Abstract
The proteasome inhibitor bortezomib has been shown to possess promising antitumor activity and significant efficacy against a variety of malignancies. Different studies demonstrated that bortezomib breaks the chemoresistance in different tumor cells basically by altering nuclear factor–κB (NF-κB) activity. NF-κB has been shown to be constitutively active in most primary Hodgkin-Reed-Sternberg (H-RS) cells in lymph node sections and in Hodgkin lymphoma (HL) cell lines and was suggested to be a central molecular switch in apoptosis resistance in HL. Here we report a bimodal effect of bortezomib in HL cells. Whereas high-dose bortezomib induced direct cytotoxicity that correlated with decreased NF-κB activity, low-dose bortezomib sensitized HL cells against a variety of cytotoxic drugs without altering NF-κB action. Strikingly, bortezomib induced marked XIAP down-regulation at the posttranslational level that was independent of the NF-κB status. Similarly, RNA interference (RNAi)–mediated XIAP down-regulation generated susceptibility to cytostatic agents. The results identify XIAP as an NF-κB–independent target of bortezomib action that controls the chemoresistant phenotype of HL cells.
Introduction
The proteasome, a large multicatalytic proteinase complex, is responsible for the degradation of most intracellular proteins. The proteasome has a central role in catabolism of a wide variety of proteins controlling cellular division, growth, function, and death. Numerous examples of regulatory proteins including cyclins, cyclin-dependent kinases and kinase inhibitors, oncogenes, tumor suppressor genes, and transcriptional activators and inhibitors have been found to undergo proteasomal proteolysis. Inhibition of the proteasome induces the accumulation of important regulatory intracellular proteins like cytoplasmic inhibitor of NF-κB (IκBα), p53 tumor suppressor gene, and p21 and p27 cyclin-dependent kinase inhibitors, which leads to decreased NF-κB activity, increased p53-mediated transcription of genes involved in apoptosis, and dysregulation of the cell cycle.1–4 In cancer cells, the proteasome is essential to mechanisms underlying tumor cell growth, apoptosis, angiogenesis, and metastasis, thereby representing a novel target for cancer therapy.3–5
Pharmacologic inhibitors of the proteasome have been shown to possess antitumor activity and have significant efficacy against a variety of malignancies.1–2 The best characterized proteasome inhibitor, bortezomib (Velcade, previously known as PS-341; Millennium Pharmaceuticals, Cambridge, MA), is a dipeptidyl boronic acid that reversibly inhibits the chymotrypsin-like activity of the proteasome. This agent displays remarkable selectivity toward the proteasome relative to serine and cysteine proteases, and it possesses unique antitumor properties as shown in a National Cancer Institute (NCI) tumor cell line screen and in several murine xenograft models.6–11 Bortezomib is the first proteasome inhibitor that was clinically tested in patients and became a therapeutic modality for multiple myeloma.
Hodgkin lymphoma (HL) accounts for approximately 30% of all malignant lymphomas12 with the common feature that neoplastic cells constitute only a small minority of the cells in the affected tissue, often corresponding to less than 2% of the total tumor load. Classical HL (cHL), representing approximately 95% of all HLs, is a fatal disease with 90% of untreated patients dying within 2 to 3 years.12 The tumor cells of cHL, designated Hodgkin-Reed-Sternberg (H-RS) cells, are mainly derived from germinal center or post–germinal center B cells, while few (less than 2%) are derived from T cells. H-RS cells lack specific functional markers of mature B or T cells, seem to be arrested in maturation, and therefore should be physiologically prone to undergo apoptosis.13–14 The mechanisms of apoptotic resistance in H-RS cells have been intensively investigated during the last decade. It has been shown that H-RS cells are resistant to CD95-mediated apoptosis15 due to the constitutive expression of cFLIP.16 In addition, H-RS cells display a defective mitochondrial apoptotic pathway17 and uniformly show up-regulated XIAP expression,18 which together cause robust resistance to apoptosis.
In this study we investigated the molecular mechanisms of bortezomib action in apoptosis-resistant HL B-cell lines. Whereas high-dose bortezomib induced direct cytotoxicity associated with decreased NF-κB activity, low-dose bortezomib generated sensitization without altering NF-κB action in HL cells. Among different cellular targets of bortezomib, XIAP was markedly down-regulated independently of the NF-κB status and any other transcriptional regulations. Similarly, RNAi-mediated XIAP down-regulation generated susceptibility to cytostatic agents, suggesting XIAP as the key mediator of chemoresistance in HL cells.
Materials and methods
Cell lines and cell culture
The establishment and culturing of the HL and control cell lines have been previously described.15–18 L591, L428, L1309, and Jurkat cell lines were cultured in VLE RPMI 1640 supplemented with 10% FCS, 2 mM l-glutamine, 100 μg/mL streptomycin, and 100 U/mL penicillin (Biochrom, Berlin, Germany). To generate cells overexpressing XIAP (HeLa-myc-XIAP), XIAP-encoding cDNAs were obtained by reverse transcriptase–polymerase chain reaction (RT-PCR) using a first-strand cDNA derived from HeLa cells as the template and specific primers containing EcoRI and XhoI digestion sites. The PCR products were digested with EcoRI and XhoI, followed by ligation into pcDNA3 containing an N-terminal myc tag (Invitrogen, Karlsruhe, Germany). HeLa cells were stably transfected with pcDNA3 or pcDNA3-myc-XIAP by selection in medium containing 500 μg/mL G418 (Invitrogen).
Cells were treated with increasing concentrations of bortezomib, Bay 11-7085 (Bay) (Sigma, Deisenhofen, Germany), or cytostatic agents including staurosporine (STS) (0.5 μM) (Alexis, Lausen, Switzerland), etoposide (50 μM), doxorubicin (1 μM), vinblastine (0.2 μM), or cisplatin (200 μM) (Sigma) and incubated for indicated times.
Sample preparation and immunoblotting
Whole cell extracts were prepared by lysing cells in CHAPS lysis buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, 1% CHAPS, complete protease inhibitor cocktail) on ice for 20 minutes.17–18 Samples were centrifuged, and supernatants were recovered. Poly(ADP-ribose) polymerase (PARP) cleavage was assessed after incubation of pellets in urea extraction buffer (50 mM Tris [pH 6.8], 6 M urea, 3% SDS, 10% glycerol, 0.00125% bromophenol blue, 5% 2-mercaptoethanol) denatured at 100°C for 10 minutes.
Equal amounts of protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Rabbit polyclonal antiserum specific for human caspase-9 and Bax and mouse monoclonal antibodies specific for XIAP, Bcl2, PARP, and cytochrome c were obtained from BD Laboratories (Heidelberg, Germany). Rabbit anti–caspase-3, rabbit anti-Mcl1, and mouse anti-Smac antibodies were obtained from Cell Signaling Technology (Beverly, MA). Affinity purified goat anti-cIAP2 and rabbit anti-cIAP1 antibodies were obtained from R&D Systems (Wiesbaden-Nordenstadt, Germany).
Electrophoretic mobility shift assay (NF-κB activity)
NF-κB activity was assessed as described previously.19 Briefly, nuclear extracts were prepared and normalized for protein content. The NF-κB–specific oligonucleotides (Applied Biosystems, Darmstadt, Germany) were end-labeled with γ-32P-ATP (Amersham, Freiburg, Germany) using polynucleotide kinase (Roche Applied Sciences, Mannheim, Germany). Electrophoretic mobility shift assays were performed by incubating 6 μg of nuclear extracts for 20 minutes at room temperature with 500 ng poly(dT-dC) (Pharmacia, Freiburg, Germany) in a binding buffer (5 mM HEPES [pH 7.8], 5 mM MgCI, 50 mM KCI, 5 mM dithiothreitol, 10% glycerol, 20 μL final volume). Then end-labeled double-stranded oligonucleotide probe (2 × 104 cpm) was added, and the reaction mixture was incubated for 7 minutes. The samples were fractionated by electrophoresis through a 6% polyacrylamide gel in low-ionic-strength buffer (0.25× TBE).
Northern blotting
Total mRNAs were isolated from cells using NucleoSpin RNAII Kit and NucleoTrap mRNA Mini Kit (Macherey-Nagel, Düren, Germany). Samples were loaded onto a 1.2% denaturing agarose gel, separated for 2 to 3 hours at 70 V, and blotted on a positively charged nylon membrane. XIAP and β-actin were detected by specific radioactive probes labeled by rediprimeII Kit (Invitrogen).
siRNA and lentiviral gene transfer
To silence XIAP expression, pENTR construct was generated with a pair of oligonucleotides derived from XIAP mRNA (the sequence was obtained from Ambion Europe [ID: 2733], Huntingdon, United Kingdom), which includes the unique N-19 target as described in pSUPER RNAi System Manual (OligoEngine, Seattle, WA).20 The vector uses the polymerase-III H1-RNA gene promoter. After generating an entry clone, the pLenti6/V5DEST XIAP-siRNA–expressing vector was created using LR recombination. The viral particles were produced according to the instructions of the manufacturers (ViraPower Lentiviral expression system; Invitrogen). The recombinant lentiviral constructs were transduced into the cells, and stable cell lines were generated by blasticidin (Invitrogen) selection.
Cell viability
Cells (105 per well) were incubated in 96-well plastic plates at 37°C in full medium and treated with bortezomib, Bay 11-7085, or cytostatic agents for indicated times. Cell viability was assessed by XTT test (Cell Proliferation Kit II [XTT]; Roche Applied Sciences, Manheim, Germany). Briefly, after treatments cells were incubated with the XTT reagents at 37°C for 4 hours according to the instructions of the manufacturer. The absorbance of the samples was measured with an enzyme-linked immunosorbent assay (ELISA) reader (wavelength, 450 nm; reference wavelength, 620 nm). The data are mean values from 3 different experiments in triplicate. Blank absorbance from wells that lacked cells was subtracted from that of the samples, and the difference between the absorbance is referred to as “% cell viability” (100% in untreated cells).
DNA fragmentation
For DNA fragmentation assays, 107 cells were pelleted at 1000g, lysed in 200 μL lysis buffer (100 mM Tris-Cl [pH 8.5], 200 mM NaCl, 0.2% [wt/vol] SDS, 5 mM EDTA, and 2 mg/mL proteinase K), and incubated for 30 minutes at 56°C. After 2 successive phenol-chloroform-isoamylalcohol (24:24:1, pH 8.8) extractions, polynucleotides were precipitated with 1:10 vol 3 M sodium acetate (pH 5.8) and 2.5 vol ethanol. The DNA precipitate was washed once with 70% (vol/vol) ethanol and air-dried for 5 minutes and resuspended in 100 μL TE buffer (10 mM Tris-Cl [pH 8.0], 1 mM EDTA). RNA was removed by addition of 0.2 mg/mL RNase A and incubation at 37°C for 1 hour. DNA of approximately 5 × 105 cells was loaded with 10% Ficoll onto a 1.8% agarose gel and visualized with ethidium bromide under UV light.21
Results
Bortezomib-induced direct cytotoxicty is associated with decreased NF-κB activity in HL B-cell lines
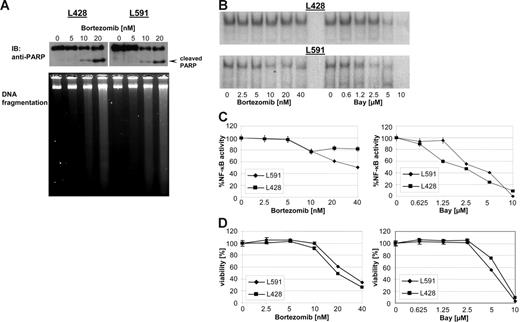
Several studies have described bortezomib as a selective mediator of apoptosis in malignant cells. HL B-cell lines have been shown to be resistant to different apoptotic stimuli. To determine the cytotoxic capacity of bortezomib in HL 2 HL B-cell lines, L428 and L591 were treated with increasing concentrations of bortezomib (0 to 20 nM) for 48 hours. PARP cleavage and degradation of DNA were used to monitor the ongoing apoptotic process after bortezomib treatment (Figure 1A). Increasing concentrations of bortezomib resulted in cleavage of PARP and degradation of DNA, demonstrating the capability of bortezomib to reactivate the defective apoptotic machinery in HL B-cell lines. By preventing the degradation of IκBα, bortezomib has been shown to inhibit NF-κB.22 NF-κB is constitutively active in H-RS cells and is suggested to be a central molecular switch in apoptosis resistance in HL.23–24 Therefore, we first analyzed the potency of bortezomib-inhibiting NF-κB activity in HL B cells by electrophoretic mobility shift assay (Figure 1B). Increasing amounts of bortezomib or Bay 11-7085 (Bay), an irreversible inhibitor of IκBα phosphorylation and degradation, reduced or completely abrogated NF-κB activity in both HL B-cell lines tested, respectively (Figure 1B-C). Similarly, both bortezomib and Bay exerted cytotoxic effects in a dose-dependent manner, suggesting an antiproliferating activity of high-dose bortezomib correlating with reduction of NF-κB activity (Figure 1D).
Bortezomib exerts cytotoxicity in HL B-cell lines. HL B-cell lines L591 and L428 were treated with increasing amount of bortezomib. (A) After 48 hours PARP was detected in nuclear extracts by mouse anti-PARP antibody. DNA of approximately 5 × 105 cells was loaded with 10% Ficoll onto a 1.8% agarose gel and visualized with ethidium bromide under UV light. (B) After 24 hours nuclear extracts were prepared and analyzed for NF-κB binding activity. (C) NF-κB binding activity was determined using a phosphoimager (molecular imager; Bio-Rad, Hercules, CA). Signal intensities were quantified and are presented as percentage of the mean levels in untreated cells. The experimental values represent mean ± SD values from 3 individual experiments performed. (D) Viability was determined by XTT assay after 48 hours and expressed as mean ± SD values from 3 individual experiments performed in triplicate.
Bortezomib exerts cytotoxicity in HL B-cell lines. HL B-cell lines L591 and L428 were treated with increasing amount of bortezomib. (A) After 48 hours PARP was detected in nuclear extracts by mouse anti-PARP antibody. DNA of approximately 5 × 105 cells was loaded with 10% Ficoll onto a 1.8% agarose gel and visualized with ethidium bromide under UV light. (B) After 24 hours nuclear extracts were prepared and analyzed for NF-κB binding activity. (C) NF-κB binding activity was determined using a phosphoimager (molecular imager; Bio-Rad, Hercules, CA). Signal intensities were quantified and are presented as percentage of the mean levels in untreated cells. The experimental values represent mean ± SD values from 3 individual experiments performed. (D) Viability was determined by XTT assay after 48 hours and expressed as mean ± SD values from 3 individual experiments performed in triplicate.
Sensitization of HL B cells against cytostatic agents without NF-κB inhibitory action
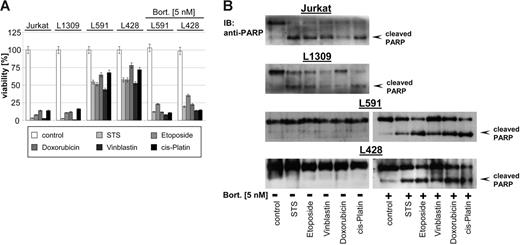
Different studies demonstrated that bortezomib potentiates the activity of other chemotherapeutics, in part by down-regulating chemoresistance pathways.1–2 As shown in Figure 2, HL B-cell lines L428 and L591 were resistant to staurosporine (STS), etoposide, doxorubicin, vinblastine, and cisplatin. In contrast, treatment of Jurkat T cells or the control B-cell line L1309 with all cytotoxic agents resulted in cell death (Figure 2A) associated with PARP cleavage (Figure 2B) indicating apoptotic cell death. Pretreatment with low-dose bortezomib (5 nM) for 48 hours that did not exert any direct cytotoxicity (Figure 1D) or NF-κB inhibitory activity (Figure 1B-C) significantly enhanced the potency of cytotoxic agents (up to 50% more cytotoxicity) (Figure 2A), which was accompanied by cleavage of PARP as a sign of ongoing apoptosis in HL B cells (Figure 2B). These data suggest that bortezomib breaks the chemoresistance phenotype of HL B cells by targeting molecular mechanisms other than NF-κB.
Bortezomib promotes cytotoxicity of cytostatic agents. Control B-cell line L1309, control Jurkat T-cell line, and HL B-cell lines (untreated or pretreated with bortezomib, 5 nM, 48 hours) were treated with STS (0.5 μM), etoposide (50 μM), doxorubicin (1 μM), vinblastine (0.2 μM), or cisplatin (200 μM) and incubated for 24 hours. (A) Viability was assayed by XTT test. The means ±SD are from 3 individual experiments performed in triplicate. (B) PARP cleavage was detected in nuclear extracts by mouse anti-PARP antibody.
Bortezomib promotes cytotoxicity of cytostatic agents. Control B-cell line L1309, control Jurkat T-cell line, and HL B-cell lines (untreated or pretreated with bortezomib, 5 nM, 48 hours) were treated with STS (0.5 μM), etoposide (50 μM), doxorubicin (1 μM), vinblastine (0.2 μM), or cisplatin (200 μM) and incubated for 24 hours. (A) Viability was assayed by XTT test. The means ±SD are from 3 individual experiments performed in triplicate. (B) PARP cleavage was detected in nuclear extracts by mouse anti-PARP antibody.
Bortezomib-induced XIAP down-regulation without NF-κB inhibition
In various tumor cell types bortezomib has been described to overcome the antiapoptotic program by altering the expression levels of different modulators of apoptosis including Bcl-2, Bax, Mcl1, and inhibitor of apoptosis proteins (IAPs).1–2 To identify possible targets of bortezomib action, HL B cells were treated with increasing amounts of bortezomib for 48 hours, and the expression levels of different apoptotic modulators were examined. As shown in Figure 3A, bortezomib treatment selectively reduced XIAP expression (95% to 100% reduction; Figure 3B) in HL B-cell lines, whereas expression levels of Bax, Bcl2, Mcl1, cIAP1, cIAP2, cytochrome c, and actin were not affected. IAPs including cIAP1, cIAP2, and XIAP have been identified as gene targets of NF-κB transcriptional activity.25–26 Indeed, unlike bortezomib, Bay dose-dependently reduced XIAP, cIAP1, and cIAP2 levels (Figure 3C), which correlated with NF-κB inhibition as expected (Figure 1B). These findings suggest that bortezomib inhibits XIAP expression by mechanisms not involving NF-κB. The control of XIAP expression by bortezomib was further examined by quantifying XIAP messenger RNA (mRNA) after bortezomib treatment by Northern blot analysis (Figure 3D). No reduction of XIAP mRNA was observed under low-dose bortezomib treatment excluding the transcriptional regulation of XIAP expression by bortezomib. XIAP expression has been shown to be additionally regulated at the translational level, specifically through an internal ribosome-entry site (IRES).27 We therefore established HeLa-myc-XIAP cell lines stably transfected with DNA constructs containing the open reading frame of XIAP without an IRES element under the control of the cytomegalovirus (CMV) promoter expressing an N-terminally myc-tagged XIAP. As shown in Figure 3E, bortezomib reduced endogenous XIAP levels as well as ectopically expressed myc-XIAP in HeLa and HeLa-myc-XIAP cells. Because bortezomib reduced XIAP irrespective of the transcriptional origin, these data suggest that bortezomib controls XIAP at the posttranslational level.
Bortezomib reduces XIAP expression. (A) HL B-cell lines were treated for 48 hours with increasing concentrations of bortezomib. cIAP2, cIAP1, XIAP, actin, Bcl2, Bax, Mcl1, and cytochrome c were detected in total cell extracts by Western blotting using specific antibodies. (B) Quantitative analysis of Western blot of XIAP expression. Graphs of mean band intensity of XIAP from Western blot images were acquired on an Alpha Innotech documentation station (Biozym; Hess, Oldendorf, Germany). All values were normalized to the actin expression levels and are presented as percentage of the mean levels in untreated cells (100%). The SD was calculated from up to 3 individual experiments. (C) HL B-cell lines were treated for 48 hours with increasing concentrations of Bay. cIAP2, cIAP1, XIAP, and actin were detected in total cell extracts by specific antibodies. (D) Quantitative analysis of XIAP mRNA. Total mRNA of HL B-cell lines was prepared and analyzed by Northern blotting using XIAP and β-actin probes. The quantification of XIAP mRNA was performed using a phosphoimager (molecular imager; Bio-Rad) by measurement of signal intensities normalized for β-actin mRNA content and is presented as percentage of the mean levels in untreated cells (100%). The data show 1 representative of 3 independent experiments. (E) HeLa and HeLa-myc-XIAP cell lines were treated for 48 hours with increasing concentrations of bortezomib. XIAP, myc-XIAP, and actin were detected in total cell extracts by Western blotting using specific antibodies.
Bortezomib reduces XIAP expression. (A) HL B-cell lines were treated for 48 hours with increasing concentrations of bortezomib. cIAP2, cIAP1, XIAP, actin, Bcl2, Bax, Mcl1, and cytochrome c were detected in total cell extracts by Western blotting using specific antibodies. (B) Quantitative analysis of Western blot of XIAP expression. Graphs of mean band intensity of XIAP from Western blot images were acquired on an Alpha Innotech documentation station (Biozym; Hess, Oldendorf, Germany). All values were normalized to the actin expression levels and are presented as percentage of the mean levels in untreated cells (100%). The SD was calculated from up to 3 individual experiments. (C) HL B-cell lines were treated for 48 hours with increasing concentrations of Bay. cIAP2, cIAP1, XIAP, and actin were detected in total cell extracts by specific antibodies. (D) Quantitative analysis of XIAP mRNA. Total mRNA of HL B-cell lines was prepared and analyzed by Northern blotting using XIAP and β-actin probes. The quantification of XIAP mRNA was performed using a phosphoimager (molecular imager; Bio-Rad) by measurement of signal intensities normalized for β-actin mRNA content and is presented as percentage of the mean levels in untreated cells (100%). The data show 1 representative of 3 independent experiments. (E) HeLa and HeLa-myc-XIAP cell lines were treated for 48 hours with increasing concentrations of bortezomib. XIAP, myc-XIAP, and actin were detected in total cell extracts by Western blotting using specific antibodies.
XIAP knock-down restores susceptibility of HL B cells to cytotoxic agents
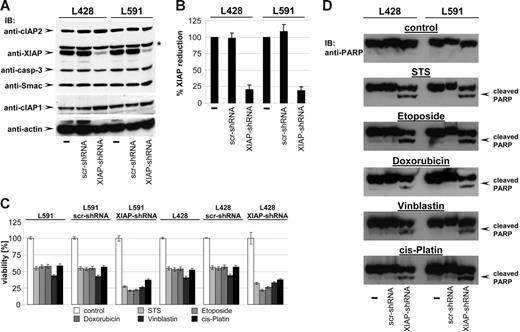
One of the hallmarks of H-RS cells is the high expression level of XIAP, a potent inhibitor of caspases and apoptosis, which has been suggested to be one of the key mediators of apoptosis resistance in H-RS cells.18 Thus, XIAP appeared to be a likely candidate target of bortezomib action creating susceptibility against cytostatic drugs. If XIAP were the key mediator of chemoresistance in HL B cells, knock-down of XIAP by RNAi should sensitize HL B cells to cytotoxic drugs. XIAP expression was specifically down-regulated by generating HL B-cell lines stably expressing small hairpin RNA (shRNA) targeting XIAP mRNA using lentiviral gene transfer. As shown in Figure 4A, only HL cells expressing shRNA against XIAP (L428-XIAP-shRNA and L591-XIAP-shRNA) displayed down-regulated XIAP expression (up to 82% reduction; Figure 4B), while scrambled (scr) shRNA (L428-scr-shRNA and L591-scr-shRNA) remained ineffective. The specificity of XIAP knock-down was revealed by unaltered expression of caspase-3, Smac, cIAP1, cIAP2, and actin (Figure 4A).
Knock-down of XIAP by RNA interference sensitizes HL B-cell lines for cytostatic agents. (A) cIAP2, XIAP, caspase-3, Smac, cIAP1, and actin were detected in total cell extracts of L428, L428-scr-shRNA, L428-XIAP-shRNA, L591, L591-scr-shRNA, and L591-XIAP-shRNA by Western blot analysis using specific antibodies. (B) Quantitative analysis of panel A. Graphs of mean band intensity of XIAP from Western blot images were acquired on an Alpha Innotech documentation station. All values were normalized to actin expression levels and are presented as percentage of the mean levels in untreated cells (100%). The SD values were calculated from 3 individual experiments. (C-D) HL B-cell lines L428, L428-scr-shRNA, L428-XIAP-shRNA, L591, L591-scr-shRNA, and L591-XIAP-shRNA were left untreated or treated for 24 hours with STS (0.5 μM), etoposide (50 μM), doxorubicin (1 μM), vinblastine (0.2 μM), or cisplatin (200 μM). Viable cell number was determined using an XTT assay (C). PARP cleavage was detected in nuclear extracts by mouse anti-PARP antibody (D).
Knock-down of XIAP by RNA interference sensitizes HL B-cell lines for cytostatic agents. (A) cIAP2, XIAP, caspase-3, Smac, cIAP1, and actin were detected in total cell extracts of L428, L428-scr-shRNA, L428-XIAP-shRNA, L591, L591-scr-shRNA, and L591-XIAP-shRNA by Western blot analysis using specific antibodies. (B) Quantitative analysis of panel A. Graphs of mean band intensity of XIAP from Western blot images were acquired on an Alpha Innotech documentation station. All values were normalized to actin expression levels and are presented as percentage of the mean levels in untreated cells (100%). The SD values were calculated from 3 individual experiments. (C-D) HL B-cell lines L428, L428-scr-shRNA, L428-XIAP-shRNA, L591, L591-scr-shRNA, and L591-XIAP-shRNA were left untreated or treated for 24 hours with STS (0.5 μM), etoposide (50 μM), doxorubicin (1 μM), vinblastine (0.2 μM), or cisplatin (200 μM). Viable cell number was determined using an XTT assay (C). PARP cleavage was detected in nuclear extracts by mouse anti-PARP antibody (D).
We finally examined whether XIAP down-regulation results in increased susceptibility to cytotoxic drugs by analyzing cell viability and PARP cleavage. As shown in Figure 4C-D, cytotoxic agents induced significantly increased cell death of L428-XIAP-shRNA and L591-XIAP-shRNA and PARP cleavage. As already shown in Figure 2A, parental L428 and L591 or derivatives expressing scrambled shRNA were only partially affected and did not show any PARP cleavage. Apparently, selective down-regulation of XIAP recapitulates the chemosensitivity phenotype of bortezomib.
Discussion
In this study, we scrutinized bortezomib as an antiproliferative agent in Hodgkin lymphoma B-cell lines. We describe here a bimodal action of bortezomib where high-dose bortezomib induces direct cytotoxicity and inhibition of NF-κB activity. At lower doses bortezomib was neither cytotoxic nor did it alter NF-κB activity, yet it established a marked sensitization against a variety of cytotoxic drugs. A major observation of this study is that bortezomib selectively and independently of NF-κB down-regulates XIAP, previously shown to be a key mediator of apoptosis resistance in HL cells.18,28
Proteasome inhibitors first drew clinical interest as potential antineoplastic agents owing to their ability to inhibit NF-κB activity.1–2 NF-κB has been shown to be constitutively active in H-RS cells and is suggested to be a central molecular switch in apoptosis resistance in HL.23–24 However, our data shown in Figure 2 demonstrate that low-dose bortezomib but not Bay (data not shown) significantly down-regulates the chemoresistance in HL B cells. Notably, under this condition neither direct cytotoxicity nor NF-κB inhibition was observed in bortezomib-pretreated HL B cells, suggesting that NF-κB inhibition does not account for chemosensitization of HL B cells pretreated with bortezomib. Further, only high-dose bortezomib exerted direct cytotoxicity, which correlated with reduction of NF-κB activity. In other studies, treatment of chronic lymphocytic leukemia (CLL) cells, solid tumor cell lines, and lymphoma cells with proteasome inhibitors induced apoptosis but did not lead to NF-κB inhibition,1 suggesting that even direct cytotoxicity of bortezomib might not be linked to its effects on NF-κB activity. Bortezomib has been shown to induce cell cycle arrest and apoptosis in HL cell lines irrespective of Iκ-B gene mutation, suggesting that additional activities may be mediated by NF-κB–independent mechanisms.29 Rather, given the broad range of proteasome substrates, it seems likely that proteasome inhibitor–mediated sensitization/apoptosis in HL B cells involves factors other than NF-κB modulators. Moreover, whereas several clinical trials evaluated the use of bortezomib as single-agent treatment of various refractory and relapsed malignancies,1 the clinical effectivity of bortezomib in tumors including renal cell carcinoma, neuroendocrine tumors, sarcomas, acute leukemias, and melanoma has been less promising, and combination therapies were proposed.30–34 This is an important observation because a recent pilot study of bortezomib in patients with relapsed and refractory classical HL demonstrated that in these patients bortezomib has minimal single-agent activity, suggesting a need for bortezomib-based combination therapy in HL.35
Altered proliferation in cancer cells is usually in a delicate balance with the apoptotic option. Bortezomib has been described to induce susceptibility in tumor cells by multifactorial mechanisms involving the altered turnover of different critical cellular proteins involved in an apoptotic program including Bcl2 family proteins and IAPs.1–2 Our results demonstrated a marked down-regulation of XIAP in HL B-cell lines (L428 and L591, Figure 3; KMH2 and L1236, data not shown) upon bortezomib treatment, which is in line with previous reports suggesting XIAP as a possible target for bortezomib in different tumors.36–38 Through its ability to inhibit caspases, XIAP has been suggested to be a key mediator of apoptosis resistance in H-RS cells.18,28 As a target gene of NF-κB transcriptional activity,26 XIAP has been recently shown to be down-regulated by inhibition of NF-κB in HL cell lines that promoted caspase-dependent cell death.39 However, bortezomib-induced XIAP reduction in HL B cells occurred independently of NF-κB activity. Analysis of XIAP mRNA after bortezomib treatment of HL B cells excluded the transcriptional control of XIAP down-regulation by bortezomib. Further, XIAP expression is additionally regulated at the translational level, specifically through an internal ribosome-entry site (IRES),27 which might be targeted by bortezomib treatment. Analysis of bortezomib's impact on cap-dependent ectopically expressed myc-XIAP or IRES-regulated endogenous XIAP in HeLa cells revealed reduction of both myc-XIAP and endogenous XIAP irrespective of transcriptional origin. Thus, bortezomib modulates XIAP in HL B cells at the posttranslational level.
The observations that bortezomib-induced reduction of XIAP as well as down-regulation of XIAP by RNAi promoted cytotoxicity of a variety of cytostatic drugs and the fact that XIAP is strongly expressed in most HL cases18,40 argue for XIAP as the possible chemoresistance factor in HL B cells. The results of this study suggest that a combination of cytostatic agents and bortezomib might become a valuable therapeutic modality in HL.
Authorship
Contribution: H.K. designed and performed the experiments, analyzed the data, and wrote the paper; A.D., J.-M.S., B.Y., K.W., D.H., and C.P. participated in performing the research; M.K. controlled and analyzed the data; and all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hamid Kashkar, Institute for Medical Microbiology, Immunology and Hygiene, University of Cologne, Goldenfelsstrasse 19-21, 50935 Köln, Germany; e-mail: h.kashkar@uni-koeln.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work is supported by grants from Deutsche Krebshilfe (M.K.) and Köln Fortune Program/Faculty of Medicine, University of Cologne (H.K.).


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal