Abstract
Antiendothelial cell antibodies (AECAs) are commonly detectable in diseases associated with vascular injury, including systemic lupus erythematosus (SLE), systemic sclerosis, Takayasu arteritis, Wegener granulomatosis, Behçet syndrome, and transplant arteriosclerosis. Here, we explore the hypothesis that these antibodies might augment polymorphonuclear leukocyte (PMN) adhesion to endothelium in inflammation. Initially, we established that a mouse IgG mAb bound to endothelial cells (ECs) significantly increased PMN adhesion to cytokine-stimulated endothelium in an FcγRIIa-dependent manner. Neutralizing antibodies, and adenoviral transduction of resting ECs, demonstrated that the combination of E-selectin, CXCR1/2, and β2 integrins is both necessary and sufficient for this process. We observed an identical mechanism using AECA IgG isolated directly from patients with SLE. Assembled immune complexes also enhanced PMN adhesion to endothelium, but, in contrast to adhesion because of AECAs, this process did not require CXCR1/2, was not inhibited by pertussis toxin, and was FcγRIIIb rather than FcγRIIa dependent. These data are the first to demonstrate separate nonredundant FcγRIIa and FcγRIIIb-mediated mechanisms by which EC-bound monomeric IgG and assembled immune complexes amplify leukocyte adhesion under dynamic conditions. Furthermore, the observation that FcγRIIa and CXCR1/2 cooperate to enhance PMN recruitment in the presence of AECAs suggests a mechanism whereby AECAs may augment tissue injury during inflammatory responses.
Introduction
The recruitment of circulating polymorphonuclear leukocytes (PMNs) to sites of inflammation is mediated by a series of adhesion and activation steps, known as the “adhesion cascade.”1 Thus, selectins are largely responsible for initial tethering of flowing PMNs to endothelium and also mediate subsequent PMN rolling on the endothelial surface. Activation of rolling PMNs leads to leukocyte arrest via modulation of the affinity and avidity of β2 integrins, with LFA-1 (CD11a/CD18) acting primarily to slow rolling and to promote arrest, and Mac-1 (CD11b/CD18) acting to stabilize adhesion.2–5 The capacity of endothelial cells (ECs) to support these interactions with PMNs is stimulated by cytokines such as TNFα and IL-1β, which induce expression of a large number of adhesion molecules, chemottractant, and other proinflammatory genes, including E-selectin, chemokines (eg, IL-8), and ICAM-1.6,7
Antibodies that react with the surface of vascular endothelial cells (antiendothelial cell antibodies, AECAs) are found in a variety of diseases associated with vascular injury, including systemic lupus erythematosus (SLE), systemic sclerosis, Takayasu arteritis, Wegener granulomatosis, Behçets syndrome, and transplant arteriosclerosis,8,9 A number of mechanisms have been proposed whereby IgG binding to ECs may exert pathogenic effects, including the induction of EC inflammatory activation and thrombogenicity, the stimulation of leukocyte free-radical production and cellular cytotoxicity, and the induction of EC apoptosis.10–14
Interactions between circulating human PMNs and immune complexes are mediated via 2 low-affinity Fcγ receptors, FcγRIIa (CD32a) and FcγRIIIb (CD16b), which are both thought to form homodimers and have distinct membrane-anchoring and -signaling capacities.15–19 The cytoplasmic domain of FcγRIIa has a specialized immunoreceptor tyrosine-based activation motif, which on receptor cross-linking becomes tyrosine-phosphorylated by Src-family tyrosine kinases such as Fgr and Lyn.20–22 Subsequent receptor association with Syk kinase and phosphoinositide 3-kinase results in phagocytosis, degranulation, respiratory burst, and antibody-dependent cellular cytotoxicity.23 In contrast to FcγRIIa, FcγRIIIb is glycophosphatidyl inisotol linked to the cell membrane and may signal via activating distinct Src-family tyrosine kinases such as Hck, as well as by raising the intracellular Ca++ concentration.21,24,25 In most assay systems, FcγRIIa and FcγRIIIb cooperate in stimulating PMN effector functions.26–31 FcγRIIa and FcγRIIIb have overlapping IgG isotype-binding specificities, preferentially recognizing human IgG1 and IgG3, and reacting well with murine IgG2a.16,32
Cross-talk between PMN Fcγ receptors and β2 integrins, particularly Mac-1 (CD11b/CD18, CR3), is well known to occur.33 Under static conditions, initial adhesion to surfaces coated with immune complexes is independent of β2 integrins, but β2 integrins are needed for cell spreading and the maintenance of adhesion.34–36 However, Coxon et al37 reported a much faster effect of FcγRIIIb ligation on PMN adhesion to a glass surface coated with immune complexes, showing that FcγRIIIb is expressed on the tips of PMN microvilli and well placed to interact with IgG under dynamic conditions.37 Furthermore, they found that FcγRIIIb mediates selectin-independent capture of PMNs under flow conditions and the immediate triggering of Mac-1–dependent arrest.37 This has suggested an important mechanism whereby immune complexes may trigger vascular injury.
In this study, we have addressed the issue of whether AECAs influence PMN recruitment under dynamic conditions. We show for the first time that perfusion of AECAs over ECs results in IgG binding to ECs and subsequently to enhanced leukocyte adhesion, but only if ECs are cytokine activated. This ability of monomeric IgG bound to ECs to augment PMN recruitment to cytokine-activated cells is dependent on E-selectin and occurs through the ability of FcγRIIa and CXCR-1/2 to synergize in activating β2 integrins. This mechanism is distinct from FcγRIIIb-mediated PMN recruitment, which can take place in the absence of chemokine-receptor costimulation but does not occur when ECs are coated simply with monomeric IgG.
Materials and methods
Antibodies and reagents
Mouse antiendoglin (mAb RMAC8), anti–L-selectin (mAb DREG-56), anti–E-selectin (mAb 1.2B6), anti–ICAM-1 (mAb 6.5B6), anti-FcγRIIIb (mAb 3G8), anti-FcγRIIa (mAb IV.3), anti-CD18 (mAb TS1/18), and anti–LFA-1 (mAb TS1/22) were purified from hybridoma supernatants. Anti-CD11b mAb ICRF-44 was a gift from Dr Nancy Hogg (Cancer Research UK, London, United Kingdom). Anti–CXCR-1 (clone 501) and anti–CXCR-2 (clone 19) were from Biosource (Nivelles, Belgium). Anti–E-selectin mAb SPLAT-1 (human IgG4) was a gift from Dr Martyn Robinson (UCB Celltech, Slough, United Kingdom). Control mouse mAb MOPC21 was from Sigma-Aldrich (Poole, United Kingdom). Fab fragments of mAb 3G8 were generated using the ImmunoPure Fab preparation kit (Pierce, Rockford, IL). Biotinylated anti–human IgG/IgM and anti–human IgG was from Jackson Immunological (West Grove, PA). FITC-conjugated goat anti–mouse Ig and sheep anti–rabbit Ig were from Dako (Ely, United Kingdom). Polyclonal rabbit anti–mouse Ig serum (RbAMIg) was obtained by immunizing rabbits with mouse IgG. Biotinylated antibodies against human IgG1, IgG2, IgG3, and IgG4 were from Sigma-Aldrich. AlexaFluor-conjugated streptavidin, anti–mouse Ig, and anti–rabbit Ig antisera were from Invitrogen (Carlsbad, CA). TNFα was obtained from UCB Celltech. Other cell culture reagents were from Sigma-Aldrich.
Generation of adenoviral constructs
E-selectin (adE-selectin) and IL-8 (adIL-8) adenoviral constructs were generated using the ViraPower adenoviral system (Invitrogen). An ICAM-1 adenovirus construct (AdICAM-1) was donated by Dr Charlotte Lawson (Imperial College).
Cell culture
Immortalized human microvascular ECs (HMEC-1) were maintained in MCBD131 (Invitrogen) with 15% heat-inactivated fetal calf serum (FCS), 100 IU/mL penicillin/streptomycin, 1 mM L-glutamine, 10 U/mL heparin, and 10 ng/mL epidermal growth factor. HL60 cells were cultured at a density of 2 × 105 to 2 × 106/mL in RPMI/10% FCS.
PMN isolation
PMNs were purified from healthy volunteers by centrifugation over a Histopaque (Sigma-Aldrich) gradient followed by sedimentation in 2% Dextran. After lysis of erythrocytes using sterile water, PMNs were resuspended in Hanks balanced saline solution (HBSS) with calcium and magnesium prior to use.
Pretreatment of HMEC-1 monolayers
The human dermal microvsacular EC line HMEC-1 cells (donated by Dr Edwin Ades, CDC, Atlanta, GA) was grown to confluency on 9 cm2 Nunc Flaskettes (VWR) and activated with TNFα (10 ng/mL) for 5 hours where indicated. For adenoviral infection, HMEC-1 cells at 80% confluency were infected with adenovirus in serum-free MCDB131 for 3 hours and were then maintained in fresh medium for a further 48 hours. Slides were then assembled into the flow chamber apparatus for experiments. IgG was deposited on ECs by perfusion with antiendoglin mAb RMAC8, or SLE IgG, for 5 minutes at 1.5 dynes/cm2 at 37°C. Immune complexes were assembled by perfusion of RbAMIg (1:250 dilution) for 5 minutes after mAb RMAC8.
Flow cytometry
For cell-surface expression of proteins, 105 cells were added to each well of a 96-well plate and spun at 212 g for 1 minute. Primary antibody (50 μL at 10 μg/mL) was added, and cells were left on ice for 15 minutes. After washing, appropriate secondary conjugated antibody (50 μL) was added at the recommended dilution and left for 20 minutes on ice in the dark prior to washing and fixation with 2% paraformaldehyde. Cells were then analyzed with an EPICS XL flow cytometer (Beckman Coulter, High Wycombe, United Kingdom). Relative fluorescent intensity (RFI) was calculated by dividing the mean fluorescent intensity of test antibody by the fluorescent intensity of an isotype-matched negative control antibody, where no expression has an RFI of 1.00.
Confocal microscopy
HMEC-1 cells were fixed with 1.25% paraformaldehyde for 10 minutes while inside the flow chamber. They were then washed and incubated with AlexaFluor-conjugated antibodies or biotinylated anti–human IgG for 45 minutes followed by streptavidin AlexaFluor 488 for 30 minutes, followed by washing and mounting in antifade mounting media (Dako). Images were visualized using a Zeiss Axiovert 200 LSM 510 confocal microscope, with a Plan-NeoFluor 40×/1.3 NA oil DIC objective (Zeiss, Welwyn Garden City, United Kingdom). Images were acquired using the Zeiss LSM software and processed using Zeiss LSM image browser 3.5 and Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA).
Adhesion assays under flow
Adhesion assays were conducted as described.38 Briefly, HMEC-1 monolayers were assembled in a parallel plate flow chamber and placed onto the stage of a 37°C prewarmed inverted Nikon (supplied by Microscope Services and Sales, Engham, United Kingdom) Diaphot 300 florescence microscope connected to a JVC (supplied by Microscope Services and Sales, Engham, United Kingdom)) TK-C1360 color video camera. PMNs (106/mL) or HL60 cells (3 × 105/mL) were perfused over EC monolayers at the indicated shear. After 2 minutes, 10 fields of view were recorded for 10 seconds and analyzed for leukocyte adhesive interactions using Cell Motion tracking software (Ed Marcus Laboratories, Newton, MA). HL60 rolling on E-selectin was determined to be between 3 and 100 μm/seconds, whereas PMNs roll at a slower rate and thresholds were set at 2 to 50 μm/seconds. In antibody-blocking experiments, cells were preincubated with antibody for 20 minutes prior to the assay.
SLE sera
Patients with SLE satisfied the American College of Rheumatology Criteria.39 Informed consent was obtained in accordance with the Declaration of Helsinki, and the project was approved by the Hammersmith Hospital's Trust Research Ethics Committee. Following venipuncture, blood was allowed to clot and then centrifuged at 2000g for 10 minutes prior to aspiration of serum and storage at −70°C. To purify IgG, samples were precleared using a Sepharose C10 column (GE Healthcare, Little Chalfont, Bucks, United Kingdom) and passed over Sepharose-conjugated protein G (Amersham). IgG was eluted using 0.1 M glycine (pH 2.5) and protein concentration determined by measuring absorbance at 280 mm. Purity of IgG fractions was confirmed by high-performance liquid chromatography.
AECA ELISA
An enzyme-linked immunoabsorbent assay (ELISA) was performed on unfixed HMEC-1 cells to detect AECAs. Cells were seeded (2 × 104 per well) on 96-well plates for 48 hours. Plates were then blocked with HBSS + 1% BSA for 1 hour at 37°C before incubation with patient serum (75 μL diluted 1:5 in HBSS) or purified IgG for 1 hour at 37°C. After washing, cells were incubated with biotinylated goat anti–human IgG/IgM or anti–human IgG isotype specific antibodies for 1 hour. After 3 washes, streptavidin-HRP (1:200) was added for 20 minutes followed by incubation with TMB liquid substrate (Sigma, St Louis, MO). The OD was measured at 450 mm using an ELISA reader (supplied by Thermo Fisher Scientific, Hemel Hempstead, United Kingdom).
Statistical analysis
Data are expressed as the mean ± SEM. Statistical comparison of means was performed by 2-tailed unpaired Student t test.
Results
IgG bound to ECs enhances leukocyte adhesion to TNFα-activated ECs
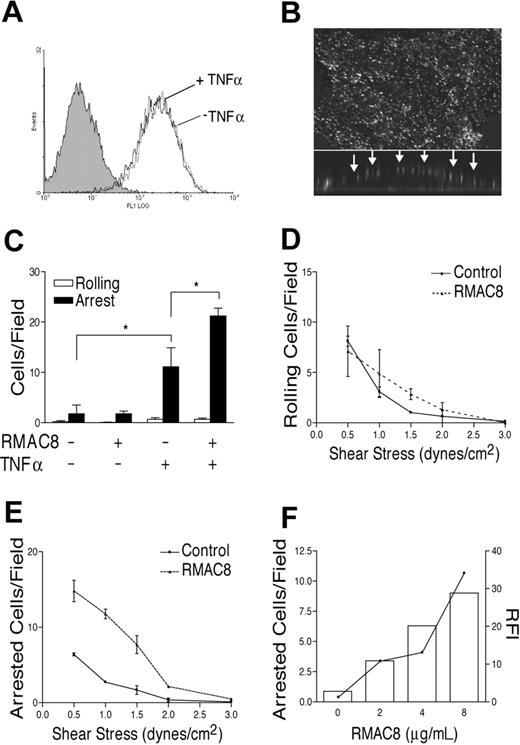
Mouse anti–human endoglin mAb RMAC8 (IgG2a) was chosen as a model antibody for this study because endoglin has a high level of constitutive expression on the apical surface of HMEC-1 cells, with no change following TNFα stimulation. Perfusion of mAb RMAC8 at a shear comparable to that in postcapillary venules (1.5 dynes/cm2) led to similar binding to unstimulated or TNFα-activated HMEC-1 cells (Figure 1A). No nonspecific IgG binding occurred when control mAb MOPC21 was perfused over EC monolayers in the same manner. Confocal microscopy of MCEC-1 cells following perfusion of mAb RMAC8 showed a granular distribution of IgG over the apical surface of the cells (Figure 1B).
IgG binding to ECs augments TNFα-mediated leukocyte recruitment. (A) Mouse antiendoglin mAb RMAC8 (10 μg/mL) was perfused over HMEC-1 cells at 0.83 mL/minute for 5 minutes, after which antibody binding was detected by flow cytometry using FITC rabbit anti–mouse Ig. The figure shows similar mAb RMAC8 binding to unstimulated (black line) and TNFα (10 ng/mL, 5 hours)–activated HMEC-1 cells (gray line). The filled histogram shows lack of binding of control mAb MOPC21. (B) Confirmation by confocal imaging of the deposition of RMAC8 on the surface of an EC, with the main panel showing composite images of Z-stack views and the lower insert showing cell cross-section. Arrows point to deposition of antibody on the apical surface. (C) PMNs were perfused at 1.5 dynes/cm2 over ECs precoated with ± RMAC8, with or without prestimulation with TNFα. The figure shows that mAb RMAC8 enhanced PMN adhesion, but only if ECs were TNFα activated. Values are mean + SEM of 3 experiments (*P < .01). HL60 cell rolling (D) and arrest (E) on TNFα-stimulated ECs, showing increased arrest but not rolling on mAb RMAC8-coated ECs over a range of 0.5 to 1.5 dynes/cm2; (F) shows that increasing RMAC8 deposition resulted in increasing HL60 cell arrest on TNFα-stimulated ECs. Relative fluorescent intensities of RMAC8 binding by flow cytometry are depicted by the line (right axis), with HL60 arrest shown in bars (left axis).
IgG binding to ECs augments TNFα-mediated leukocyte recruitment. (A) Mouse antiendoglin mAb RMAC8 (10 μg/mL) was perfused over HMEC-1 cells at 0.83 mL/minute for 5 minutes, after which antibody binding was detected by flow cytometry using FITC rabbit anti–mouse Ig. The figure shows similar mAb RMAC8 binding to unstimulated (black line) and TNFα (10 ng/mL, 5 hours)–activated HMEC-1 cells (gray line). The filled histogram shows lack of binding of control mAb MOPC21. (B) Confirmation by confocal imaging of the deposition of RMAC8 on the surface of an EC, with the main panel showing composite images of Z-stack views and the lower insert showing cell cross-section. Arrows point to deposition of antibody on the apical surface. (C) PMNs were perfused at 1.5 dynes/cm2 over ECs precoated with ± RMAC8, with or without prestimulation with TNFα. The figure shows that mAb RMAC8 enhanced PMN adhesion, but only if ECs were TNFα activated. Values are mean + SEM of 3 experiments (*P < .01). HL60 cell rolling (D) and arrest (E) on TNFα-stimulated ECs, showing increased arrest but not rolling on mAb RMAC8-coated ECs over a range of 0.5 to 1.5 dynes/cm2; (F) shows that increasing RMAC8 deposition resulted in increasing HL60 cell arrest on TNFα-stimulated ECs. Relative fluorescent intensities of RMAC8 binding by flow cytometry are depicted by the line (right axis), with HL60 arrest shown in bars (left axis).
Negligible adhesive interactions were observed when PMNs or HL60 cells were passed over unstimulated HMEC-1 cells at 1.5 dynes/cm2, but as expected recruitment was stimulated by preactivation of HMEC-1 cells with TNFα. The deposition of mAb RMAC8 had no detectable effect on leukocyte interactions with resting unstimulated HMEC-1 cells but led to significantly increased arrest of PMNs on TNFα-stimulated ECs over and above that seen on TNFα-stimulated cultures without antibody (Figure 1C). Thus, PMN arrest on mAb RMAC8-coated TNFα-activated ECs was 22.36 + 1.58 cells/field compared with 11.09 + 2.66 on uncoated cells (means + SEM of 3 experiments; P < .01). Experiments altering the level of shear showed that, whereas the presence of mAb RMAC8 did not significantly influence HL60 cell rolling on TNFα-activated HMEC-1 cells (Figure 1D), HL60 cell arrest was enhanced over a range of 0.5 to 1.5 dynes/cm2 (Figure 1E). IgG-mediated HL60 cell arrest was dependent on the concentration of RMAC8 perfused over the monolayers (Figure 1F).
IgG-enhanced leukocyte adhesion to TNFα-activated ECs requires E-selectin, β2 integrins, and FcγRIIa but not FcγRIIIb
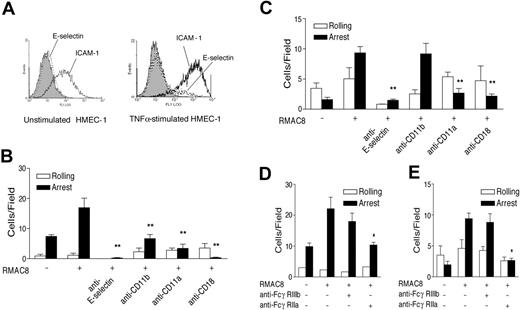
To define the adhesion molecules active in this model, PMNs, HL60 cells, and HMEC-1 cells were analyzed by flow cytometry. PMNs expressed readily detectable CD11a and CD11b, whereas only CD11a was detectable on HL60 cells. Stimulation of HMEC-1 cells with TNFα led to a large increase in ICAM-1 expression with the RFI increasing from 3.8 to 63.2 (Figure 2A). TNFα also increased expression of E-selectin on HMEC-1 cells (Figure 2A), although the change in expression was very modest compared with that seen with human umbilical cord vein endothelial cells (not shown). Nevertheless, IgG-augmented PMN arrest on TNFα-stimulated HMEC-1 cells at 1.5 dyne/cm2 was fully inhibited by neutralizing antibodies to E-selectin or CD18 and significantly inhibited by either anti-CD11a or anti-CD11b (Figure 2B). Similar observations were made with HL60 cells, although, consistent with the lack of Mac-1 expression, no inhibition was observed with anti-CD11b (Figure 2C).
IgG-enhanced leukocyte recruitment requires E-selectin and β2 integrins. (A) Flow cytometry showing basal expression of E-selectin (mAb 1.2B6, dotted line) and ICAM-1 (mAb 6.5B5, black line) and increased expression following TNFα stimulation (10 ng/mL for 4 hours). The filled histograms show lack of reactivity of control mAb MOPC21. (B-E) Inhibition of RMAC8-mediated PMN (B,D) and HL60 cell (C,E) adhesion at 1.5 dynes/cm2 to TNFα-stimulated ECs by (B,C) anti-E-selectin (mAb SPLAT-1), anti-β2 integrin (mAb TS1/18), anti-αL (mAb TS1/22), or anti-αM (mAb 44), or (D,E) anti-FcγRIIa (IV.3), but not by fab′ anti-FcγRIIIb (3G8). Values are mean ± SEM of 3 experiments (*P < .01, **P < .03).
IgG-enhanced leukocyte recruitment requires E-selectin and β2 integrins. (A) Flow cytometry showing basal expression of E-selectin (mAb 1.2B6, dotted line) and ICAM-1 (mAb 6.5B5, black line) and increased expression following TNFα stimulation (10 ng/mL for 4 hours). The filled histograms show lack of reactivity of control mAb MOPC21. (B-E) Inhibition of RMAC8-mediated PMN (B,D) and HL60 cell (C,E) adhesion at 1.5 dynes/cm2 to TNFα-stimulated ECs by (B,C) anti-E-selectin (mAb SPLAT-1), anti-β2 integrin (mAb TS1/18), anti-αL (mAb TS1/22), or anti-αM (mAb 44), or (D,E) anti-FcγRIIa (IV.3), but not by fab′ anti-FcγRIIIb (3G8). Values are mean ± SEM of 3 experiments (*P < .01, **P < .03).
HL60 cells had a comparable surface density of FcγRIIa on flow cytometry to that on PMNs (RFI 11.3 and 10.2, respectively) but had much lower expression of FcγRIIIb (RFI 2.1 on HL60 cells compared with 97.6 on PMNs). Using inhibitory antibodies to FcγRIIa and FcγRIIIb, we found that RMAC8-mediated adhesion of both PMNs and HL60 cells to TNFα-stimulated ECs was fully abrogated by anti-FcγRIIa, whereas anti-FcγRIIIb mAb 3G8 had no significant inhibitory effect (Figure 2D-E). Taken together, these data therefore indicate that FcγRIIa is the principal Fcγ receptor involved in mAb RMAC8-enhanced adhesion to TNFα-stimulated EC monolayers under flow.
Expression of E-selectin and ICAM-1 via adenovirus infection does not substitute for TNFα
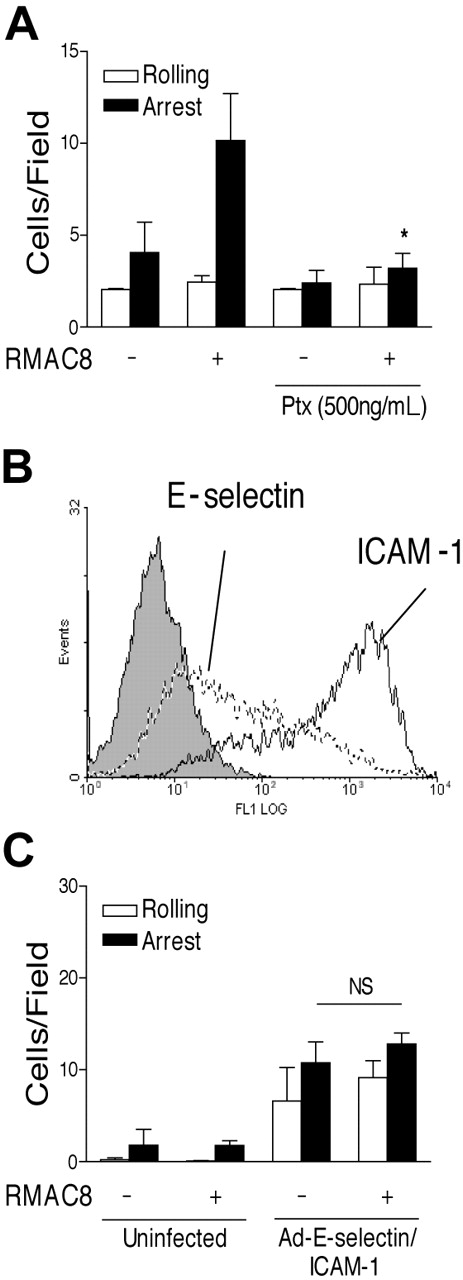
Surprisingly, the RMAC8-mediated enhancement of both HL60 cell (Figure 3A) and PMN (not shown) adhesion to TNFα-activated HMEC-1 cells was significantly inhibited by pertussis toxin, suggesting the possible requirement for G-protein–coupled receptor (GPCR) signaling. Cells were still viable after treatment with pertussis toxin, as shown by their trypan blue exclusion and calcium flux in response to FcγRIIa cross-linking (data not shown). We therefore used adenovirus constructs to test whether E-selectin and ICAM-1 expression was sufficient to allow FcγRIIa-mediated adhesion to occur. Infection with adE-selectin (100 MOI) and adICAM-1 (50 MOI) adenovirus constructs led to induction of E-selectin expression and up-regulation of ICAM-1, as shown by flow cytometry (Figure 3B). No increased expression of these molecules or increased adhesion of leukocytes was observed following infection with adenovirus expressing β-galactosidase at up to 400 MOI, indicating that adenoviral infection per se did not induce up-regulation of endogenous adhesion mechanisms in HMEC-1 cells (not shown). Infection with adE-selectin and ICAM-1 led to increases in leukocyte interactions with HMEC-1 cells at a shear stress of 1.5 dynes/cm2 that were comparable to those seen on TNFα-activated ECs. However, up-regulation of E-selectin and ICAM-1 expression was not sufficient to allow mAb RMAC8 to influence adhesion of PMNs (Figure 3C) or HL60 cells (not shown). It was possible to increase the number of PMNs arrested on mAb RMAC8-coated ECs from 12.8 cells/field to 24.9 cells/field by preactivation with TNFα (not shown), demonstrating that the failure of mAb RMAC8 to increase adhesion on mAb RMAC8-coated HMEC-1 cells infected with adE-selectin and ICAM-1 was not due to adhesion being maximal.
Expression of E-selectin and ICAM-1 is not sufficient to allow IgG-enhanced leukocyte adhesion to ECs. (A) HL60 cells were preincubated with pertussis toxin (500 μg/mL for 5 hours) before perfusion at 1.5 dynes/cm2 over TNFα-stimulated ECs coated with mAb RMAC8. (B) Flow cytometry profiles of E-selectin (mAb 1.2B6, dashed line), ICAM-1 (mAb 6.5B5, black line), and antibody control (mAb MOPC21, filled line) on HMEC-1 cells coinfected with adE-selectin (100 MOI) and adICAM-1 (50 MOI). Note increased expression of E-selectin and ICAM-1 compared with Figure 2A. (C) Lack of effect is shown of mAb RMAC8 on PMN interactions at 1.5 dynes/cm2 on resting ECs, despite increasing E-selectin and ICAM-1 expression by coinfection with adE-selectin (100 MOI) and adICAM-1 (50 MOI). Values are mean + SEM of 3 experiments (*P < .01, NS = not significantly different).
Expression of E-selectin and ICAM-1 is not sufficient to allow IgG-enhanced leukocyte adhesion to ECs. (A) HL60 cells were preincubated with pertussis toxin (500 μg/mL for 5 hours) before perfusion at 1.5 dynes/cm2 over TNFα-stimulated ECs coated with mAb RMAC8. (B) Flow cytometry profiles of E-selectin (mAb 1.2B6, dashed line), ICAM-1 (mAb 6.5B5, black line), and antibody control (mAb MOPC21, filled line) on HMEC-1 cells coinfected with adE-selectin (100 MOI) and adICAM-1 (50 MOI). Note increased expression of E-selectin and ICAM-1 compared with Figure 2A. (C) Lack of effect is shown of mAb RMAC8 on PMN interactions at 1.5 dynes/cm2 on resting ECs, despite increasing E-selectin and ICAM-1 expression by coinfection with adE-selectin (100 MOI) and adICAM-1 (50 MOI). Values are mean + SEM of 3 experiments (*P < .01, NS = not significantly different).
Expression of E-selectin and IL-8 via adenovirus infection allows IgG-mediated leukocyte adhesion
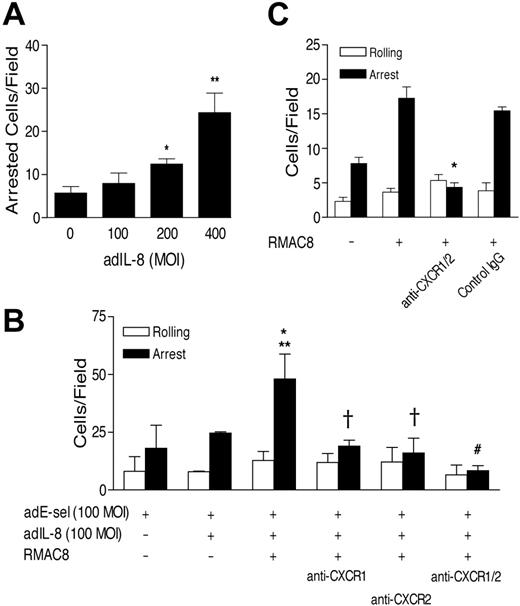
We therefore next explored whether provision of IL-8 by infection of MCEC-1 cells with an IL-8 adenovirus construct (adIL-8) would allow RMAC8-stimulated adhesion to occur on ECs expressing E-selectin, maintaining ICAM-1 expression at basal levels. In preliminary experiments, adIL-8 was titrated in HMEC-1 cells coinfected with adE-selectin and shown to lead to IL-8 release (as measured by ELISA, not shown), and to enhance PMN arrest at 200 or more MOI (Figure 4A). As shown in Figure 4B, mAb RMAC8 significantly augmented adhesion to ECs infected with adE-selectin and a subthreshold concentration of adIL-8 (100 MOI), and this was significantly inhibited by antibodies to the IL-8 receptors CXCR-1 and CXCR-2. As shown in Figure 4C, anti–CXCR-1 and anti–CXCR-2 were subsequently found to inhibit fully the mAb RMAC8-dependent PMN adhesion to TNFα-stimulated HMEC-1 cells. Thus, the cooperation of E-selectin, FcγRIIa, CXCR-1/2, and β2 integrins is both necessary and sufficient for mAb RMAC-8–augmented adhesion to ECs under flow conditions.
Expression of IL-8 with E-selectin allows mAb RMAC8-enhanced adhesion to ECs. (A) the ability of adIL-8 is shown at increasing MOI to stimulate PMN arrest on HMEC-1 cells coinfected with E-selectin (100 MOI), when assayed at 1.5 dynes/cm2 (*P < .03, **P < .01). (B) adIL-8 (100 MOI) synergized with mAb RMAC8 is shown to increase adhesion (at 1.5 dynes/cm2) to HMEC-1 cells coinfected with E-selectin (100 MOI) and that this was inhibited by anti–CXCR-1 and/or -2 (*P < .05 compared with no adIL8; **P < .04 compared with adIL-8 but no RMAC8; #P < .03, †P < .05 compared with adhesion in the absence of blocking antibody). (C) PMN adhesion to RMAC8-coated TNFα-activated HMEC-1 cells was inhibited by anti-CXCR-1 and -2 (*P < .001). All values are mean + SEM of 3 experiments.
Expression of IL-8 with E-selectin allows mAb RMAC8-enhanced adhesion to ECs. (A) the ability of adIL-8 is shown at increasing MOI to stimulate PMN arrest on HMEC-1 cells coinfected with E-selectin (100 MOI), when assayed at 1.5 dynes/cm2 (*P < .03, **P < .01). (B) adIL-8 (100 MOI) synergized with mAb RMAC8 is shown to increase adhesion (at 1.5 dynes/cm2) to HMEC-1 cells coinfected with E-selectin (100 MOI) and that this was inhibited by anti–CXCR-1 and/or -2 (*P < .05 compared with no adIL8; **P < .04 compared with adIL-8 but no RMAC8; #P < .03, †P < .05 compared with adhesion in the absence of blocking antibody). (C) PMN adhesion to RMAC8-coated TNFα-activated HMEC-1 cells was inhibited by anti-CXCR-1 and -2 (*P < .001). All values are mean + SEM of 3 experiments.
Assembly of immune-complexes on EC leads to IL-8-independent FcγRIIIb-mediated leukocyte recruitment
Although our data so far indicated that FcγRIIa rather than FcγRIIIb mediates the increase in PMN adhesion to ECs coated with monomeric IgG, a previous study investigating PMN adhesion to glass coated with immune-complexes has shown that FcγRIIIb can mediate selectin-independent PMN capture under flow conditions and subsequently trigger Mac-1–dependent arrest.37 We therefore assembled immune complexes on the EC surface, by perfusing RbAMIg over HMEC-1 cells coated with mAb RMAC8. Flow cytometry confirmed the binding of RbAMIg (Figure 5A), and confocal microscopy showed a similar surface distribution of rabbit Ig to that of mouse Ig on cells perfused with RMAC8 alone (not shown). Addition of RbAMIg to RMAC8 had no detectable effect on PMN interactions with resting unstimulated HMEC-1 cells, when studied at 1.5 dynes/cm2. However mAb RbAMIg + RMAC8 immune complexes were fully capable of stimulating recruitment at 1.5 dynes/cm2 when assembled on plastic (Figure 5B). As previously observed by Coxon et al37 using immune complexes on glass, PMN adhesion to mAb RMAC8 + RbAMIg-coated plastic was very rapid, with little rolling. We did not observe PMN adhesion to plastic coated with rabbit Ig (50 μg/mL) alone, demonstrating that immune-complex assembly was necessary for this phenomenon.
Assembly of immune complexes on the surface of ECs results in FcγRIIIb-mediated PMN recruitment. (A) Rabbit anti–mouse Ig (RbAMIg; 1:250) was perfused at 0.83 mL/minute for 5 minutes after perfusion of mAb RMAC8, after which deposition on the surface of ECs was demonstrated by staining with FITC-conjugated goat anti–rabbit-Ig by flow cytometry. The profiles are for HMEC-1 perfused with RMAC8 (filled line), RbAMIg alone (gray line), or RMAC8 followed by RbAMIg (black line). (B) PMNs were perfused at 1.5 dynes/cm2 over unstimulated HMEC-1 cells precoated with mAb RMAC8 alone or with mAb RMAC8 followed by RbAMIg, or over plastic coated with mAb RMAC8 (50 μg/mL) followed by RbAMIg (50 μg/mL). Immune complexes enhanced PMN arrest when coated on plastic but not when presented by ECs (*P < .001. (C) RMAC8 + RbAMIg stimulated PMN arrest at 1.5 dynes/cm2 on resting ECs infected with adE-selectin (100 MOI) and adICAM-1 (50 MOI) (*P < .005), and (D) this was inhibited by fab′ anti-FcγRIIIb (3G8) but not by anti-FcγRIIa (IV.3) on PMNs (*P < .01). (E) In contrast, RMAC8 + RbAMIg stimulated PMN adhesion at 1.5 dynes/cm2 to TNFα-stimulated ECs was inhibited by anti-FcγRIIa, but not significantly by anti-FcγRIIIb (*P < .004). All values are mean + SEM of 3 experiments.
Assembly of immune complexes on the surface of ECs results in FcγRIIIb-mediated PMN recruitment. (A) Rabbit anti–mouse Ig (RbAMIg; 1:250) was perfused at 0.83 mL/minute for 5 minutes after perfusion of mAb RMAC8, after which deposition on the surface of ECs was demonstrated by staining with FITC-conjugated goat anti–rabbit-Ig by flow cytometry. The profiles are for HMEC-1 perfused with RMAC8 (filled line), RbAMIg alone (gray line), or RMAC8 followed by RbAMIg (black line). (B) PMNs were perfused at 1.5 dynes/cm2 over unstimulated HMEC-1 cells precoated with mAb RMAC8 alone or with mAb RMAC8 followed by RbAMIg, or over plastic coated with mAb RMAC8 (50 μg/mL) followed by RbAMIg (50 μg/mL). Immune complexes enhanced PMN arrest when coated on plastic but not when presented by ECs (*P < .001. (C) RMAC8 + RbAMIg stimulated PMN arrest at 1.5 dynes/cm2 on resting ECs infected with adE-selectin (100 MOI) and adICAM-1 (50 MOI) (*P < .005), and (D) this was inhibited by fab′ anti-FcγRIIIb (3G8) but not by anti-FcγRIIa (IV.3) on PMNs (*P < .01). (E) In contrast, RMAC8 + RbAMIg stimulated PMN adhesion at 1.5 dynes/cm2 to TNFα-stimulated ECs was inhibited by anti-FcγRIIa, but not significantly by anti-FcγRIIIb (*P < .004). All values are mean + SEM of 3 experiments.
We next used adenovirus constructs to examine further the adhesion of PMNs to unstimulated HMEC-1 cells coated with RMAC8 + RbAMIg. Unlike with RMAC8 alone, infection with adE-selectin (100 MOI) and adICAM-1 (50 MOI) was sufficient to allow mAb RMAC8 + RbAMIg to significantly enhance PMN adhesion at a shear stress of 1.5 dynes/cm2, increasing adhesion by approximately 3-fold (Figure 5C). RMAC8 + RbAMIg–mediated PMN arrest under these conditions was completely abrogated by anti–E-selectin, anti–LFA-1, or anti–Mac-1 but not affected by anti–CXCR-1 and/or anti–CXCR-2, or by pertussis toxin (not shown). In contrast to mAb RMAC8-mediated adhesion to TNFα-activated ECs, RMAC8 + RbAMIg immune complex–stimulated adhesion to unstimulated HMEC-1 cells coinfected with E-selectin + ICAM-1 was inhibited fully anti-FcγRIIIb, whereas anti-FcγRIIa had no significant effect (Figure 5D). HL60 cells, which have a low expression of FcγRIIIb, did not show enhanced adhesion to RMAC8 + RbAMIg–coated ECs infected with adE-selectin and adICAM-1 (data not shown). Taken together, these observations confirm that ligation of FcγRIIIb by immune complexes is able to directly stimulate β2 integrin–mediated adhesion but indicate that when ECs are used as substrate there is a requirement for initial selectin-mediated capture. When PMN adhesion was tested on TNFα-stimulated ECs coated with RMAC8 + RbAMIg, immune complex–stimulated adhesion was completely inhibited by anti-FcγRIIa, whereas anti-FcγRIIIb had a lesser inhibitory effect that did not reach statistical significance in 3 experiments (Figure 5E). FcγRIIa therefore appears to be dominant when ECs have been activated by TNFα.
AECA found in SLE augment PMN recruitment via cooperation between FcγRIIa and CXCR1/2
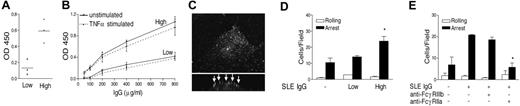
Our data therefore demonstrate separate FcγRIIa- and FcγRIIIb-mediated mechanisms for IgG-mediated leukocyte adhesion to ECs and suggest that the critical determinants are whether ECs are cytokine activated and whether ECs are coated with monomeric IgG or assembled immune complexes. We therefore predicted that SLE antibodies would influence PMN-EC interactions by the FcγRIIa-mediated mechanism we had identified using mAb RMAC8. Sera from 17 patients with SLE were screened for EC reactivity by a cell-based ELISA, using isotype-specific conjugates. The IgG reacting with HMEC-1 cells in the sera of these patients was shown to be entirely IgG1 (not shown). Three sera with high AECA activity and 3 sera with low EC binding (Figure 6A) were separately pooled, and IgG was purified from them on a Protein G column. Figure 6B shows concentration-dependent AECA reactivity of IgG fractions from the pooled sera, with TNFα prestimulation of HMEC-1 cells having no influence on binding. In addition, preincubation of HMEC-1 cells with IgG containing high EC-binding activity for either 4 or 16 hours did not induce E-selectin or ICAM-1 expression (not shown). Perfusion of IgG containing high EC-binding activity over HMEC-1 cells under flow resulted in binding to the apical surface with a similar distribution to that seen with RMAC8 (Figure 6C). As with mAb RMAC8, deposition of SLE IgG with high-AECA activity had no effect on PMN adhesion to HMEC-1 cells coinfected with E-selectin (100 MOI) and ICAM-1 (50 MOI) (not shown) but significantly increased PMN arrest on TNFα-activated ECs (Figure 6D). Furthermore, human AECA-stimulated PMN recruitment under these conditions was ablated by anti-FcγRIIa and not significantly influenced by anti-FcγRIIIb (Figure 6E). As expected, human AECA-stimulated PMN recruitment on TNFα-activated ECs was also inhibited by anti–E-selectin, anti-CD18, or a combination of anti-CXCR1 and anti-CXCR2 (not shown). Therefore, these experiments show for the first time that AECA found in SLE augment β2 integrin–dependent PMN adhesion to cytokine-activated ECs by an FcγRIIa-mediated mechanism requiring costimulation via CXCR-1/2.
IgG from patients with SLE augments PMN recruitment via FcγRIIa. (A) HMEC-1 cell binding of SLE sera with high- and low-binding activity, as tested by ELISA. (B) Titration of IgG fractions with high- and low-AECA activity on unstimulated and TNFα-stimulated HMEC-1 cells, as tested by ELISA. Values are mean of triplicates ± standard deviation. (C) Purified IgG from sera of patients with SLE with high-level AECA activity was perfused at 400 μg/mL over HMEC-1 cells at 0.83 mL/minute for 5 minutes, after which antibody binding was determined by confocal imaging with biotinylated-conjugated anti–human IgG and AlexaFluor 488 streptavidin. Main panel shows composite Z-stacks and the lower inserts show cell cross-section. Arrows point to the deposition of human Ig on the apical surface of the cells. (D) Deposition of SLE IgG (400 μg/mL) with high-level anti-EC activity enhanced PMN adhesion at 1.5 dynes/cm2 to TNFα-stimulated HMEC-1 cells (*P < .03 compared with either no AECA or IgG with low-AECA activity). Values are mean + SEM of 3 experiments. Panel E shows that AECA-stimulated PMN adhesion at 1.5 dynes/cm2 was fully inhibited by anti-FcγRIIa (IV.3) but not by fab′ anti-FcγRIIIb (3G8) (*P < .02). All values are mean + SEM of 3 experiments.
IgG from patients with SLE augments PMN recruitment via FcγRIIa. (A) HMEC-1 cell binding of SLE sera with high- and low-binding activity, as tested by ELISA. (B) Titration of IgG fractions with high- and low-AECA activity on unstimulated and TNFα-stimulated HMEC-1 cells, as tested by ELISA. Values are mean of triplicates ± standard deviation. (C) Purified IgG from sera of patients with SLE with high-level AECA activity was perfused at 400 μg/mL over HMEC-1 cells at 0.83 mL/minute for 5 minutes, after which antibody binding was determined by confocal imaging with biotinylated-conjugated anti–human IgG and AlexaFluor 488 streptavidin. Main panel shows composite Z-stacks and the lower inserts show cell cross-section. Arrows point to the deposition of human Ig on the apical surface of the cells. (D) Deposition of SLE IgG (400 μg/mL) with high-level anti-EC activity enhanced PMN adhesion at 1.5 dynes/cm2 to TNFα-stimulated HMEC-1 cells (*P < .03 compared with either no AECA or IgG with low-AECA activity). Values are mean + SEM of 3 experiments. Panel E shows that AECA-stimulated PMN adhesion at 1.5 dynes/cm2 was fully inhibited by anti-FcγRIIa (IV.3) but not by fab′ anti-FcγRIIIb (3G8) (*P < .02). All values are mean + SEM of 3 experiments.
Discussion
In this paper, we show that FcγRIIa and FcγRIIIb are both able to respond to surface-bound IgG under dynamic conditions thought to be present in postcapillary venules (∼ 1.5 dyne/cm2) and that both receptors can stimulate an increase in β2 integrin–mediated PMN adhesion to ECs. Our data indicate however that FcγRIIa has a specialized nonredundant capability of reacting with EC-bound monomeric IgG, providing there is costimulation via CXCR-1 and/or -2. In contrast, although FcγRIIIb can mediate GPCR-independent adhesion to ECs, it appears to require the assembly of immune complexes. Thus, PMNs respond to EC-bound AECAs from patients with SLE via FcγRIIa.
FcγRIIa shows an intermediate level of expression on PMNs, with an RFI of 10.2, which in our hands is slightly higher than that of LFA-1 (RFI, ∼ 8). Moreover, FcγRIIa expression has been located both on leukocyte microvilli and on the cell body.40 However, a key question arising from our work is how FcγRIIa is able to enhance β2 integrin–mediated adhesion under dynamic conditions in response to surface-bound monomeric IgG, whereas FcγRIIIb does not. FcγRIIIb has an approximately 10-fold greater expression than FcγRIIa on the surface of unstimulated PMNs, with a receptor density of approximately 135.00/cell.41 Furthermore, the affinities of FcγRIIa and FcγRIIIb for human IgG1, the isotype of the lupus AECA purified in the study, are similar, each having an affinity constant (KA) of 10−6 to 10−7 M−1.16,32,42 IL-8 is known to augment the capacity of human PMN FcγRIIa to bind IgG-coated erythrocytes43 and also to lead to the rapid clustering of LFA-1 into high-avidity patches on the PMN surface.44 This raises the possibility that FcγRIIa might also cluster in response to IL-8 stimulation, thereby increasing avidity and the chance of receptor cross-linking by surface-bound IgG. We have however performed confocal microscopy on PMNs over the course of 120 seconds following IL-8 stimulation and have not observed membrane FcγRIIa redistribution (not shown). Another possibility is that contact with E-selectin and/or IL-8 receptor ligation stimulates the inclusion of a critical signaling intermediate(s) into lipid rafts, in which FcγRIIa is known to reside constitutively (at least in monocytes).45 It should also be borne in mind that combined ligation of CXCR1/2 and FcγRIIa may lead to synergistic activation of PI3K, which is critically involved in modulating PMN β2 integrin function.23,44
Although there may be cross-reactivity of AECAs between human and mouse ECs,46 it is not yet clear to what degree AECAs occur in murine models of lupus. Furthermore, testing the effects of AECAs on PMN-EC interactions in mice is not straightforward because mouse PMNs lack FcγRIIa. Instead, mouse PMNs express FcγRIIb in which the cytoplasmic domain has an immunoreceptor tyrosine-based inhibitory motif, and it remains to be determined whether this cooperates with GPCRs to increase β2 integrin–mediated adhesion in the same way. If not, an alternative approach to in vivo validation will be to test the effects of AECAs on PMN recruitment in human FcγRIIa transgenic mice, in which FcγRIIa has apparently normal ligand binding and signaling function.47
Coxon et al37 have proposed that the ability of FcγRIIIb to mediate selectin-independent capture and GPCR-independent activation of Mac-1 under flow is related to the localization of FcγRIIIb on the tips of PMN microvilli, which may be the initial points of contact with immune complex.37 We were able to confirm that FcγRIIIb can mediate GPCR-independent activation of Mac-1 and to extend the paradigm to LFA-1. However, although FcγRIIIb-mediated selectin-independent primary capture on plastic coated with immune complexes, we found that an endothelial selectin was required for FcγRIIIb-stimulated adhesion to immune complex–coated ECs. This disparity might be due to the relative densities of immune complexes on the different substrates, or possibly might relate to the repulsive charge of the EC surface membrane. Although our experiments used E-selectin for initial PMN capture, it is possible that P-selectin would be equally able to facilitate FcγRIIIb-mediated adhesion. If so, FcγRIIIb could trigger rapid PMN arrest on endothelium coated with immune complexes on translocation of P-selectin in response to rapidly acting agonists such as histamine or complement C5a.48,49 Note however that enhanced adhesion to TNFα-activated ECs (rather than unstimulated ECs) coated with immune complexes in the form of mAb RMAC8 + RbAMIg was more dependent on FcγRIIa than FcγRIIIb. Although this has not yet been further explored, it is possible that FγRIIIb function may be lost by receptor shedding in response to CXCR1/2-mediated activation.50
Two technical points relating to the study merit discussion. The MCEC-1 cell line is derived from human cutaneous microvessels and provides an appropriate model of endothelium for a study on AECAs in SLE. Although MCEC-1 cells respond well to TNFα with respect to up-regulation of ICAM-1 expression, only a subset of cells was induced to express E-selectin. Nevertheless, E-selectin was found to be critical for both FcγRIIa- and FcγRIIIb-mediated adhesion in response to IgG/AECAs or immune complexes, suggesting that an individual EC expressing E-selectin is probably sufficient for PMN capture in this system. Second, we used a combination of adenovirus infection and neutralizing antibodies to establish that IL-8/CXCR1/2 interactions are necessary and sufficient to allow FcγRIIa-mediated PMN adhesion to ECs. Although adenovirus-mediated gene expression has been used previously to study the effects of endothelial adhesion molecules in the absence of global activation by TNFα or other cytokines,51 we are not aware of previous studies using adenoviral infection to test the effects of chemokines on leukocyte-EC interactions. The introduction of IL-8 into the assays by adenoviral infection of ECs allows local synthesis and mimics the native mode of IL-8 presentation to flowing leukocytes. This strategy avoids possible inhibitory effects on leukocyte activation/adhesion of presenting IL-8 in solution52 and should prove useful for studying the effects of other EC-derived chemoattractants on leukocyte-EC interactions.
It was not the intention of the present study to define the antigens with which AECAs react, but rather to prove the principle that AECAs could influence leukocyte recruitment under physiologic flow conditions. By isolating IgG from patients with SLE we have shown that the basic mechanisms revealed with our model system apply also to putatively pathologic human antibodies. Our study was predominantly conducted using a shear of 1.5 dynes/cm2 and was intended to model flow in postcapillary venules, for example, in skin. Because neither AECAs nor assembled immune complexes had any influence on leukocyte-endothelial cell interactions in the absence of E-selectin, our data suggest that endothelial-bound IgG may not influence leukocyte-EC interactions significantly in postcapillary venules under resting conditions but may amplify inflammatory responses when endothelium becomes minimally activated. This suggestion fits well with the pattern of expression of endothelial adhesion molecules known to occur in the skin of patients with SLE, with E-selectin expression largely restricted to active disease.53 It should however be noted that different rules may apply in capillary beds with lower shear, as in renal glomeruli, where FcγRIIa and/or FcγRIIIb could well trigger β2 integrin–mediated adhesion without requiring initial selectin-mediated capture.
Whether or not the increase in PMN recruitment related to Fcγ receptor ligation accelerates or delays PMN arrival into the extravascular space and subsequent survival, and whether it is associated with vascular cell injury by release of proteases and reactive oxygen awaits further study. Antineutrophil cytoplasmic antibodies, which activate PMNs via ligation of Fcγ receptors, have been found to enhance rather than delay PMN transmigration through endothelial cell monolayers, and it is possible that IgG attached to ECs could have a similar effect.54–56 Frohlich et al12 found that interactions of PMNs with immobilized Ig under rotating conditions led to a marked oxidative burst over the course of 1 to 2 hours, but extrapolating from their study to our own is difficult because of significant differences in the 2 assay systems.12
The observation that AECAs found in SLE can amplify PMN-EC interactions suggests that patients with these antibodies may have disproportionate leukocyte recruitment in relation to the degree of endothelial activation. Inappropriate amplification of PMN-EC interactions may contribute to microvascular dysfunction and injury at times of systemic cytokine release, such as during disease flares. The data also suggest a mechanism whereby acute lupus flares may be triggered by infections.57 However, our studies have used healthy donors for isolating PMNs, on which FcγRIIa and FcγRIIIb are the principal Fcγ receptors, and it should be borne in mind that FcγRI (CD64) can be detected on circulating PMNs during sepsis.58 Whether or not FcγRI makes an additional contribution to leukocyte-EC interactions during infections in SLE awaits investigation. A further consideration is whether AECA-mediated adhesion of SLE PMNs to ECs is influenced by the FcγRIIa 131H/R polymorphism, with the R/R131 allotype being linked to lupus nephritis.59,60 This polymorphism is thought to particularly determine the capacity of FcγRIIa to bind human IgG261 and is not relevant to the ability of PMNs to respond to the IgG1 AECAs isolated in this study. However, a larger sample of SLE sera may reveal the presence of IgG2 AECAs in some patients.
In conclusion, our data lead to a new paradigm for understanding PMN interactions with IgG-coated surfaces and suggest a novel means by which PMNs may adhere to ECs in SLE and other diseases in which AECAs are present. Although FcγRIIIb triggers GPCR-independent adhesion to immune complex–coated surfaces, FcγRIIa mediates adhesion to cytokine-activated ECs coated with AECAs by a mechanism requiring costimulation with CXCR-1 and/or CXCR-2. Our observations suggest that AECAs can amplify PMN-EC interactions and may lead to disproportionate leukocyte recruitment in relation to the degree of endothelial activation. The further dissection of the signaling events involved, and the possible downstream adverse consequences, both in vitro and in vivo, may lead to greater insight into the molecular mechanisms of vascular injury.
Authorship
Contribution: O.J.F. helped design the study and performed the research; M.J. and O.O.E. helped perform the research, J.C.M. helped design the research, and D.O.H. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dorian O. Haskard, BHF Cardiovascular Medicine Unit, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, Du Cane Rd, London W12 ONN, United Kingdom; e-mail: d.haskard@imperial.ac.uk.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Charlotte Lawson and Professor Marlene Rose for the gift of adICAM-1, and Drs Nancy Hogg, Alex Ivetic, William Muller, and Ravi Rao for helpful discussion on the manuscript.
This work was supported by grants from the Wellcome Trust, British Heart Foundation, and Arthritis Research Campaign.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal