and 16 weeks after transplantation and analyzed for CD45.2 expression by flow cytometry; mean donor chimerism was determined. *P > .001 relative to wild-type BM. (B) To demonstrate a typical lineage analysis of the Gab2−/− engraftment, CD45.2 positive cells costaining for Gr-1, B220, Ter119, or CD4 are shown for lethally irradiated adult C57BL/6 CD45.1 recipient mice analyzed 16 weeks later. Error bars represent the average plus or minus a standard deviation. (C) The average multilineage analysis data for both experiments at 16 weeks after transplantation is shown. *P > .001 relative to wild-type BM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/1/10.1182_blood-2006-11-060707/2/m_zh80130703160005.jpeg?Expires=1769177510&Signature=A9N0uV4Xl7Lsbbo-cKi1KGap~jsZdwOhQMwfpwDd0b-WNl7RtlYPRH5yjk3mTIXFpt~x8EDXICAlqIf9BzqMQ2XqN5ZthV1nOvR6t1dcqvBqv5hs5i6IBPLhJPh-8alG5I6WtL2MG0rgPnScUSRZ9AIy3V4yItAYI~PEytVdtz46M0MLQhuIzttVTT7SDV9vH6u9gwLmUKsyPDT5-m3AAC~fX45EqJ0GaCqmHjeznV7KkKSd-DW01lX9ulVyO~KrsSVjI~gWaVB7U11qd2epa~A8wnXl-PztiZ~n2L67c1Ug3RG0DESJjF-YIngmni3AUhLCTjEQwd5j3OqaKx2ppg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal