Abstract
Early B lymphopoiesis in mammals is induced within the bone marrow (BM) microenvironment, but which cells constitute this niche is not known. Previous studies had shown that osteoblasts (OBs) support hematopoietic stem cell (HSC) proliferation and myeloid differentiation. We now find that purified primary murine OBs also support the differentiation of primitive hematopoietic stem cells through lymphoid commitment and subsequent differentiation to all stages of B-cell precursors and mature B cells. Lin−Sca-1+Rag-2− BM cell differentiation to B cells requires their attachment to OBs in vitro, and this developmental process is mediated via VCAM-1, SDF-1, and IL-7 signaling induced by parathyroid hormone (PTH). Addition of cytokines produced by nonosteoblastic stromal cells (c-Kit ligand, IL-6, and IL-3) shifted the cultures toward myelopoiesis. Confirming the role of OBs in B lymphopoiesis, we found that selective elimination of osteoblasts in Col2.3Δ-TK transgenic mice severely depleted pre-pro-B and pro-B cells from BM, preceding any decline in HSCs. Taken together, these results demonstrate that osteoblasts are both necessary and sufficient for murine B-cell commitment and maturation, and thereby constitute the cellular homolog of the avian bursa of Fabricius.
Introduction
It has long been hypothesized that early B lymphopoiesis in mammals is induced within the bone marrow (BM) microenvironment, but which cell or cells specifically constitute this B-cell niche is not known. Osteoblasts are the differentiated stromal cell type unique to the bone marrow environment, and therefore are a candidate for a cell type to play a specific role in perhaps multiple stages in hematopoiesis, including perhaps B lymphopoiesis. Previously, we found that purified human osteoblasts play a pivotal role in myelopoiesis, both by secreting numerous hematopoietic cytokines that support terminal neutrophil maturation and support the in vitro expansion of primitive long-term culture-initiating cells (LTCICs).1,2 More recently, analyses of bone morphogenetic protein receptor 1A–deficient (BMPR1A−/−) mice suggest that increased osteoblast number and/or activity increases the pool size of repopulating hematopoietic stem cells (HSCs) in vivo,3 suggesting that osteoblasts' stimulatory effect may extend as far back in hematopoietic differentiation to the level of the uncommitted HSCs.4,5 Whether osteoblasts play a specific role in supporting and/or directing the differentiation of HSCs to lymphoid commitment and differentiation is, however, less clear.
We sought to test directly whether osteoblasts play a pivotal role in B lymphopoiesis over and above any role in HSC maintenance. To ask whether osteoblasts are sufficient to support and induce HSCs to B lymphopoiesis, we have measured the production of early B-cell precursors from enriched HSCs cultured on purified osteoblasts in vitro. Conversely, to ask whether osteoblasts are necessary to support and induce HSCs to B lymphopoiesis and whether osteoblasts are necessary for early B lymphopoiesis, we have examined early alterations in hematopoiesis immediately following osteoblast depletion in Col2.3Δ-TK transgenic mice treated with GCV.6 The results of these studies show that osteoblasts are both necessary and sufficient for the support of all stages of B lymphopoiesis, from the first transition from HSC to common lymphoid progenitor to the generation of mature IgM+ B cells. We therefore conclude that the osteoblast comprises a key component of the bone marrow B-cell niche.
Materials and methods
Chemicals, monoclonal antibodies, and cell lines
Rat PTH was purchased from Bachem Biochemicals (Torrance, CA). Dexamethasone, insulin, ascorbic acid, and β-glycophosphate were purchased from Sigma (St Louis, MO). Murine c-Kit ligand, IL-3, IL-6, IL-7, and BMP-2 and antibodies, including lineage-specific antibodies for HSC isolation as well as blocking antibodies against IL-7, SDF-1, TSLP, M-CSF, and VCAM-1, were obtained from R&D Systems (Minneapolis, MN). Blocking antibodies against mouse integrin α4 and ICAM-1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). S17 stromal cells (provided by Dr John Monroe, University of Pennsylvania) were cultured first in αMEM + 20% FBS and then in DMEM + 10% FBS. MS1 endothelial cells (provided by Dr Mark Kahn, University of Pennsylvania) were cultured in DMEM + 5% FBS. All stromal cultures were maintained in 25-cm2 flasks and passaged prior to confluence.
Mice
Female and male B6.SJL-PtprcaPep3b/BoyJ (SJL, CD45.1+) and C57Bl/6J (B6, CD45.2+) mice at 8 to 16 weeks of age were purchased from The Jackson Laboratory (Bar Harbor, ME). RAG2 GFP NG BAC mice (B6) were kindly provided by Dr David Allman (Department of Pathology, University of Pennsylvania, Philadelphia, PA). Transgenic mice bearing a fusion gene composed of the 2.3-kb fragment of the rat type I collagen α1 (Col1α1) promoter (whose activity is largely restricted to differentiated osteoblasts) and HSV-TK (Col2.3Δ-TK)7 were graciously provided by Dr David W. Rowe (Department of Genetics and Developmental Biology, University of Connecticut Health Center, Farmington, CT).
Osteoblast deletion in Col1α1TK transgenic mice
Six-week-old mice bearing the Col2.3ΔTK transgene and aged-matched control CD-1 mice were injected intraperitoneally daily for up to 28 days with 5 mg/kg gancyclovir (GCV; Roche Pharmaceutical, Nutley, NJ) in saline. Mice were killed at weekly intervals and hematopoietic cells harvested from all long bones and spleens. Loss of osteoblasts was confirmed by measurement of osteocalcin mRNA in situ.
Osteoblast isolation and culture
Osteoblasts were isolated from calvaria of newborn mice by collagenase digestion. Briefly, calvaria of 6 to 8 mice from whom soft tissue was removed were pretreated in 50 mL of 10 mg/mL collagenase P for 20 minutes at 37°C (Roche Applied Science, Indianapolis, IN) to remove residual connective tissue debris. After washing, the treated calvaria were minced into (approximately 1 mm2) fragments and digested 3 times with 50 mL of 10 mg/mL collagenase P at 37°C for 10 to 15 minutes. The supernatants collected from all 3 digestions were pooled together and centrifuged. The pelleted cells were resuspended in 10% FCS/α-medium supplemented with ascorbic acid and β-glycerophosphate and kept at culture conditions of 37°C and 5% CO2. Under these conditions, the cells quickly adhered to the plastic and grew to confluence in 3 to 4 days. Aliquots of the adherent cells were released by digestion with 1 mg/mL collagenase P and 0.25% trypsin for immunostaining and in situ mRNA analyses. The maturation and purity of osteoblasts were examined by the in situ detection of osteopontin, as well as by flow cytometric detection of multiple antigens including CD45, PECAM-1, Pan-endo, and VCAM-1.
To compare osteoblastic versus adipocytic differentiation of primary calvarial cells, osteoblast differentiation was induced by the addition of 10 mM β-glycerol phosphate, 50 μM ascorbate-2-phosphate, 10−7 M dexamethasone, and 100 ng/mL human BMP-2 into 10% FCS/α-medium for 5 to 6 days until the cells reached confluence, followed by digestion, and replating in the presence of ascorbic acid and β-glycerophosphate as well as 1 × 10−7 M PTH for 4 to 5 days. For adipocytic induction, the primary calvarial cells were cultured in the presence of 10−7 M dexamethasone and 10 μg/mL human insulin for 10 to 14 days.8,9
Analysis of osteoblasts by immunohistochemistry, ELISA, and intracellular cytokine production
Osteoblasts were preseeded onto sterile glass cover slides in 6-well plate for 5 days. Then the glass slides were picked up and fixed and stained by standard immunochemistry procedure. Antibodies against osteopontin, osteocalcin, and control IgG were purchased from Santa Cruz Biotechnology. Stained cells were visualized with a Nikon Alpha phot-2 Y52 microscope (Nikon, Tokyo, Japan) under a 160/0.17 (100×) objective lens. The measurement of IL-7 or SDF-1 concentration by enzyme-linked immunosorbent assay (ELISA) in the supernatant of monolayer culture was performed as per the manufacturer's protocols (R&D Systems). Intracellular staining of IL-7 was performed following the protocol provided by Cytofix/Cytoperm Plus Kit (Pharmingen, San Diego, CA).
HSC purification
Freshly isolated BM cells from the hind legs of 8- to 10-week-old mice were washed once with 2% FCS in PBS, and their cell concentration was adjusted to 5 × 107/mL. To this suspension was added APC-coupled c-Kit antibody, FITC-coupled Sca-1 antibody, and PE-coupled cocktail of lineage antibodies including those against Mac-1, Gr-1, B220, Ter119, and CD3 (Pharmingen). The Lin−Sca-1+ or Lin−Sca-1+c-Kit++ HSCs were isolated on a MoFlo cell sorter (Dako, Carpinteria, CA).
To isolate RAG2− HSCs, Sca-1+ cells were first isolated by magnetic sorting (VarioMACS; Miltenyi Biotec, Auburn, CA) of bone marrow mononuclear cells from RAG2 GFP NG BAC mice. The positively selected Sca-1+ cells were then stained with Lin-PE and c-Kit-APC. GFP− LSK (0.4% of total Sca-1+ BM cells) and GFP+ LSK (0.1% of total Sca-1+ BM cells) cells were purified by fluorescence-activated cell sorting (FACS).
Osteoblasts/hematopoietic coculture
Osteoblasts and osteoblastic or endothelial cell lines were plated into 6-well plates in 10% FCS/α-medium supplemented with ascorbic acid, β-glycerophosphate, and 1 × 10−7 M PTH for 3 to 4 days, allowing the cells to grow to confluence and forming a gellike collagen pad. Then FACS-sorted Lin− (B220, Mac-1, Gr-1, CD3, Ter119) Sca-1+ (CD45.1+) BM cells from SJL mice were seeded into the precultured osteoblasts with or without cytokines in 10% FCS/α-medium supplemented with ascorbic acid and β-glycophosphate for 5 to 6 days. The adherent hematopoietic cells were released by digestion with 1 mg/mL collagenase P and 0.25% trypsin for 10 minutes at 37°C. For noncontact coculture, a 0.4-μm Transwell membrane insert (Corning Company, Acton, MA) was placed into the well of precultured osteoblasts, and the HSCs were added to the insert chamber.
Isolation of OB-adherent B220+ cells and transplantation
Hematopoietic cells adherent to osteoblast monolayers were released into suspension with 1 mg/mL collagenase P and 0.25% trypsin for 10 minutes at 37°C. The remaining gel pad and debris were removed by passaging the digested solution through a 70-mm screen. The recovered cells containing both hematopoietic and osteoblastic cells were stained with APC-labeled CD45.1 antibody, FITC-labeled B220 antibody, and PE-labeled combined antibodies against Mac-1, Gr-1, Ter119, and NK1.1. The FACS-sorted CD45.1+B220+Mac-1/Gr-1/Ter119/NK1.1− cells were verified to be CD19−. Then, 2 × 103 sorted CD45.1+ cells were injected into the tail veins of sublethally irradiated B6 mouse. Three weeks after transplantation, the hematopoietic tissues were collected, and CD45.1+ cells were detected and analyzed for lineage-specific markers.
Results
To ask whether osteoblasts are able to play a role in supporting lymphopoiesis, we examined the ability of murine osteoblasts to support the differentiation of lineage-depleted Sca+ (Lin−Sca-1+) BM cells to B-cell precursors. We first purified murine osteoblasts from newborn C57Bl/6 mice (CD45.2) in the presence of 10−7 M PTH.10,11 Cytospins of these cultured cells demonstrated a morphologically homogenous population of cells that expressed membrane-associated osteoblast-related protein osteopontin (Figure 1A). These cells were not detectably contaminated by either hematopoietic cells (CD45.2+) or endothelial cells (Figure 1B). Of interest, the osteoblasts expressed VCAM-1, which has been hypothesized to mediate early B-precursor adhesion to a previously uncharacterized population of BM stromal cells,12,13 as well as Sca-1, CD61, and ICAM-1 (Figure 1C).
Characterization of primary cultured osteoblasts. (A) May-Giemsa–stained cytospin of osteoblasts after in vitro culture for 5 days of calvarial cells of newborn mice, in the presence of 10−7 M PTH. (B, upper panel) Immunostaining of in vitro–cultured osteoblasts with IgG control or an osteopontin-specific antibody. (B, lower panel) Flow cytometric analysis of in vitro–cultured osteoblasts demonstrates no staining with antibodies to hematopoietic (CD45) or endothelial (PECAM-1, Pan-endo) cells, but does show staining with VCAM-1. (C) Flow cytometric analysis of osteoblast monolayers confirms the uniform presence of cell surface proteins known to be present on osteoblasts (Sca-1, CD61, and ICAM-1), but no staining with CD62L, CD40, CD69, or CD44.
Characterization of primary cultured osteoblasts. (A) May-Giemsa–stained cytospin of osteoblasts after in vitro culture for 5 days of calvarial cells of newborn mice, in the presence of 10−7 M PTH. (B, upper panel) Immunostaining of in vitro–cultured osteoblasts with IgG control or an osteopontin-specific antibody. (B, lower panel) Flow cytometric analysis of in vitro–cultured osteoblasts demonstrates no staining with antibodies to hematopoietic (CD45) or endothelial (PECAM-1, Pan-endo) cells, but does show staining with VCAM-1. (C) Flow cytometric analysis of osteoblast monolayers confirms the uniform presence of cell surface proteins known to be present on osteoblasts (Sca-1, CD61, and ICAM-1), but no staining with CD62L, CD40, CD69, or CD44.
When Lin−Sca-1+ (LS) BM cells from adult congenic SJL mice (CD45.1) were added to these osteoblast monolayers, the numbers of hematopoietic cells increased 20-fold over 6 days in culture. This hematopoietic proliferation was strongly dependent on contact with osteoblasts, as interposition of a porous (Transwell) membrane prevented more than 95% of the inductive effect (Figure 2A). Most of the cells produced in the Lin−Sca+/OB contact cultures were myeloid precursors, but approximately 5% of the hematopoietic cells recovered from the adherent monolayer following collagenase/trypsin digestion were B220+. In addition to these adherent hematopoietic cells, a similar number of B220+ cells were found released into the culture supernatant (Figure 2A right-hand panel). No B220+ cells were found in Transwell noncontact cultures. The adherent B220+ cells recovered from the adherent compartment of the Lin−Sca+/OB contact cultures expressed low to medium levels of B220+ but no CD19 or IgM. Of these, 14% were CD43+CD24−/lo pre-pro-B cells and 45% were CD43+CD24+ pro-B cells.14 In contrast to the adherent cells, B-lymphoid cells found in suspension were more mature, expressing higher levels of B220 and including subpopulations expressing CD19 (7%) and IgM (9%) (Figure 2B).
Primary osteoblasts stimulate BM B lymphopoiesis in vitro. (A) Hematopoietic proliferation during hematopoietic-osteoblast coculture is dependent on OB contact. (A, left-hand panel) Lin−Sca+ BM cells (CD45.1+) were isolated by FACS and cultured either directly on (CD45.2+) osteoblast monolayers (OB) or separated from osteoblast monolayers by a Transwell membrane (OB + Trans). (A, right-hand panel) Hematopoietic proliferation was nearly completely abrogated in the Transwell cultures, and no B220+ cells were produced. In the LS BM-OB contact cultures, 4% to 8% of all CD45.1+ cells produced were B220+, and these B220+ cells were found approximately equally portioned between cells adherent to the OB monolayer (OB-Ad) and nonadherent cells readily decanted from the suspension culture (OB-Susp). Data are shown as means ± SD; n = 4. (B) Flow cytometric analyses of B-lymphocyte precursors generated from Lin−Sca+ BM-osteoblast (non-Transwell) cocultures, recovered from both OB-adherent (OB-adh) and suspension cell (OB-susp) fractions. Nearly 80% of B220+ cells found adherent to the OB monolayer expressed CD24, 60% expressed CD43, and 44% were CD24+CD43+, but none expressed surface IgM. B220+ cells recovered from the culture supernatants expressed lower levels of CD43, and included (9%) B cells expressing surface IgM. (C) PTH stimulation increases the survival and proliferation of both B220+CD19− and B220+CD19+ cells in osteoblast cocultures. IgM−Mac1−NK1.1− (CD45.1+) BM cells were further FACS purified to isolate B220+CD19− and CD19−B220+CD19+ cells, which were then cultured with (CD45.2+) osteoblasts for 6 days in the presence or absence of PTH. At the end of the cultures, the cells were collected and cells with the phenotype of the input cells (B220+CD19− or B220+CD19+) were measured by flow cytometry. Data shown are expressed as the ratio of output/input B220+CD19− cells in the culture initiated with B220+CD19− cells (C, bars on left), or the ratio of output/input B220+CD19+ cells in the culture initiated with B220+CD19+ cells (C, bars on right). (D) Osteoblasts support B-lymphoid transitions from pre-pro B cells, pro-B cells, and B cells. Primary BM B220+CD19− pre-pro-B cells, B220+CD19+ pro-B cells, and B220++CD19+ pre-B cells were identified by flow cytometry and isolated by FACS, and each fraction was separately cultured with osteoblasts. Flow cytometric analysis after 5 days demonstrates further continued maturation in each of these compartments along B lymphopoiesis, including the acquisition of cell surface CD19 and/or IgM.
Primary osteoblasts stimulate BM B lymphopoiesis in vitro. (A) Hematopoietic proliferation during hematopoietic-osteoblast coculture is dependent on OB contact. (A, left-hand panel) Lin−Sca+ BM cells (CD45.1+) were isolated by FACS and cultured either directly on (CD45.2+) osteoblast monolayers (OB) or separated from osteoblast monolayers by a Transwell membrane (OB + Trans). (A, right-hand panel) Hematopoietic proliferation was nearly completely abrogated in the Transwell cultures, and no B220+ cells were produced. In the LS BM-OB contact cultures, 4% to 8% of all CD45.1+ cells produced were B220+, and these B220+ cells were found approximately equally portioned between cells adherent to the OB monolayer (OB-Ad) and nonadherent cells readily decanted from the suspension culture (OB-Susp). Data are shown as means ± SD; n = 4. (B) Flow cytometric analyses of B-lymphocyte precursors generated from Lin−Sca+ BM-osteoblast (non-Transwell) cocultures, recovered from both OB-adherent (OB-adh) and suspension cell (OB-susp) fractions. Nearly 80% of B220+ cells found adherent to the OB monolayer expressed CD24, 60% expressed CD43, and 44% were CD24+CD43+, but none expressed surface IgM. B220+ cells recovered from the culture supernatants expressed lower levels of CD43, and included (9%) B cells expressing surface IgM. (C) PTH stimulation increases the survival and proliferation of both B220+CD19− and B220+CD19+ cells in osteoblast cocultures. IgM−Mac1−NK1.1− (CD45.1+) BM cells were further FACS purified to isolate B220+CD19− and CD19−B220+CD19+ cells, which were then cultured with (CD45.2+) osteoblasts for 6 days in the presence or absence of PTH. At the end of the cultures, the cells were collected and cells with the phenotype of the input cells (B220+CD19− or B220+CD19+) were measured by flow cytometry. Data shown are expressed as the ratio of output/input B220+CD19− cells in the culture initiated with B220+CD19− cells (C, bars on left), or the ratio of output/input B220+CD19+ cells in the culture initiated with B220+CD19+ cells (C, bars on right). (D) Osteoblasts support B-lymphoid transitions from pre-pro B cells, pro-B cells, and B cells. Primary BM B220+CD19− pre-pro-B cells, B220+CD19+ pro-B cells, and B220++CD19+ pre-B cells were identified by flow cytometry and isolated by FACS, and each fraction was separately cultured with osteoblasts. Flow cytometric analysis after 5 days demonstrates further continued maturation in each of these compartments along B lymphopoiesis, including the acquisition of cell surface CD19 and/or IgM.
The production of IgM+ B lymphocytes from Lin−Sca-1+ cell/osteoblast cocultures suggests that osteoblasts induce all developmental transitions required for full B-cell development. However, since many other cell types are generated during these cocultures, it is theoretically possible that the later stages of B-lymphocyte maturation are stimulated indirectly, by other hematopoietic or stromal cells derived from the multipotential Lin−Sca-1+ cells. To examine whether osteoblasts alone directly stimulate these stages of B-cell development, we isolated IgM−Mac1−NK1.1−B220+CD19− pre-pro-B and IgM−Mac1−NK1.1−B220+CD19+ pro-B/pre-B cells from normal BM and directly seeded them onto congenic osteoblast monolayers. As shown in Figure 2C, osteoblasts stimulated the survival and expansion of B220+CD19− pre-pro-B and B220+CD19+ pro-B/pre-B cells over 6 days, and pretreatment of the osteoblast monolayer with PTH enhanced this ability. In addition, a substantial fraction of both purified B220+CD19+ pro-B and B220++CD19+ pre-B cells differentiated to become IgM+ B lymphocytes (Figure 2D). Purified B220+CD19− pre-pro-B generated very few IgM+ B lymphocytes over these same 6 days, but one fifth became CD19+. These data demonstrate that osteoblasts play a direct role in stimulating all stages of BM B lymphopoiesis, from the commitment of HSCs to lymphopoiesis to the terminal differentiation of IgM+ B lymphocytes.
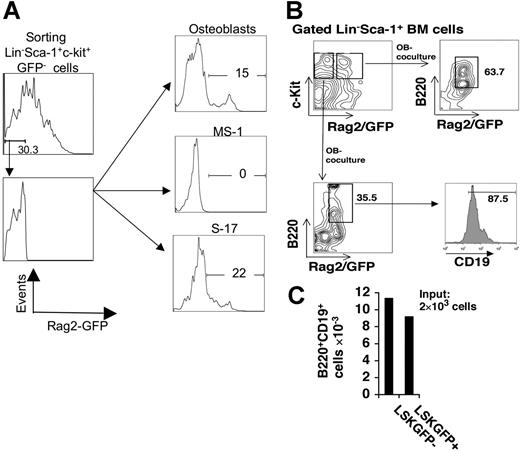
Next we asked whether osteoblasts support the earliest lineage decisions of primitive HSCs to B-lymphoid commitment per se. To focus on this early step, we initiated cultures with more highly purified early hematopoietic stem cells that excluded common lymphoid or committed lymphoid progenitors. For this purpose, we isolated GFP− Lin−Sca+c-Kit+ (LSK) BM cells from RAG2 GFP NG BAC mice (B6),15 a recombinant B6 transgenic strain in which all cells that express recombinase activate a GFP transgene. Three-thousand sorted GFP− LSK cells were cultured on either primary osteoblasts, the endothelial cell line MS-1, or the permissive stromal line S17. Over 14 days in coculture with osteoblasts, 15% of the cells generated from these GFP− LSK cells became GFP+, nearly identical to the numbers of GFP+ cells produced by GFP− LSK cells cultured with S17 cells (1.64 × 104 versus 1.67 × 104). In contrast, no GFP− LSK cells cultured on endothelial monolayers became GFP+ (Figure 3A). Of note, these GFP− LSK cells cultured on OB-generated B220+CD19+GFP+ B-lymphocyte precursors, at efficiency very similar to that of cultured GFP+ LSK cells, which already expressed recombinase (Figure 3B-C). Thus, osteoblasts stimulate early transitions from HSCs to committed lymphoid progenitors. Taken together, these data demonstrate that osteoblasts play a direct role in stimulating all stages of BM B lymphopoiesis, from the commitment of HSCs to lymphopoiesis to the differentiation to IgM+ B lymphocytes.
Osteoblasts support the differentiation of RAG2− LSK cells to RAG2+ cells, and to B-lymphoid precursors. (A) BM LSK cells from RAG2 GFP NG BAC mice were analyzed for GFP expression by FACS, and sorted into GFP− and GFP+ populations. GFP− LSKs were cultured for 14 days on either primary osteoblasts, MS-1 endothelial cells, or S17 stromal cells, after which the hematopoietic cells were recovered, stained with nonfluorescein dyes for B220 and CD19, and analyzed for Rag2−GFP, CD19, and B220 expression by flow cytometry. (A) Rag-2−GFP− BM cells generate EGFP-bright cells after 14-day coculture with osteoblasts, and S-17 cells, but not MS-1 endothelial cells. Each coculture was initiated with 3000 LSK Rag2− cells. After 14 days, 1.09 × 105 total hematopoietic cells were recovered from the OB coculture, of which 1.64 × 104 were EGFP bright, whereas none of the 1.2 × 105 cells recovered from the MS-1 coculture was EGFP bright. S17 cocultures generated 7.6 × 104 total cells, of which 1.67 × 104 were EGFP bright. Percent of output cells with green fluorescence higher than control (nontransgenic and transgenic, sorted, Rag-2 GFP−) cells is shown in the figure. (B) GFP+B220+CD19+ B lymphocytes are generated from both GFP− LSK cells and GFP+ LSK cells isolated from RAG2 GFP NG BAC mice, following 14-day cultures on osteoblast monolayers. (C) Absolute numbers of B-lymphocyte precursors produced from cocultures initiated with 105 LSK cells.
Osteoblasts support the differentiation of RAG2− LSK cells to RAG2+ cells, and to B-lymphoid precursors. (A) BM LSK cells from RAG2 GFP NG BAC mice were analyzed for GFP expression by FACS, and sorted into GFP− and GFP+ populations. GFP− LSKs were cultured for 14 days on either primary osteoblasts, MS-1 endothelial cells, or S17 stromal cells, after which the hematopoietic cells were recovered, stained with nonfluorescein dyes for B220 and CD19, and analyzed for Rag2−GFP, CD19, and B220 expression by flow cytometry. (A) Rag-2−GFP− BM cells generate EGFP-bright cells after 14-day coculture with osteoblasts, and S-17 cells, but not MS-1 endothelial cells. Each coculture was initiated with 3000 LSK Rag2− cells. After 14 days, 1.09 × 105 total hematopoietic cells were recovered from the OB coculture, of which 1.64 × 104 were EGFP bright, whereas none of the 1.2 × 105 cells recovered from the MS-1 coculture was EGFP bright. S17 cocultures generated 7.6 × 104 total cells, of which 1.67 × 104 were EGFP bright. Percent of output cells with green fluorescence higher than control (nontransgenic and transgenic, sorted, Rag-2 GFP−) cells is shown in the figure. (B) GFP+B220+CD19+ B lymphocytes are generated from both GFP− LSK cells and GFP+ LSK cells isolated from RAG2 GFP NG BAC mice, following 14-day cultures on osteoblast monolayers. (C) Absolute numbers of B-lymphocyte precursors produced from cocultures initiated with 105 LSK cells.
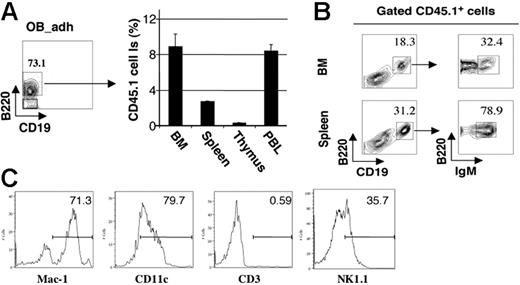
To document that the adherent population of B220+CD19− cells induced and maintained by osteoblasts included true functional precursors that develop into mature B cells, we transplanted 2 × 103 FACS-purified B220+CD19− (CD45.1+) cells from LS/OB cocultures, released and purified from 10-day osteoblast cocultures, into sublethally irradiated congenic (CD45.2) mice. After 3 weeks, a substantial contribution of CD45.1+ cells was found in peripheral blood, spleen, and bone marrow, but not thymus (Figure 4A), and flow cytometric analysis confirmed the presence of CD45.1+ mature B220++CD19+IgM+ B lymphocytes in both BM and spleen (Figure 4B). Of interest, we also detected donor-derived B220−CD19− non-B cells in recipient BM, most of which were Mac-1+CD11c+CD3−NK1.1−/lo (Figure 4C). This suggests that the adherent B220+CD19− population also includes precursors for macrophages and dendritic cells, whether originating from a distinct or inherently bilineage precursor, as indicated by previous studies of Pax5−/− lymphoid progenitors.16–18
B220+CD19− adherent cells from differentiated LSK cells cultured on OBs in vitro differentiate into IgM+ B cells in vivo. LSK cells from CD45.1+ mice were cultured on (CD45.2+) OB monolayers for 10 days, and the OB-adherent hematopoietic cells (OB-adh) were recovered. B220+CD19− pre-pro-B cells were isolated by FACS and transplanted into sublethally irradiated CD45.2 mice. (A) CD45.1+ cell contributions to recipient bone marrow, spleen, thymus, and blood after 21 days (means ± SD, n = 5). (B) Costaining with CD45.1 and B-cell differentiation markers, demonstrating the presence of donor-derived CD45.1+B220+CD19+IgM+ immature B lymphocytes in BM and spleen (n = 5). (C) Phenotypic characterization of (CD45.1+) donor-derived B220−CD19− cells in recipient (CD45.2+) bone marrow.
B220+CD19− adherent cells from differentiated LSK cells cultured on OBs in vitro differentiate into IgM+ B cells in vivo. LSK cells from CD45.1+ mice were cultured on (CD45.2+) OB monolayers for 10 days, and the OB-adherent hematopoietic cells (OB-adh) were recovered. B220+CD19− pre-pro-B cells were isolated by FACS and transplanted into sublethally irradiated CD45.2 mice. (A) CD45.1+ cell contributions to recipient bone marrow, spleen, thymus, and blood after 21 days (means ± SD, n = 5). (B) Costaining with CD45.1 and B-cell differentiation markers, demonstrating the presence of donor-derived CD45.1+B220+CD19+IgM+ immature B lymphocytes in BM and spleen (n = 5). (C) Phenotypic characterization of (CD45.1+) donor-derived B220−CD19− cells in recipient (CD45.2+) bone marrow.
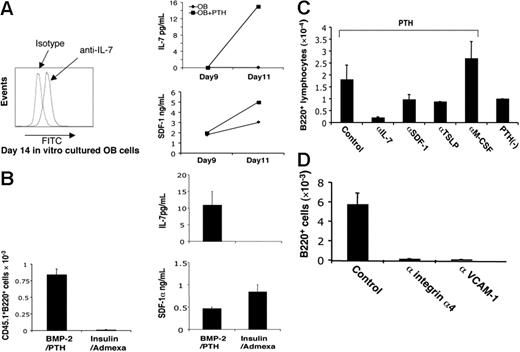
Next, we investigated which cytokines and adhesion molecules derived from osteoblasts are required for B-cell commitment and maturation.19,20 Previously, we had demonstrated that SDF-1 is secreted by human osteoblasts, as well as in other BM stromal and endothelial cells.21 In addition to high concentrations of SDF-1, we found that the cultivated osteoblasts synthesized and secreted measurable quantities of IL-7 when PTH was added (Figure 5A), confirming previous reports that IL-7 is required for early B lymphopoiesis.20 To examine the relationship between osteoblast development and their ability to synthesize IL-7 and SDF-1, we either induced (by the addition of BMP-2 plus PTH) or inhibited (by the addition of adipocyte-inducing agents insulin plus dexamethasone) osteoblastic differentiation from freshly isolated calvarial cells. Whereas PTH-stimulated, fully differentiated osteoblasts secreted both SDF-1 and IL-7, and induced B lymphopoiesis, inhibition of osteoblast differentiation and support of adipocyte differentiation with insulin and dexamethasone blocked IL-7 production and differentiation of B220+ precursors, despite continued elevated level of SDF-1 (Figure 5B). Conversely, inclusion of neutralizing antibodies to control LS/OB cocultures confirmed that IL-7 and SDF-1 as well as TSLP (thymic stromal cell–derived lymphopoietin), but not MCSF, were required for the stimulation of B-cell development by osteoblasts (Figure 5C). In addition, we found that inclusion of antibody against either VCAM-1 or integrin α4 significantly reduced the generation of B220+ B lymphocytes, emphasizing the importance of the VCAM-1/integrin α4 ligation-mediated signaling in the contact-mediated induction of B lymphopoiesis by OBs (Figure 5D).
IL-7, SDF-1, and integrin α4/VCAM-1 ligation mediate the B generation from PTH-stimulated cocultures. (A, left-hand panel) Intracellular staining of IL-7 in osteoblasts cultivated in vitro. (A, right-hand panel) PTH treatment enhances the production of IL-7 and SDF-1 from osteoblast cultures. (B) Il-7 and SDF secretion, and B-cell differentiation are favored by PTH-driven osteogenesis and inhibited by induction of adipogenesis. CD45.2+ osteoblasts cultured in β-glycerol phosphate, ascorbate, and dexamethasone were digested and replated in either the same medium, or induced to adipocyte differentiation by the addition of 10−7 M dexamethasone and 10 μg/mL human insulin for 10 days. CD45.1+ LSK cells were added to each monolayer culture, and IL-7, SDF-1 secretion, and B220 cell production from the LSK cells measured on day 10 (mean ± SD; n = 3). (C) Inclusion of neutralizing antibody against IL-7, SDF-1, TSLP, or M-CSF in the coculture modulates the production of B220+ cells (means ± SD, n = 3). (D) Neutralizing antibody against integrin α4 or VCAM-1 inhibits the production of B220+ cells from coculture (means ± SD, n = 3).
IL-7, SDF-1, and integrin α4/VCAM-1 ligation mediate the B generation from PTH-stimulated cocultures. (A, left-hand panel) Intracellular staining of IL-7 in osteoblasts cultivated in vitro. (A, right-hand panel) PTH treatment enhances the production of IL-7 and SDF-1 from osteoblast cultures. (B) Il-7 and SDF secretion, and B-cell differentiation are favored by PTH-driven osteogenesis and inhibited by induction of adipogenesis. CD45.2+ osteoblasts cultured in β-glycerol phosphate, ascorbate, and dexamethasone were digested and replated in either the same medium, or induced to adipocyte differentiation by the addition of 10−7 M dexamethasone and 10 μg/mL human insulin for 10 days. CD45.1+ LSK cells were added to each monolayer culture, and IL-7, SDF-1 secretion, and B220 cell production from the LSK cells measured on day 10 (mean ± SD; n = 3). (C) Inclusion of neutralizing antibody against IL-7, SDF-1, TSLP, or M-CSF in the coculture modulates the production of B220+ cells (means ± SD, n = 3). (D) Neutralizing antibody against integrin α4 or VCAM-1 inhibits the production of B220+ cells from coculture (means ± SD, n = 3).
Two recent reports suggest that HSCs may localize preferentially to BM endothelial cells.22,23 These results, combined with the previous observations of the role of osteoblasts in maintaining HSC pool size, raise the possibility that the HSC niches may be complex, requiring the interaction of osteoblasts, endothelial cells, and perhaps other mesenchymal components. To begin to test this concept functionally, we measured the effects of adding a cocktail of cytokines produced by nonosteoblast BM stromal cells, including c-Kit ligand, IL-3, and IL-6 into osteoblast/Lin−Sca+ (LS) BM cell cocultures. In these cultures, cytokine addition significantly augmented the ability of osteoblastic monolayer to support the survival and expansion of LS cells, but inhibited the production of B220+ B lymphocytes. Cultures initiated on OB monolayers with 104 LS cells produced 1.0 × 103 Sca+Kit+ cells and 1.4 × 103 B220+ cells (of 1.2 × 105 total adherent plus nonadherent hematopoietic cells), whereas cultures initiated on OB monolayers supplemented with c-KitL/IL-3/IL-6 produced 1.8 × 104 Sca+Kit+ cells and 0.8 × 103 B220+ cells (of 2.6 × 105 total hematopoietic cells) (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). These results suggests the possibility of specific microniches on the osteoblastic surface that support varying degrees of HSC self-renewal, myelopoiesis, or B lymphopoiesis depending on the relative proximity of other BM stromal cells such as endothelial cells.
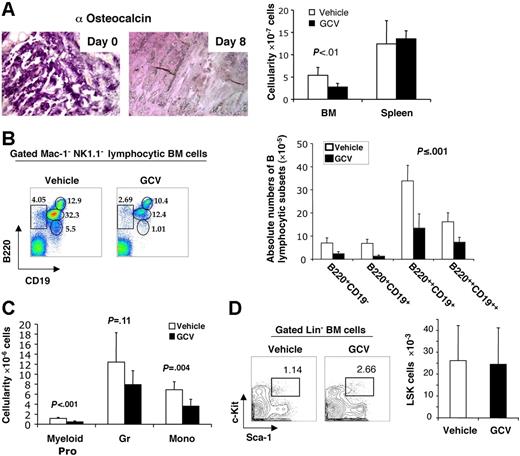
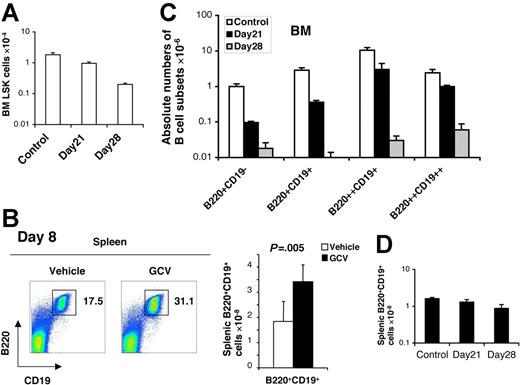
If the ability of osteoblasts to support B lymphopoiesis in vitro reflects a key role in vivo, we hypothesized that elimination of osteoblasts in vivo would result in loss of B-cell precursors, beyond any effect on HSC numbers per se. Recently, Visnjic et al6 performed elegant studies demonstrating that conditional ablation of endosteal osteoblasts in Col2.3Δ-TK transgenic mice by GCV administration in vivo for 21 to 28 days resulted in the depletion of a wide range of hematopoietic cells from the bone marrow, including HSCs as well as more mature myeloid and B-lymphoid precursors. Since BM HSCs were themselves depleted, the simplest explanation for the loss of myeloid and lymphoid precursors was the loss of the HSCs from which they derive. However, an alternative possibility was that OBs might play a more direct role in B lymphopoiesis in vivo, beyond the level of HSC support. Given the present findings that cultured OBs directly stimulate all stages of B lymphopoiesis, we sought to directly test this hypothesis by examining early alterations in hematopoiesis immediately following osteoblast depletion, prior to HSC loss, by GCV in Col2.3Δ-TK transgenic mice. BM biopsies from untreated mice documented robust hematopoietic activity, and showed abundant immunostaining signals for the osteoblast-specific matrix protein osteocalcin. (Figure 6). Osteocalcin-specific osteoblasts were depleted as early as 8 days following GCV treatment. In parallel, total BM hematopoietic cellularity, but not splenic cellularity, declined by 50% during this 8-day GCV treatment (Figure 6A). Flow cytometric analysis of small mononuclear cells (Mac-1−NK1.1−) from BM of control mice identified 4 distinct stages of B-lineage development: B220+CD19− pre-pro-B cells, B220+CD19+ pro-B cells, B220++CD19+ pre-B cells, and B220++CD19++ immature B cells. Osteoblast ablation resulted in the depletion of each of these B-lymphocyte subsets by day 8, with the greatest reduction in pre-pro-B (64%) and pro-B (81%) cells (Figure 6B). Consistent with previous findings showing that osteoblasts support in vitro myeloid progenitor survival/expansion,2 the number of Gr-1loc-Kit+ myeloid progenitors in the BM was also reduced (60%), as were the number of mature monocytes and granulocytes by 28% and 42%, respectively (Figure 6C). Erythroid (gly A+) and megakaryocytic (CD41+) cells remained at 90% to 100% of controls. Despite these effects on B lymphopoiesis, and to a lesser extent on myelopoiesis, the number of LSK cells, which includes both long-term and short-term HSCs, was not yet reduced by day 8 after osteoblast depletion with GCV (Figure 6D). In contrast, LSK cells were not detectably depleted until day 21 (Figure 7A). 6 Of note, the number of mature B lymphocytes in the spleen did not decline at day 8 (Figure 7B), underscoring the organ-specific depletion of early stages of B cells from BM tissue by GCV treatment. These results confirm that osteoblasts play a direct role in BM B-cell precursor production, beyond simply supporting HSC survival. Continued evaluation over 28 days following osteoblast depletion confirmed that BM pre-pro B and pro-B subsets continued to decline further and more rapidly than LSK, with BM pre-pro and pro-B cells falling to less than 1% of control by 28 days (Figure 7A, C-D). These data indicate that acute depletion of osteoblasts causes an abrupt decline in B-cell precursors in the bone marrow, confirming their ability to directly support B lymphopoiesis.
Osteoblast depletion leads to early loss of B lymphopoiesis in bone marrow. Col2.3Δ-TK transgenic mice were treated with vehicle (□) or GCV (▪) for 8 days (n = 5). (A) Osteocalcin staining of bone tissue section at day 0 and day 8 after GCV treatment (left-hand panel), as well as day-8 BM (femurs and tibiae) and spleen cellularities of vehicle- or GCV-treated mice (right-hand panel). (B) Flow cytometric analyses of B-lymphocyte precursors recovered from the BM of vehicle- or GCV-treated mice, with the percent of each subpopulation within the mononuclear bone marrow fractions noted (left-hand panel); absolute numbers of different B-lymphocyte precursor populations within BM after treatment (means ± SD, n = 5) (left-hand panel). (C) Absolute numbers of Gr-1loc-Kit++ myeloid progenitors, Gr-1++Mac-1++ granulocytes, and Gr-1+Mac-1++ monocytes within the BM of vehicle- or GCV-treated mice (means ± SD, n = 5). (D) Detection of Lin−Sca+Kit+ BM cells in vehicle- and GCV-treated mice by flow cytometry, with percent LSK cells noted (left-hand panel), and absolute numbers (right-hand panel) of LSK cells detected in total BM, as determined by multiplying percent LSK cells × total cellularity/femurs + tibias (means ± SD).
Osteoblast depletion leads to early loss of B lymphopoiesis in bone marrow. Col2.3Δ-TK transgenic mice were treated with vehicle (□) or GCV (▪) for 8 days (n = 5). (A) Osteocalcin staining of bone tissue section at day 0 and day 8 after GCV treatment (left-hand panel), as well as day-8 BM (femurs and tibiae) and spleen cellularities of vehicle- or GCV-treated mice (right-hand panel). (B) Flow cytometric analyses of B-lymphocyte precursors recovered from the BM of vehicle- or GCV-treated mice, with the percent of each subpopulation within the mononuclear bone marrow fractions noted (left-hand panel); absolute numbers of different B-lymphocyte precursor populations within BM after treatment (means ± SD, n = 5) (left-hand panel). (C) Absolute numbers of Gr-1loc-Kit++ myeloid progenitors, Gr-1++Mac-1++ granulocytes, and Gr-1+Mac-1++ monocytes within the BM of vehicle- or GCV-treated mice (means ± SD, n = 5). (D) Detection of Lin−Sca+Kit+ BM cells in vehicle- and GCV-treated mice by flow cytometry, with percent LSK cells noted (left-hand panel), and absolute numbers (right-hand panel) of LSK cells detected in total BM, as determined by multiplying percent LSK cells × total cellularity/femurs + tibias (means ± SD).
BM B-cell precursor depletion following GCV treatment of Col2.3Δ-TK transgenic mice precedes depletion of BM LSK cells and is not seen in the spleen. (A) Total LSK cells recovered from the BM (2 hind legs) of GCV-treated and control mice at day 21 and day 28 (means ± SD; n = 5). (B) Flow cytometric detection (left-hand panel) and total numbers (right-hand panel) of B220+CD19+ B lymphocytes in the spleens 28 days after treatment with vehicle or GCV (means ± SD, n = 5). (C-D) The absolute numbers of recovered BM B-lymphocyte subsets (C) (B220+CD19− pre-pro-B, B220+CD19+ pro-B, B220++CD19+ and B220++CD19++ pre-B, and immature B cells), as well as those of splenic mature B cells (D) at day 21 and day 28 after GCV treatment (means ± SD, n = 4).
BM B-cell precursor depletion following GCV treatment of Col2.3Δ-TK transgenic mice precedes depletion of BM LSK cells and is not seen in the spleen. (A) Total LSK cells recovered from the BM (2 hind legs) of GCV-treated and control mice at day 21 and day 28 (means ± SD; n = 5). (B) Flow cytometric detection (left-hand panel) and total numbers (right-hand panel) of B220+CD19+ B lymphocytes in the spleens 28 days after treatment with vehicle or GCV (means ± SD, n = 5). (C-D) The absolute numbers of recovered BM B-lymphocyte subsets (C) (B220+CD19− pre-pro-B, B220+CD19+ pro-B, B220++CD19+ and B220++CD19++ pre-B, and immature B cells), as well as those of splenic mature B cells (D) at day 21 and day 28 after GCV treatment (means ± SD, n = 4).
Discussion
These studies provide direct evidence that osteoblasts are necessary and sufficient for supporting all stages of BM B lymphopoiesis, via inductive signals that include VCAM-1–mediated adhesion and locally secreted IL-7 and SDF-1. Thus, whereas in birds specialized cells in the bursa of Fabricius may form the B-cell niche, in mice this role has been subsumed by BM osteoblasts.
The present direct in vitro culture studies show that osteoblasts support HSC differentiation to B lymphopoiesis. Moreover, by isolating specific developmental stages and assaying cellular differentiation on osteoblasts, we have found that osteoblasts support each stage of differentiation from HSC to IgM+ B cell. In particular, osteoblasts support the transition from Rag2− HSC to Rag2+ committed lymphoid progenitor, thereby dictating B-lymphoid commitment per se as well as subsequent steps in B-lymphoid differentiation. Of note, this inductive effect is not shared by adipocytes or endothelial cells, but only by osteoblasts, and the inductive effect of osteoblasts is comparable with that seen with the S17 line, a stromal cell line of uncertain lineage with known B-cell inductive properties. Of note, our data also show that the early B-cell precursors produced in HSC-osteoblast cocultures are completely developmentally competent, as they are able to proliferate and expand to produce mature B cells after transplantation in vivo. Thus, osteoblasts appear to be able to support all developmental transitions in B lymphopoiesis. What molecular events are required for each step is at present uncertain, but the present system should allow for its careful molecular dissection in the future.
Several previous studies had suggested that osteoblasts contribute to both HSC proliferation and myeloid differentiation.1–3,10 The present results also confirm and extend the studies of Visnjic et al, who showed that osteoblasts are required for HSCs in vivo, as their postnatal depletion leads to widespread hematopoietic failure, including the loss of HSCs, myeloid progenitors and precursors, and B lymphoid precursors over one month.6 Although the most parsimonious explanation for these findings was that HSC loss led to multilineage hematopoietic failure, the present studies demonstrate that the loss of B-cell precursors following osteoblast depletion actually precedes any detectable loss of HSCs, and is detectable as soon as maximal osteoblast depletion can be documented, at day 8. In addition, throughout the lifetime of the mice after osteoblast depletion, the degree of B-precursor depletion exceeds the reduction in HSC numbers. These results strongly suggest that osteoblasts are essential for some aspects of B lymphopoiesis, over and above any role they may play in HSC maintenance and/or proliferation. Of course, our data also demonstrate that OBs support the production of myeloid precursors in vitro, and acute depletion of OBs in vivo results in rapid declines in the numbers of BM granulocytic and monocytic cells. Thus, OBs clearly have multiple roles in BM hematopoiesis, of which supporting the differentiation of LSKs to B-lineage commitment and subsequent support of B-precursor differentiation is only one.
Of course, the present results do not exclude the contribution of other BM stromal cell types to B lymphopoiesis, either adjacent to or separated from the endosteal region,23 in a sense analogous to Kiel et al's recent report that BM and spleen sinusoidal endothelium might serve as additional HSC niches when HSCs are mobilized.24 In fact, we believe that multiple stromal cell types are likely to be critical in the HSC niche per se, since several cytokines implicated in HSC proliferation (eg, c-Kit ligand) are secreted by whole BM stromal cultures, but not in pure human osteoblast cultures.2,10 Thus HSC maintenance, which is clearly required for the continued production of B-cell precursors in the bone marrow, may indeed require a complex multicellular niche. However, for the developmental steps leading from the HSC to mature B cells, osteoblasts appear to play the major necessary and sufficient roles.
One key implication of these data is that bone formation is directly linked to the integrity of the humoral immune system. This finding therefore raises the possibility that deficiencies in osteoblast number or function might underlie deficiencies in B-cell production. Such a decline could, for example, contribute to the decline in humoral immunity seen in aging. At a more speculative level, since osteoblast depletion prevents the earliest detectable transitions from the HSC compartment, it is likewise formally possible that declines in osteoblast function might lead to declines in the number of available T-cell progenitors available for emigration to the thymus. Overall, our data suggest that the further characterization of the diverse effects of osteoblasts on HSC fate decision, survival, and differentiation will greatly improve our understanding of local topological mechanisms regulating stem cell lineage commitment and differentiation.
Authorship
Contribution: J.Z., R.G., Y.C., E.H., R.T., and S.G.E. designed experiments; J.Z., Y.J., N.K., J.W., and G.J.J. performed experiments; J.Z., R.G., Y.Z., E.H., R.T., and S.G.E. interpreted results; J.Z., R.G., R.T., and S.G.E. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen G. Emerson, Division of Hematology/Oncology, University of Pennsylvania School of Medicine, Maloney 510, 3600 Spruce St, Philadelphia, PA 19104; e-mail: emersons@mail.med.upenn.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal