Abstract
Minor histocompatibility antigen (MiHAg) differences between donor and recipient in MHC-matched allogeneic hematopoietic stem cell transplantation (allo-HSCT) often result in graft-versus-host disease (GVHD). While MiHAg-specific T-cell responses can in theory be directed against a large number of polymorphic differences between donor and recipient, in practice, T-cell responses against only a small set of MiHAgs appear to dominate the immune response, and it has been suggested that immunodominance may predict an important contribution to the development of GVHD. Here, we addressed the feasibility of graft engineering by ex vivo removal of T cells with 1 or more defined antigen specificities in a well-characterized experimental HSCT model (B6 → BALB.B). We demonstrate that immunodominant H60- and H4-specific CD8+ T-cell responses can be effectively suppressed through MHC class I tetramer–mediated purging of the naive CD8+ T cell repertoire. Importantly, the development of GVHD occurs unimpeded upon suppression of the immunodominant MiHAg-specific T-cell response. These data indicate that antigen-specific graft engineering is feasible, but that parameters other than immunodominance may be required to select T-cell specificities that are targeted for removal.
Introduction
MHC-matched allogeneic hematopoietic stem cell transplantation (allo-HSCT) in combination with donor lymphocyte infusion is an effective therapy for hematologic malignancies such as chronic myeloid leukemia.1,2 While donor-derived T cells are essential for graft-versus-leukemia (GVL) responses, these cells are also responsible for graft-versus-host disease (GVHD).3–5 GVHD remains a major cause of morbidity and mortality upon allo-HSCT and can therefore be considered the dose-limiting factor in donor lymphocyte infusion (DLI). Consequently, approaches that can prevent the development of GVHD but preserve GVL effects would be highly desirable. Both GVL and GVHD are caused by T-cell recognition of minor histocompatibility antigens (MiHAgs), peptide antigens that are derived from polymorphic genes and that are presented in the context of MHC class I and class II molecules. Because the number of polymorphisms between any 2 nonhomozygous individuals is in the order of millions,6 an extremely high amount of peptide sequences differ between a donor-recipient pair and could hence function as potential MiHAgs.7,8 However, it has been shown in murine models that relatively few MiHAgs dominate the immune response,9 possibly due to the same factors that determine immunodominance in antiviral responses.10 Elegant experiments by Shlomchik and colleagues have demonstrated that the main target organ and severity of GVHD differ substantially depending on the MHC alleles that present the MiHAgs;11 these data thereby suggest that T-cell responses against a small set of antigens are responsible for the distinct types of GVHD. Likewise, reactivity against leukemic cells also appears dominated by responses against a small set of MiHAgs in patients that have undergone allo-HSCT.12 However, to date it is unknown whether the MiHAgs that lead to GVHD development are the same as the immunodominant antigens identified in the immunologic assays.
To date, studies in humans that address possible associations of T-cell responses against specific MiHAgs with clinical GVHD show mixed results. In some reports, disparity at the HA-1 locus was found to be associated with the development of GVHD,13,14 whereas other groups did not observe a significant correlation between GVHD and HA-1 status.15,16 More in general, while the occurrence of GVL and GVHD are closely correlated, several MiHAg-specific T-cell responses appear to be mainly associated with GVHD, whereas others appear to be for the most part linked to GVL (reviewed in Bleakley and Riddell17 ).
Murine models have been useful to dissect the mechanisms of GVH responses. In the B6 → BALB.B transfer model, transfer of B6 splenocytes into irradiated BALB.B mice leads to massive B6 anti-BALB.B T-cell responses that are directed toward MiHAgs presented by H-2b MHC molecules, and this transplantation regimen results in severe GVHD and death of the mice within several weeks.18 In the past years, the epitopes that are largely responsible for the B6 anti-BALB.B CD8+ T-cell responses have been identified.19–24 Remarkably, a large part of the total B6 anti-BALB.B CD8+ T-cell response is directed against a single MiHAg, H60.9,20 In addition, smaller contributions are made by H4-, H28-, H13-, and (in case of female-to-male transplantations) HY-specific T-cell responses. Of these MiHAgs, H13 and HY are ubiquitously expressed, whereas H60 is exclusively expressed in hematopoietic cells, including professional antigen-presenting cells (APCs).21,25 H4 is expressed in hematopoietic cells,26 but also in lung and neuronal cells, and H28 is expressed in interferon-stimulated lymphoid tissue.26
Based on the strong immunodominance of the H60 MiHAg, it has been suggested that the H60-specific T-cell response could be a main contributor to GVHD upon B6 → BALB.B transfer.19 Because H60 is exclusively expressed in hematopoietic cells, such an involvement would likely be based on recognition of tissue-resident hematopoietic cells by H60-specific T cells, leading to a local inflammatory cascade. In support of this concept, it has been shown in a murine transplantation model that expression of MiHAgs by recipient APCs can be sufficient for the development of acute GVHD.27
To address whether immunodominance of a MiHAg is indicative of an essential role in GVHD, we developed a methodology for ex vivo antigen-specific graft engineering. We used this method to assess the role of the immunodominant MiHAg H60 in the development of GVHD in the B6 → BALB.B transfer model. Importantly, despite the documented effective inhibition of the immunodominant H60-specific T-cell response and contrary to previously published data,28 this treatment has no effect on either the incidence or severity of GVHD. These data indicate that antigen-specific graft engineering is feasible but that immunodominance does not necessarily translate into an essential role in GVHD.
Materials and methods
Mice
C57BL/6 (B6), C.B10-H2b/LiMcdJ (BALB.B), T-cell receptor α (TCRα)–deficient mice29 (C57BL/6 background), influenza A NP366-374-specific TCR (F5)–transgenic mice,30 and MiHAg HY738-746-specific TCR-transgenic mice31 were obtained from the experimental animal department of The Netherlands Cancer Institute. Recipient and donor mice used for HSCT were bred and kept under specific pathogen–free conditions. Animal experiments were performed in accordance with institutional and national guidelines and were approved by the Experimental Animal Committee of The Netherlands Cancer Institute.
Peptides and MHC tetramers
The peptides NP366-374 (amino acid [aa]: ASNENMDAM), HY738-746 (aa: KCSRNRQYL), H13 (aa: SSVIGVWYL), H4 (aa: SGIVYIHL), H60 (aa: LTFNYRNL), H28 (aa: IZENFPRL), and OVA257-264 (aa: SIINFEKL) were produced using standard 9-fluorenylmethoxycarbonyl (FMOC) chemistry. PE- or APC-labeled MHC tetramers were produced as described previously.32,33 H2-Kb MHC tetramers were generated with human β2-microglobulin (β2m).
Antigen-specific T-cell depletion
Spleens from naive mice were harvested and leukocytes were purified over a Lympholyte-M (Cedarlane Laboratories, Hornby, ON, Canada) gradient. Splenocytes (100 × 106/mL) were incubated with approximately 25 μg/mL of PE- or APC-labeled MHC tetramers for 20 minutes at room temperature in complete medium (IMDM; Life Technologies, Bethesda, MD) supplemented with 8% FBS, 10 μM β-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin). Cells were washed and incubated with anti-PE or anti-APC beads (Miltenyi Biotec, Bergisch-Gladbach, Germany) according to the manufacturer's protocol. Negative selection was performed by autoMACS (Miltenyi Biotec) according to manufacturer's guidelines. Mock depletion of splenocytes comprised the same procedure, only lacking incubation with MHC tetramers. For T-cell transfer, depleted cells were washed twice with HBSS (Life Technologies) and injected intravenously.
Peptide vaccination and T-cell restimulation
Mice were vaccinated with 100 μg peptide combined with intraperitoneal anti-CD40 (FGK4.5) treatment as previously described.34 Immunization with 2 peptides was performed with 2 single-peptide emulsions, injected at opposite flanks.
At day 10 after vaccination, mononuclear splenocytes were harvested, and 2 × 106 cells/mL were restimulated in complete medium in the presence of 20 CU/mL recombinant interleukin-2 (rIL-2; Chiron BV, Amsterdam, the Netherlands) and peptide as indicated, or with 0.5 × 106 CD8-depleted, irradiated (30 Gy) splenocytes for 13 to 14 days. At day 7 of culture, medium was supplemented with 20 CU/mL rIL-2. Live cells were purified by Ficoll gradient and used for analysis.
Flow cytometry
MHC class I tetramer staining was performed as described previously.33,35 For intracellular IFN-γ staining, 1 × 105 splenocytes were stimulated with 0.1 μg/mL of the indicated peptide and 10 CU/mL rIL-2 for 5 hours, or with 1 × 105 CFSE-labeled B6 or BALB.B splenocytes that were pretreated overnight with 20 CU/mL rIL-2 and 10 ng/mL rIFNγ (R&D Systems, Minneapolis, MN).21 All mAbs were from BD Pharmingen, except PE-conjugated anti-CD8α mAb (Caltag, Burlingame, CA). In case of MHC tetramer stainings, propidium iodide (Sigma, St Louis, MO) was used to exclude dead cells. Data acquisition and analysis were done on a FacsCalibur (Becton Dickinson, MountainView, CA) with CellQuest (BD Biosciences, San Jose, CA) or FCS express software (De Novo Software, Thornhill, ON, Canada).
In vivo GVHD model
Transplantation of female B6 bone marrow (BM) and splenocytes into lethally irradiated male BALB.B recipients was performed as described previously.19 Briefly, BALB.B mice (8-16 weeks old, randomized for age) received total body irradiation in 2 split doses of 4.5 Gy, with a time interval of 4 hours, and with a cumulative dose of 9 Gy, 1 day before HSCT. Female B6 splenocytes (25 × 106 or 2 × 106) depleted for H60/H4-specific CD8+ T cells, or mock-depleted, were injected together with 5 × 106 T-cell–depleted BM cells (depletion efficiency, > 98%). At days 6 to 9 after transfer, chimerism was confirmed by staining of blood samples with an allotype-specific α-mouse β2m antibody (Ly-m11; BD Pharmingen, San Diego, CA), labeled with Alexa Fluor 647 (Molecular Probes, Eugene, OR).
Blood samples were analyzed for antigen-specific T-cell responses by intracellular IFNγ staining or MHC tetramer staining. For analysis of organ-residing antigen-specific T cells, liver T cells were isolated as described previously.36
Mice were monitored daily, and killed when 1 or more of the following parameters occurred: (1) weight loss of more than 25% of the starting body weight; (2) development of ascites as assessed by weight gain of more than 1 g/day; (3) persistent diarrhea; or (4) overall weak appearance for more than 2 days.
Extensive necropsy was performed for all killed mice. Tissues (skin, liver, salivary glands, gastrointestinal tract, spleen, pancreas, heart and lungs, urogenital system, secondary sex glands, head, extremities, and spinal cord) were sampled in buffered formalin. Sections were stained with hematoxylin and eosin and examined blindly for indications of GVHD pathology, with a special emphasis on liver, skin, and intestine. Sections were reviewed with a Zeiss Axioskop2 Plus microscope (Carl Zeiss Microscopy, Jena, Germany) equipped with Plan-Apochoma (5×/0.16, 10×/0.45, 20×/0.60); and 40×/0.95 and Plan-Neofluar (2.5×/0.075) objectives. Within the body of the microscope, an additional enlargement device was included. Images were captured with a Zeiss AxioCam HRc digital camera and processed with AxioVision 4 software (both from Carl Zeiss Vision, Munchen, Germany).
Results
Removal of transgenic and endogenous antigen-specific T cells with MHC class I tetramers
The goal of this project was to develop a method to remove T cells specific for 1 or multiple defined antigens from polyclonal T-cell populations, and to use such technology to assess the role of immunodominant MiHAg-specific T-cell responses in GVHD induction. Multimeric forms of MHC molecules have been used previously to enrich antigen-specific T cells from a variety of sources.37 However, the requirements for effective enrichment and removal are distinct (specificity versus completeness), and we therefore set out to develop such a technology, using a setting where the efficiency of depletion could be readily analyzed. To this purpose, TCR-transgenic CD8+ T cells specific for the influenza A nucleoprotein (NP366) epitope were mixed with C57/BL6 splenocytes, and incubated with PE-labeled Db-NP tetramers (Figure 1A; “NP Before”). Subsequent depletion with anti-PE magnetic-activated cell-sorting (MACS) beads led to a reduction of NP366-specific CD8+ T cells of 98% or greater (data not shown). A second round of selection further enhanced the efficiency of depletion, resulting in an overall depletion efficiency of 99% (Figure 1A; “NP after”). Comparable results were obtained when TCR-transgenic HY-specific CD8+ T cells were depleted from a mixed splenocyte population (Figure 1A; bottom). The recovery of splenocytes upon autoMACS depletion was approximately 50%.
Effective MHC class I tetramer–mediated depletion of antigen-specific CD8+ T cells. (A) Splenocytes of mice transgenic for the NP366-374-specific F5 TCR (top), or HY738-746-specific TCR (bottom) were mixed with wild-type splenocytes and subjected to 2 rounds of MHC tetramer–based depletion with Db-NP or Db-HY tetramers. Prior to depletion (Before), and after depletion (After), the percentage of NP- or HY-specific CD8+ T cells (top right corner) was determined by combined staining with α-CD8 antibody and Db-NP or Db-HY tetramers. Depletion was performed by staining with PE-labeled antigen-specific Db tetramer, followed by negative selection with α-PE microbeads (Miltenyi Biotec). (B-C) Depletion of naive, polyclonal HY-specific T cells. Splenocytes from female B6 mice were depleted with Db-HY tetramers and α-PE microbeads, or mock-depleted with α-PE microbeads alone. Cells (40 × 106) were transferred into female TCRα-deficient mice, and 4 days after transfer, mice received an HY738 peptide vaccination in combination with α-CD40 antibody treatment. At day 10 after vaccination, spleens were harvested and spleen cells were restimulated in vitro for 14 days. The percentage of HY738-specific CD8+ T cells was assessed by Db-HY tetramer staining (B), and by intracellular IFNγ staining (C), upon a 5-hour in vitro restimulation with the HY738-746 peptide (P = .002), or with the irrelevant NP366-374 peptide. Each dot plot in panel B represents a single mouse, and bars in panel C represent mean responses of 3 mice ± SD (representative of at least 2 independent experiments).
Effective MHC class I tetramer–mediated depletion of antigen-specific CD8+ T cells. (A) Splenocytes of mice transgenic for the NP366-374-specific F5 TCR (top), or HY738-746-specific TCR (bottom) were mixed with wild-type splenocytes and subjected to 2 rounds of MHC tetramer–based depletion with Db-NP or Db-HY tetramers. Prior to depletion (Before), and after depletion (After), the percentage of NP- or HY-specific CD8+ T cells (top right corner) was determined by combined staining with α-CD8 antibody and Db-NP or Db-HY tetramers. Depletion was performed by staining with PE-labeled antigen-specific Db tetramer, followed by negative selection with α-PE microbeads (Miltenyi Biotec). (B-C) Depletion of naive, polyclonal HY-specific T cells. Splenocytes from female B6 mice were depleted with Db-HY tetramers and α-PE microbeads, or mock-depleted with α-PE microbeads alone. Cells (40 × 106) were transferred into female TCRα-deficient mice, and 4 days after transfer, mice received an HY738 peptide vaccination in combination with α-CD40 antibody treatment. At day 10 after vaccination, spleens were harvested and spleen cells were restimulated in vitro for 14 days. The percentage of HY738-specific CD8+ T cells was assessed by Db-HY tetramer staining (B), and by intracellular IFNγ staining (C), upon a 5-hour in vitro restimulation with the HY738-746 peptide (P = .002), or with the irrelevant NP366-374 peptide. Each dot plot in panel B represents a single mouse, and bars in panel C represent mean responses of 3 mice ± SD (representative of at least 2 independent experiments).
We next established whether MHC class I tetramer–mediated T-cell depletion could also efficiently eliminate polyclonal antigen-specific T cells. Because the precursor frequency of naive antigen-specific CD8+ T cells is less than 1 in 10 000,38,39 direct determination of the depletion efficiency is evidently impossible. Therefore, naive HY-specific CD8+ T cells were “blindly” depleted from female B6 splenocytes with Db-HY tetramers or were mock depleted, the resulting cells were transferred into female T-cell–deficient mice, and the reconstituted mice were vaccinated with the HY peptide antigen. In vitro restimulation with cognate peptide yielded in a high frequency of HY-specific CD8+ T cells in cultures of mock-depleted splenocytes (Figure 1B, bottom; Figure 1C). In contrast, HY-specific CD8+ T cells were undetectable within cultures from recipients of HY-depleted splenocytes, as judged both by MHC tetramer staining (Figure 1B; top), and by functional analysis of CD8+ T cells (Figure 1C). T-cell responses against other antigens were unaffected by this depletion procedure (data not shown). Together, these data demonstrate that MHC tetramer–mediated depletion can efficiently purge endogenous CD8+ T cells with a defined antigen specificity from a naive T-cell pool.
Effective tetramer depletion of H60- and H4-specific CD8+ T cells
In the B6 → BALB.B GVHD model, H60-specific CD8+ T cells dominate the overall anti-BALB.B T-cell response, followed by H4- and H28-specific CD8+ T cells.9,20 Given the profound dominance of H60-specific T-cell responses, it has been argued that prevention of the H60-specific T-cell response might lead to a reduction of GVHD.19 To address this hypothesis, we first established the efficiency of H60-specific CD8+ T-cell purging in T-cell–deficient mice. Because removal of a single antigen specificity is likely to be insufficient in a clinical setting, we also tested the feasibility of a simultaneous depletion of H4-specific T cells by mixing Kb-H60 and Kb-H4 tetramers during the depletion procedure. H4/H60-depleted or mock-depleted splenocytes were transferred into T-cell–deficient mice that received subsequent immunization with H4 and H60 peptides. In vitro restimulation with either the H4 or H60 peptide resulted in a high frequency of BALB.B-specific T cells in cultures from recipients of mock-depleted splenocytes (Figure 2). In sharp contrast, when H4/H60 T-cell depletion had preceded T-cell transfer, BALB.B-specific T-cell responses were not detected above background, both upon in vitro restimulation with H4 (Figure 2; top left and bottom left) or with H60 antigen (Figure 2; top right and bottom right). The efficiency of H4/H60-specific CD8+ T-cell depletion was further revealed by the lack of detectable IFNγ production of CD8+ T cells upon short-term restimulation with saturating amounts of H4 or H60 peptide (data not shown). Altogether, these results demonstrate the feasibility of simultaneous MHC tetramer–mediated depletion of CD8+ T-cell populations with at least 2 different antigen specificities in a highly stringent setting where a single antigen is offered during in vitro restimulation and antigen competition is hence minimal.
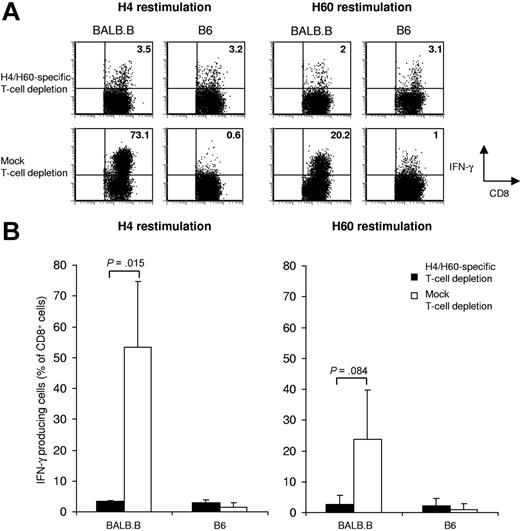
Depletion of antigen-specific T cells directed toward MiHAg H4 and H60. Splenocytes from B6 mice were depleted with Kb-H4 tetramers plus Kb-H60 tetramers, or were mock depleted. Splenocytes (25 × 106) were subsequently transferred into TCRα-deficient mice; 4 days later, mice received both H4 and H6 peptide vaccination. After vaccination (10 days), splenocytes were restimulated in vitro for 14 days in the presence of 5 × 10−4 μg/mL H4 (left panels), or 5 × 10−2 μg/mL H60 peptide (right panels). Total alloreactive T-cell responses were assessed by intracellular IFNγ staining upon a 5-hour incubation with IFNγ-pretreated, CFSE-labeled BALB.B splenocytes (left plots), or with B6 control splenocytes (right plots). (A) Dot plots show the results of representative mice within each group with the percentage of IFNγ-producing CD8+ T cells depicted in the top right corner. (B) Graph shows the mean percentage of IFNγ-producing CD8+ T cells of 3 mice (H4: P = .015; H60: P = .084 ± SD).
Depletion of antigen-specific T cells directed toward MiHAg H4 and H60. Splenocytes from B6 mice were depleted with Kb-H4 tetramers plus Kb-H60 tetramers, or were mock depleted. Splenocytes (25 × 106) were subsequently transferred into TCRα-deficient mice; 4 days later, mice received both H4 and H6 peptide vaccination. After vaccination (10 days), splenocytes were restimulated in vitro for 14 days in the presence of 5 × 10−4 μg/mL H4 (left panels), or 5 × 10−2 μg/mL H60 peptide (right panels). Total alloreactive T-cell responses were assessed by intracellular IFNγ staining upon a 5-hour incubation with IFNγ-pretreated, CFSE-labeled BALB.B splenocytes (left plots), or with B6 control splenocytes (right plots). (A) Dot plots show the results of representative mice within each group with the percentage of IFNγ-producing CD8+ T cells depicted in the top right corner. (B) Graph shows the mean percentage of IFNγ-producing CD8+ T cells of 3 mice (H4: P = .015; H60: P = .084 ± SD).
Identical course of GVHD in the B6 → BALB.B transplantation model upon removal of H4/H60-specific T cells
Having established a method to efficiently remove antigen-specific T cells from a naive precursor T-cell pool, we evaluated the role of the immunodominant MiHAg H60 in the B6 → BALB.B transfer model.19 BALB.B mice were lethally irradiated, and reconstituted with 25 × 106 H4/H60 T-cell–depleted or mock-depleted B6 splenocytes in combination with T-cell–depleted BM cells. To analyze the effect of antigen-specific T-cell depletion on immune responses toward the H60 antigen and other MiHAgs, pooled peripheral blood samples were analyzed for antigen-specific IFNγ production. We assessed T-cell responses based on cytokine production to ensure detection of functionally active MiHAg-specific T cells that could potentially escape detection by MHC tetramer staining, as has been observed for recently activated T cells40 (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). Blood samples of mice that had received mock-depleted splenocytes showed a massive H60-specific T-cell response, with a peak at day 9 after transfer of 14.8% (Figure 3A-B). In contrast, in pooled samples from mice that had received H4/H60-specific T-cell–depleted splenocytes, the H60-specific T-cell response was close to background levels. We were not able to detect H4-specific T-cell responses ex vivo in both groups. However, subsequent experiments showed that H4-specific T-cell depletion was also effective (see the section on the overall magnitude of the B6 anti-BALB.B response). In mice that received mock-depleted splenocytes, no substantial T-cell responses against the H28, H13, and HY MiHAgs could be observed (Figure 3A-B), confirming the previously described immunodominance of H60 in this GVHD model.19 Interestingly, in mice that received H4/H60 T-cell–depleted splenocytes, T-cell responses toward these subdominant MiHAgs were also absent (Figure 3A-B).
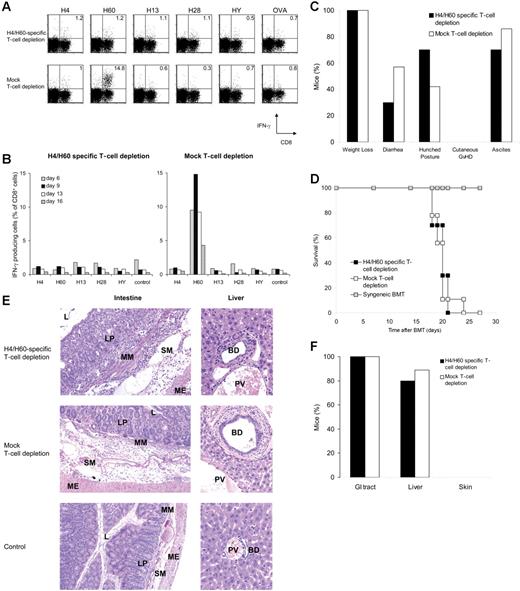
H4/H60-specific depletion abolishes in vivo T-cell responses but not GVHD. Splenocytes from female B6 mice were depleted with Kb-H4 and Kb-H60 tetramers, or mock depleted. Splenocytes (25 × 106) together with 5 × 106 T-cell–depleted BM cells were transferred into lethally irradiated male BALB.B recipients. Peripheral blood was obtained at indicated days after transfer and pooled, and the percentage of CD8+ T cells specific for the different antigens was determined by intracellular IFNγ staining upon a 5-hour peptide restimulation with the indicated MiHAg peptides, or with OVA peptide as a control. (A) Dot plots represent blood samples on day 9 after transfer obtained from mice that received H4/H60-specific T-cell–depleted splenocytes (n = 10; top), or mock depleted splenocytes (n = 9; bottom). Numbers in upper right quadrants reflect the percentage of IFNγ-producing CD8+ T cells of total CD8+ T cells. (B) Kinetics of antigen-specific T-cell responses against the different antigens, measured as described in panel A. (C) Clinical signs of GVHD scored daily starting from day 14 after transfer, when the mice had recovered from irradiation-induced weight loss. (D) Survival plot of male mice that received H4/H60-specific T-cell–depleted splenocytes (▪), mock-depleted splenocytes (□), or female mice that received a syngeneic transfer from female BALB.B donor mice (⊡; n = 4). (E) Histopathologic analysis of intestinal and liver sections obtained after the onset of clinical GVHD. Note that lymphocytic infiltrates are present throughout the intestinal wall and in the periportal area of BALB.B recipients receiving B6 splenocytes (top and middle rows) compared with sections from mice receiving a syngeneic transplant (bottom row). Magnifications: 10 × 1.25 (left panels); × 20 (right panels). L indicates lumen; LP, lamina propria; MM, muscularis mucosae; SM, submucosa; ME, muscularis externa; BD, bile duct; and PV, portal vein. (F) Incidence of histopathologic signs of GVHD in the classical target organs.
H4/H60-specific depletion abolishes in vivo T-cell responses but not GVHD. Splenocytes from female B6 mice were depleted with Kb-H4 and Kb-H60 tetramers, or mock depleted. Splenocytes (25 × 106) together with 5 × 106 T-cell–depleted BM cells were transferred into lethally irradiated male BALB.B recipients. Peripheral blood was obtained at indicated days after transfer and pooled, and the percentage of CD8+ T cells specific for the different antigens was determined by intracellular IFNγ staining upon a 5-hour peptide restimulation with the indicated MiHAg peptides, or with OVA peptide as a control. (A) Dot plots represent blood samples on day 9 after transfer obtained from mice that received H4/H60-specific T-cell–depleted splenocytes (n = 10; top), or mock depleted splenocytes (n = 9; bottom). Numbers in upper right quadrants reflect the percentage of IFNγ-producing CD8+ T cells of total CD8+ T cells. (B) Kinetics of antigen-specific T-cell responses against the different antigens, measured as described in panel A. (C) Clinical signs of GVHD scored daily starting from day 14 after transfer, when the mice had recovered from irradiation-induced weight loss. (D) Survival plot of male mice that received H4/H60-specific T-cell–depleted splenocytes (▪), mock-depleted splenocytes (□), or female mice that received a syngeneic transfer from female BALB.B donor mice (⊡; n = 4). (E) Histopathologic analysis of intestinal and liver sections obtained after the onset of clinical GVHD. Note that lymphocytic infiltrates are present throughout the intestinal wall and in the periportal area of BALB.B recipients receiving B6 splenocytes (top and middle rows) compared with sections from mice receiving a syngeneic transplant (bottom row). Magnifications: 10 × 1.25 (left panels); × 20 (right panels). L indicates lumen; LP, lamina propria; MM, muscularis mucosae; SM, submucosa; ME, muscularis externa; BD, bile duct; and PV, portal vein. (F) Incidence of histopathologic signs of GVHD in the classical target organs.
To provide an more accurate quantification of the reduction in H60-specific T-cell responses, H60-specific T-cell responses were subsequently analyzed in individual animals by either IFNγ staining or MHC tetramer staining (Figure S1). Assessment of H60-specific T-cell responses by IFNγ staining shows that the reduction of H60-specific T-cell responses is at least 20-fold, and that this number is likely to be an underestimate, due to background IFNγ production that is also seen in samples that are stimulated with control peptide (average, 0.57%; data not shown). Analysis of the same samples by MHC tetramer staining, which has a lower limit of detection (approximately 0.05%), suggests that the reduction of H60-specific T-cell responses is approximately 50-fold.
To address the effect of depletion of H4/H60-specific T cells on the development of GVHD, mice were monitored daily for clinical signs of GVHD and were analyzed after death for GVHD pathology. Despite the efficient suppression of the immunodominant H60-specific T-cell response, both the incidence and severity of GVHD were unaltered compared with that of the control group. Regardless of the nature of transferred splenocytes (ie, mock-depleted or H4/H60-depleted), all mice underwent weight loss and suffered to a similar extent from diarrhea, hunched posture, and ascites (Figure 3C), and mice had to be killed within 4 weeks after transfer due to severe GVHD (Figure 3D). Importantly, all mice that had received a syngeneic HSCT survived for the duration of the experiment (3 months [Figure 3D]; data not shown), indicating that the observed pathology is directly attributable to the allogeneic transfer.
Although the clinical signs did not suggest a shift in target organs of GVHD upon removal of H4/H60-specific T-cell responses, we addressed this in more detail by histopathologic analysis. Evaluation of GI tract sections revealed that leukocyte infiltration in the intestinal wall was equally severe in both groups. Also, in the liver, leukocyte infiltration in the periportal areas was detected in both groups (Figure 3E-F). In skin, the third classical target organ of GVHD, no signs of GVHD were observed (Figure 3C,F), in line with previous findings of Shlomchik et al.11
These data are in full contrast with a recent report by Kappel et al,28 who observed, using the identical transplantation model, that ex vivo removal of H60-specific T cells could increase survival and delay the onset of GVHD after transfer of 2 × 106 CD3+ B6 T cells. Previous patient data and mouse studies have indicated that the severity of GVHD correlates with the amount of donor T cells, and a 10-fold reduction in the number of infused T cells can already significantly alter the course of GVHD.39,41 To test whether H60-specific T-cell depletion has a beneficial effect under a condition where GVHD pathology is expected to be less severe, we transferred a lower amount of H4/H60-depleted or mock-depleted female B6 splenocytes (2 × 106 compared with 25 × 106) together with T-cell–depleted BM cells into lethally irradiated male BALB.B recipients. Also, after transfer of 1.1 log less T cells, all control mice mounted an H60-specific T-cell response, although the peak of responses was delayed (day 16 vs day 9) and of a lower magnitude (average, 6.2% vs 14.8%). Mice that had received H4/H60-depleted T cells showed H60 reactivity at background levels, again demonstrating the efficiency of the depletion strategy (Figure 4A-B).
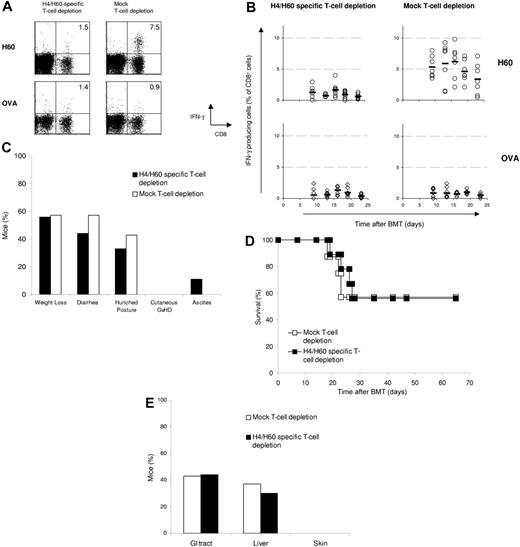
H4/H60-specific T cells are not required for GVHD independent of T-cell dose. H4/H60-specific T-cell–depleted splenocytes (2 × 106) or mock-depleted splenocytes from female B6 mice together with 5 × 106 T-cell–depleted BM cells were cotransferred into lethally irradiated male BALB.B recipients. Peripheral blood was obtained at indicated days after transfer, and the percentage of antigen-specific CD8+ T-cell responses ex vivo was determined for each individual mouse by intracellular IFNγ staining as described above. (A) Dot plots show H60-specific CD8+ T-cell responses on day 16 after transfer in peripheral blood of a representative mouse. Numbers in upper right quadrants reflect the percentage of IFNγ-producing CD8+ T cells of total CD8+ T cells. (B) Each ○ (restimulation with H60) or ⋄ (restimulation with OVA) represents 1 mouse; bars indicate averages. (C) Clinical signs of GVHD were scored daily starting from day 14 after transfer, when the mice had recovered from irradiation-induced weight loss. (D) Survival plot of mice that received H4/H60-specific T-cell–depleted splenocytes (▪; n = 9) or mock-depleted splenocytes (□; n = 7). (E) Incidence of histopathologic signs of GVHD in the classical target organs.
H4/H60-specific T cells are not required for GVHD independent of T-cell dose. H4/H60-specific T-cell–depleted splenocytes (2 × 106) or mock-depleted splenocytes from female B6 mice together with 5 × 106 T-cell–depleted BM cells were cotransferred into lethally irradiated male BALB.B recipients. Peripheral blood was obtained at indicated days after transfer, and the percentage of antigen-specific CD8+ T-cell responses ex vivo was determined for each individual mouse by intracellular IFNγ staining as described above. (A) Dot plots show H60-specific CD8+ T-cell responses on day 16 after transfer in peripheral blood of a representative mouse. Numbers in upper right quadrants reflect the percentage of IFNγ-producing CD8+ T cells of total CD8+ T cells. (B) Each ○ (restimulation with H60) or ⋄ (restimulation with OVA) represents 1 mouse; bars indicate averages. (C) Clinical signs of GVHD were scored daily starting from day 14 after transfer, when the mice had recovered from irradiation-induced weight loss. (D) Survival plot of mice that received H4/H60-specific T-cell–depleted splenocytes (▪; n = 9) or mock-depleted splenocytes (□; n = 7). (E) Incidence of histopathologic signs of GVHD in the classical target organs.
As expected, infusion of a lower number of cells led to a clear reduction in the severity and incidence of GVHD, resulting in a prolonged survival up to 2 months for around half of the mice (Figure 4C-D). The clinical signs of GVHD were confirmed in killed mice by histopathologic examination of intestine and liver (Figure 4E). Importantly, also under these conditions, where even a small reduction in T-cell responses that would otherwise lead to GVHD would be expected to have a noticeable effect, no difference in the onset of GVHD or in survival was found between mice that received H4/H60-depleted versus mock-depleted cells.
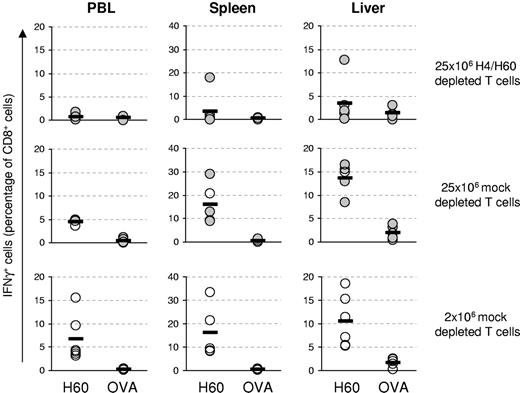
While the purging of H60-specific T cells appeared highly effective in peripheral blood samples, it was important to also measure H60-specific T-cell responses in the target organs of GVHD. To this purpose, we transferred 25 × 106 H4/H60-depleted or mock-depleted T cells, and measured H4- and H60-specific T cells responses directly ex vivo in the liver and spleen at day 7 after transfer. Whereas H60-specific T cells could readily be detected in livers of mice that received mock-depleted splenocytes, these cells were below the limit of detection in mice that received H4/H60-depleted splenocytes (Figure 5A-B). Similar results where found in the spleen, where a small IFNγ-producing T-cell population was detected after H60 peptide stimulation, which was not significant when compared to control stimulation (P = .3; Figure 5A-B).
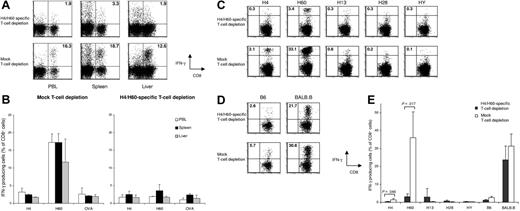
H4/H60-specific T-cell depletion reduces H4/H60-specific immune responses but does not alter total anti-BALB.B responses. Splenocytes from female B6 mice were either depleted with Kb-H4-and Kb-H60 tetramers or mock depleted. Subsequently, 25 × 106 splenocytes plus 5 × 106 T-cell–depleted BM cells were transferred into lethally irradiated male BALB.B recipients (n = 3 in both groups). Blood was sampled at day 6 after HSCT, and 1 day later, spleens and livers were harvested for T-cell isolation. Antigen-specific CD8+ T-cell responses were determined ex vivo by intracellular IFNγ staining for each individual mouse upon incubation with 0.1 μg/mL of the indicated peptide for 5 hours. (A) Dot plots show H60-specific CD8+ T-cell responses in peripheral blood (PBL), spleen, and liver samples of 1 representative mouse. (B) Graph shows the mean percentage of IFNγ-producing CD8+ T cells of 3 mice ± SD. (C-E) Splenocytes from mice analyzed in panels A and B were restimulated in vitro for 14 days with CD8+ T-cell–depleted irradiated male BALB.B target splenocytes. The restimulated cells were tested for reactivity toward the various MiHAgs (C), and for anti-BALB.B reactivity (D) by intracellular IFNγ staining. (E) Graph shows the mean percentage of IFNγ-producing CD8+ T cells of 3 mice ± SD. Numbers in upper right quadrants reflect the percentage of IFNγ-producing CD8+ T cells of total CD8+ T cells (panels A, C, and D).
H4/H60-specific T-cell depletion reduces H4/H60-specific immune responses but does not alter total anti-BALB.B responses. Splenocytes from female B6 mice were either depleted with Kb-H4-and Kb-H60 tetramers or mock depleted. Subsequently, 25 × 106 splenocytes plus 5 × 106 T-cell–depleted BM cells were transferred into lethally irradiated male BALB.B recipients (n = 3 in both groups). Blood was sampled at day 6 after HSCT, and 1 day later, spleens and livers were harvested for T-cell isolation. Antigen-specific CD8+ T-cell responses were determined ex vivo by intracellular IFNγ staining for each individual mouse upon incubation with 0.1 μg/mL of the indicated peptide for 5 hours. (A) Dot plots show H60-specific CD8+ T-cell responses in peripheral blood (PBL), spleen, and liver samples of 1 representative mouse. (B) Graph shows the mean percentage of IFNγ-producing CD8+ T cells of 3 mice ± SD. (C-E) Splenocytes from mice analyzed in panels A and B were restimulated in vitro for 14 days with CD8+ T-cell–depleted irradiated male BALB.B target splenocytes. The restimulated cells were tested for reactivity toward the various MiHAgs (C), and for anti-BALB.B reactivity (D) by intracellular IFNγ staining. (E) Graph shows the mean percentage of IFNγ-producing CD8+ T cells of 3 mice ± SD. Numbers in upper right quadrants reflect the percentage of IFNγ-producing CD8+ T cells of total CD8+ T cells (panels A, C, and D).
To examine whether depletion of H4/H60-specific T-cell responses had a discernible effect on the overall magnitude of the B6 anti-BALB.B response, splenocytes from both experimental groups were restimulated with irradiated BALB.B splenocytes and subsequently assessed for IFNγ production upon incubation with either peptide or BALB.B splenocytes. This analysis revealed that after in vitro restimulation, the percentage of H60-specific CD8+ T cells was 10-fold lower in mice that received H4/H60-depleted T cells (Figure 5C,E). This 10-fold difference after in vitro restimulation is likely to be an underestimate of the in vivo difference in H60-specific T-cell frequencies between the 2 groups, as T-cell frequencies obtained upon restimulation are nonlinear with ex vivo frequencies. For example, a difference of almost 20-fold in ex vivo T-cell frequencies (27% vs 1.5%) is compressed to a difference of less than 2-fold following in vitro restimulation (Figure S2).
Upon in vitro restimulation, H4-specific T-cell responses also were detectable in mice that received mock-depleted splenocytes, but not in mice that received H4/H60-depleted cells (P = .046). T-cell responses toward H13, H28, and HY were not substantially higher in mice that received H4/H60-depleted cells (Figure 5C,E). Importantly, when alloreactivity toward a broad range of MiHAgs was assessed through the use of BALB.B cells as target cells, T-cell reactivity in splenocyte cultures of mice that received H4/H60-depleted and mock-depleted T cells was comparable (P = .25; Figure 5D,E). These data suggest that the suppression of H60-specific T-cell responses in mice that received H60-depleted T cells is at least partially compensated by increased frequencies of alloreactive T cells with a yet unknown specificity.
It may be argued that while MHC tetramer–assisted depletion can efficiently suppress H60-specific T-cell responses in peripheral blood (Figure 3A-B) and at the target site (Figure 5A-B), a local build-up of H60-specific T cells at a later time point could result in the development of GVHD. To address this issue, lethally irradiated BALB.B mice received either 25 × 106 H4/H60-depleted or mock-depleted T cells (high T-cell dose) or 2 × 106 mock-depleted T cells (low T-cell dose). Mice were killed at day 18 or 19, when severe GVHD had developed (5 of 6 mice in both groups that received a high T cell dose) or at day 21 in mice where no clinical signs of GVHD could be observed (1 mouse in both groups that received a high T-cell dose, and all mice that received a low T-cell dose). H60-specific T-cell responses were analyzed in peripheral blood, spleen, and liver directly ex vivo. During GVHD, H60-specific T cells were clearly detectable in recipients of a high dose of mock-depleted T cells (Figure 6; middle panels), but these cells were below the limit of detection in most of the mice (5 of 6) that received H4/H60-depleted T cells, (Figure 6; top panels). Interestingly, H60-specific T cells were also present at high frequencies in blood, spleen, and liver in mice that had received a low dose of mock-depleted T cells, even though these mice did not develop clinical signs of GVHD (Figure 6; bottom panels).
GVHD occurs independent of accumulation of H60-specific T cells within the target organs at the time of disease. Splenocytes from female B6 mice were either depleted with Kb-H4-and Kb-H60 tetramers or mock depleted. Subsequently, either 25 × 106 H4/H60-depleted T cells (top row), 25 × 106 mock-depleted T cells (middle row), or 2 × 106 mock-depleted splenocytes (bottom row) plus 5 × 106 T-cell–depleted BM cells were transferred into lethally irradiated male BALB.B recipients (n = 6 in all groups). After onset of clinical GVHD (•) or at day 21 (○) blood, spleens, and livers were harvested to determine H60-specific or OVA-specific T-cell responses by intracellular IFNγ staining. Each circle represents 1 mouse; bars indicate averages.
GVHD occurs independent of accumulation of H60-specific T cells within the target organs at the time of disease. Splenocytes from female B6 mice were either depleted with Kb-H4-and Kb-H60 tetramers or mock depleted. Subsequently, either 25 × 106 H4/H60-depleted T cells (top row), 25 × 106 mock-depleted T cells (middle row), or 2 × 106 mock-depleted splenocytes (bottom row) plus 5 × 106 T-cell–depleted BM cells were transferred into lethally irradiated male BALB.B recipients (n = 6 in all groups). After onset of clinical GVHD (•) or at day 21 (○) blood, spleens, and livers were harvested to determine H60-specific or OVA-specific T-cell responses by intracellular IFNγ staining. Each circle represents 1 mouse; bars indicate averages.
Discussion
Here we show that 1 or multiple endogenous MiHAg-specific T-cell populations can be effectively removed from a naive precursor pool by MHC tetramer–based selection. MHC tetramer–based T-cell removal was highly efficient, and based on the assay used (IFNγ secretion or MHC tetramer staining), the reduction in H60-specific T-cell responses is estimated to be greater than 95% and 98%, respectively. Our data demonstrate that although T cells reactive toward the immunodominant MiHAg H60 form a very large portion of the total B6 anti-BALB.B response, removal of H60-specific T cells prior to transfer has no effect on the development of GVHD at 2 cell doses tested (Figures 3–4). These data, combined with the observation that strong H60-specific T-cell responses are observed in the absence of GVHD in mice receiving low-dose cell transplants, demonstrate that H60-specific T-cell responses do not play a decisive role in the development of GVHD.
These results are in sharp contrast with the work of Kappel et al,28 who found in the identical transplantation model that removal of H60-specific T cells did result both in increased survival and delay of GVHD after allogeneic HSCT. Several controls in our experiments support our conclusion that the H60-specific T-cell response is not required for GVHD. First, because the frequency of H60-specific T cells in the naive T-cell repertoire is around 1:10 000,42 and MHC tetramer staining has a detection limit of approximately 1:2000, we assessed depletion efficiency after subsequent antigen exposure in vivo (Figure 2). In contrast, in the experiments by Kappel et al, depletion efficiency was inferred by MHC tetramer staining of naive polyclonal T-cell populations immediately after depletion. Although this does not explain the difference in clinical outcome, it seems difficult if not impossible to determine T-cell depletion efficiency in this manner, as the detection limit of MHC tetramer staining is higher than the frequency of H60-specific T cells in the naive repertoire.42 It is also noted that the kinetics of development of GVHD in our studies appear to be more in line with the kinetics previously described for this murine model.19 Second, our data directly demonstrate that while H60-specific T-cell responses are profoundly reduced, this does not significantly affect the magnitude of the total B6 anti-BALB.B response (Figure 5), providing an immunologic underpinning to the unaltered course of GVHD. Finally, strong genetic support for our conclusion is provided by the studies of Korngold and Wettstein on immunodominance and GVHD in this transplantation model.18 Immunodominance of defined loci in the B6 → BALB.B transplantation was first established through the use of recombinant inbred strains. Within these inbred strains a hierarchy in immunodominance was found based on in vitro generation of B6 anti-BALB.B T-cell responses. Interestingly, the CXBK recombinant strain that expresses an immunodominant MiHAg (later identified by Shastri and colleagues as the H60 antigen20 ) did not develop GVHD upon transplantation with B6 cells. Vice versa, other recombinant inbred strains that did not express H60 developed severe GVHD. Thus, the immunodominant MiHAg H60 present in the CXBK strain was neither required nor sufficient for GVHD.
Collectively, we interpret the data presented here as follows: (1) while MHC tetramer–guided graft engineering is clearly feasible, at present there is no indication that this technology can be used to alter the course of GVHD; (2) because of this, we consider the use of this technology in clinical trials not advisable until data supporting a clinically relevant effect would become available; and (3) our knowledge on the identity of T-cell antigens that lead to GVHD remains incomplete, even in this well-studied murine transplantation model, and MiHAgs that are dominant as based on immunologic assays may not be required for transplantation-related pathology.
How can we dissect GVL and GVHD by either removal or purification of T cells with defined antigen specificities? A first possibility is to selectively purify and infuse those T cells that recognize defined MiHAgs that are positively associated with GVL effects. The feasibility of MHC tetramer–assisted purification of antigen-specific T cells for adoptive therapy has in fact been demonstrated by Moss and colleagues.43 However, the number of candidate antigens for which such selective T-cell infusion seems desirable is still limited.44 Alternatively, it may be possible to identify MiHAgs that are essential for GVHD through the use of novel, more specific technologies. Traditionally, MiHAgs have been identified by mixed leukocyte reactions (MLRs). However, it seems plausible that this method may be less suitable when searching for tissue-specific GVHD-inducing MiHAgs, and this strategy may be further complicated by the stochastic participation of individual T-cell clones in individual MLRs.45 As a potential alternative, with the availability of high-quality gene expression and single nucleotide polymorphism (SNP) databases it may become feasible to predict sets of potential MiHAgs that are expressed in distinct tissue sites. The combination of such database mining with recently developed high-throughput technologies for peptide-MHC generation46 and T-cell detection on MHC microarrays47,48 may make genome-wide screens for clinically relevant MiHAgs a realistic option.
Here we established a technique that uses MHC class I tetramers to allow the efficient purging of T-cell populations of interest from a polyclonal T-cell pool. With this method we have shown in a well-established murine model for allogeneic HSCT that efficient suppression of the immunodominant T-cell response has no measurable effect on the development of GVHD. While the data shown do not exclude that an essential role in GVHD may exist for other immunodominant MiHAgs, the current data do demonstrate that immunodominance per se does not indicate an essential role in GVHD. Based on these data we suggest that identification of novel tissue-specific MiHAgs is required to allow appropriate prediction for the onset and risk of GVHD. In such studies, “pathodominance” rather than immunodominance should be the primary evaluation criterion.
Authorship
Author contributions: M.d.W. designed research, performed research, analyzed data, and wrote the paper; M.T. performed research and analyzed data; J.-Y.S. performed research and analyzed data; M.W. designed research, performed research, analyzed data, and wrote the paper; and T.S. designed research, analyzed data, and wrote the paper. M.C.W. and T.N.M.S. contributed equally to this study.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Ton N. M. Schumacher, The Netherlands Cancer Institute, Department of Immunology, Plesmanlaan 121, Amsterdam, 1066 CX, The Netherlands; e-mail: t.schumacher@nki.nl; or Monika C. Wolkers, La Jolla Institute for Allergy and Immunology, 9420 Athena Cir, La Jolla, CA 92037; e-mail: mwolkers@liai.org.
The online version of this article contains a data supplement.
Supported by grants from the Netherlands Organization for Scientific Research (Pioneer 00-03) and the Landsteiner Foundation (LSBR 0522).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The authors would like to thank the Schumacher lab, in particular R. Arens and M. van den Boom for technical help, M. van der Valk for assistance with the histopathologic analysis, and J. Borst and J. Haanen for critical reading of the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal