Abstract
CCR6, a homeostatic chemokine receptor, is shown here to characterize subsets of both central and effector memory T cells that secrete high levels of IL-2 and TNF-α in response to polyclonal and antigen-specific stimulation. CCR6+ T lymphocytes disappeared dramatically from the peripheral blood of HIV-infected patients as HIV disease progressed. The capacity of CD4+CCR6+ to secrete multiple cytokines remained intact among HIV-infected long-term nonprogressors but was partially lost from subjects with standard disease progression. CCR6+ T lymphocytes, regardless of their CCR7 expression, accumulated in the spleen of HIV-infected patients, where they died by apoptosis. Assessment of CCR6 expression allowed us to describe novel memory T-cell subpopulations capable of high cytokine production and provided evidence of a pathologic CCR6-dependent pathway of memory T-cell homing that may participate in the loss of memory response against infections.
Introduction
Lymphocyte recirculation from the blood to the secondary lymphoid organs is an essential process in the immune response. Successful trafficking of the appropriate lymphocyte population to the site of infection depends on the local production of chemokines and on timely expression of chemokine receptors on lymphocytes.1 Chemokine receptors, combined with activation and differentiation markers, are likely to define discrete functional subpopulations and participate in more precise positioning of cells in organs.2–4 Beside their role in driving leukocytes to specific sites to initiate immune response, several chemokine receptors, such as CCR5 and CXCR4, allow HIV entry into permissive cells,5,6 and direct and indirect virus-mediated alterations in lymphocyte trafficking are likely to contribute to the immunosuppressive effect of HIV.7
Immunosuppression during HIV infection may render the immune control of latent chronic infections in the host inefficient and ultimately allow virus replication.8,9 Relevant mechanisms of immune dysfunction include decreased thymic output,10 reduced naïve T-cell counts,11–13 higher proportions of late-differentiated antigen-experienced CD4+ and CD8+ T cells,14,15 and impaired T-cytotoxic functions that prevent the host from clearing viruses.16,17 SIV infection preferentially depletes memory phenotype CD4+ T cells, defined by their expression of CD45RO and CCR5.18 However, Fauci's group described a heightened state of cellular activation in HIV-infected patients, a state associated with CCR5 up-regulation and CXCR4 down-regulation on CD4+ T cells, which is known to promote propagation of macrophage-tropic viruses.19 Recently, we reported that cellular activation in individuals infected with HIV-1 is associated with up-regulation of CX3CR1 on late-stage differentiated memory regulatory CD8 T cells.15 CCR7 has been used to define several subsets of naïve and memory T lymphocytes with distinct homing and effector capacities3 as well as to monitor T-cell peripheral differentiation during HIV infection.16 Thus, the study of chemokine receptors during HIV immunopathogenesis has already led to important new concepts in immune antiviral response. Nonetheless, only limited data are currently available about the role of homeostatic chemokines during HIV infections.
CCR6, with its ligand CCL20, is another “homeostatic” driving force that may help improve our understanding of immune response mechanisms. CCR6 is expressed on immature dendritic cells, B lymphocytes, and memory T cells,20–23 and its ligand CC CCL20 has been detected in numerous organs, especially the secondary lymphoid organs, which are the major HIV replication sites.24–27 This study examines the involvement of CCR6 in regulating T-cell functions in HIV pathogenesis. Our findings indicate that CCR6 characterizes multicytokine-secreting memory T cells that may play a role in antiviral immune response.
Patients, materials, and methods
In accordance with the Declaration of Helsinki, patients and volunteers gave their informed written consent for the studies, which were approved by the Pitié-Salpêtrière ethics committee.
Patients and control subjects
Adults infected with HIV-1, treated in the infectious disease unit at Pitié-Salpêtrière Hospital (Paris, France), were tested for blood and other samples for this study (n = 22; mean CD4 T-cell count, 431 ± 63 cells/mm3; and plasma viremia, < 200-419 000 copies/mL). Ten of them had no previous history of known treatment (mean CD4 T-cell count, 299 ± 75 cells/mm3; plasma viremia, < 619-419 000 copies/mL). Whole blood from healthy donors (n = 27) was obtained from the Blood Transfusion Centre at the Pitié-Salpêtrière Hospital. Frozen peripheral blood lymphocyte samples for intracellular cytokine detection were obtained from 3 HIV-infected patient groups. The first group that presented with a disease following a standard course (SCD) were from the French IMMUNOCO cohort (n = 7; mean CD4 T-cell count, 498 ± 252 cells/mm3; plasma viremia, 5600-22 000 copies/mL). The second group consisted of 9 HIV-infected long-term nonprogressor patients (LTNPs) from the French asymptomatic long term (ALT) cohort with the following characteristics: HIV diagnosis for more than 8 years; normal CD4+ T lymphocyte counts greater than 600/mm3 at inclusion; no antiretroviral treatment; and no HIV-related symptoms (mean CD4 T-cell count, 844 ± 13 cells/mm3; plasma viremia, < 200-70 290 copies/mL). The third group of HIV patients consisted of 5 patients treated with highly active antiretroviral therapy (HAART) who manifested a suppressed viral load after treatment (mean CD4 T-cell count, 365 ± 190 cells/mm3; plasma viremia, < 200 copies/mL). Spleens were obtained from organ transplant donors28 at the Pitié-Salpêtrière Hospital following national ethical guidelines for regulating the use of human tissues. Spleens of 5 patients seropositive for HIV-1 were obtained after conducting splenectomy as a therapy for idiopathic thrombopenic purpura.29
Immunofluorescence
Cell surface staining was done with the standard procedures and the following monoclonal antibodies: CCR6-fluorescein isothiocyanate (FITC), CCR7-phycoerythrin (PE) (R&D Systems, Minneapolis, MN), CD4-phycoerythrin cyanin 7 (PE-Cy7), CD8-PC7, CD45RA-PE, CD57-PE, CD38-PE (Beckman Coulter, Miami, FL), CCR6-PE, CD3-peridin chlorophyll protein-cyanin 5.5 (PCPCy5.5), CD4-allophycocyanin (APC), CD8-APC, CD27-PE, CD28-PE, CCR5-PE, CXCR3-PE, HLA-DR-PE, CD95-PE, CD127-PE, CD62L-FITC (BD Biosciences and BD Pharmingen, San Diego, CA), and CX3CR1-FITC (MBL, Nagoya, Japan). The cells were then analyzed on the flow cytometer (FACSCalibur and LSRII, Becton Dickinson, San Diego, CA). At least 10 000 events were accumulated and analyzed with CellQuest Pro (Becton Dickinson) and Flow Jo (Tree Star, Ashland, OR) software. For cell sorting, the T-cell memory subsets were isolated on the flow cytometer ARIA (Becton Dickinson) and resuspended in RPMI containing 5% of AB human serum (AbCys, Paris, France) before stimulation.
Peptides
A total of 47 EBV-BZLF1 synthetic peptides were clustered in one pool, 160 EBV-EBNA3 peptides were clustered in 4 pools of 40 contiguous peptides, 125 HIV-GAG peptides in 11 pools of 10 to 15 peptides, and 26 HIV-Nef peptides in 3 pools of 6 to 10 peptides (INSERM “Action-Thématique-Concertée on antiviral immunity”).
Cell function assays
Peripheral blood mononuclear cells (PBMCs) were diluted in RPMI (Life Technologies, Cergy Pontoise, France) containing 5% FCS (Seromed, Berlin, Germany), 2 mM l-glutamine (Gibco BRL, Life Technologies, Paisley, United Kingdom), and antibiotics (1000 UI/mL penicillin sodium, 1 mg/mL streptomycin sulfate, and 250 ng/mL amphotericin B). Some cells were stimulated with 1 μg/mL staphylococcal enterotoxin B (SEB; Sigma-Aldrich, St Louis, MO) or with antibiotin MACSiBead particles loaded with CD2, CD3, and CD28 antibodies (Miltenyi Biotech, Bergisch Gladbach, Germany) for 12 hours at 37°C, with and without 5 μg/mL brefeldin A (Sigma Chemical, Saint Quentin, France) to detect intracellular cytokines. For antigen-specific cytokine production, cells were stimulated with a pool of 15mer peptides. The cells were then labeled for membrane staining as described under “Immunofluorescence” with APC-Cy7–conjugated antibodies against CD4, anti-CD8 Pacific blue, anti-CD3 PCPCy5.5, anti-CD45 AmCyan, anti–CCR6-PE (BD PharMingen), anti-CD45RA ECD (Beckman Coulter), and anti-CCR7 APC (R&D Systems). For additional intracellular staining of IFN-γ, TNF-α, and IL-2, cells were fixed in 4% paraformaldehyde A (PFA) for 20 minutes, washed, and permeabilized with PBS 5% FCS 0.1% saponin before the addition of specific antibodies (anti–IFN-γ Alexa700, anti–TNF-α PE-Cy7, and anti–IL-2 Alexa488 from BD PharMingen). Cells were then collected and analyzed as described under “Immunofluorescence.”
Cell culture with CCL20
PBMCs diluted in supplemented RPMI were cultured at 37°C for 24 hours with or without CCL20 (R&D Systems) at different concentrations. Cells were harvested at different points during the culture, and stained as described.
CCL20 ELISA
Nonheparinized plasma specimens or supernatant of spleen cultures were stored at less than −70°C. Aliquots were thawed and analyzed in triplicate with an enzyme-linked immunosorbent assay (ELISA) sandwich procedure that used specific CCL20 antibodies purchased from R&D Systems and ELISAs were performed following manufacturer's instruction.
Statistical analyses
For data analysis and graphic representation, Prism 4.01 (GraphPad Software, San Diego, CA) was used. Statistical comparisons used unpaired 2-sample t tests for means and the Wilcoxon test to analyze cytokine production after stimulation. Linear regression used 95% confidence intervals, whereas statistical significance was defined as P < .05.
Results
CD4+ and CD8+ T cells expressing CCR6 display a “resting memory” phenotype with distinct migratory potencies
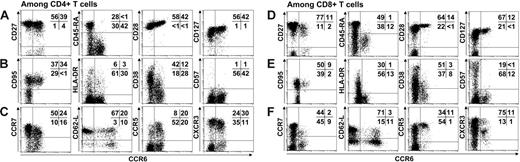
To characterize the role of CCR6+ in T-cell memory, we examined the phenotype of cells expressing CCR6, using several differentiation, activation, and homing markers on both peripheral blood CD4+ (Figure 1A-C) and CD8+ (Figure 1D-F) T cells in healthy individuals. The analysis revealed that approximately 30% of CD4+ T cells and 15% of CD8+ T cells expressed CCR6+. Among the CD4+ and CD8+ T cells, nearly all the CCR6+ lymphocytes were detected in cell subpopulations expressing CD27, CD28, and CD127 but not CD45RA, that is, in memory T cells. All CD4+ and CD8+CCR6+ lymphocytes expressed activation markers such as CD95, and only a very few were CD57+. HLA-DR was present in only a small subpopulation of CCR6+ lymphocytes, with less than one third of them being CD38dim. These data indicate that the CD4+ and CD8+ T cells expressing CCR6 have similar resting memory phenotypes.
Analysis of differentiation, activation, and migration markers on CD4+ and CD8+ T lymphocytes expressing CCR6. Differentiation markers CD27, CD45RA, CD28, CD127 (A-D), activation markers CD95, HLA-DR, CD38, and CD57 (B,E), and migration markers such as CCR7, CD62L, CCR5, and CXCR3 (C,F) were studied for their expression by CD4+ T cells (A-C) and CD8+ T cells (D-F). For flow cytometric analysis, 15 000 events were accumulated. Representative staining of PMBCs from 8 UI individuals is shown. Percentages of cells in each quadrant are indicated. Because antibody directed against CD62-L was conjugated to FITC fluorochrome, the CCR6 antibody used in the figure was conjugated to PE unlike all others stainings that used CCR6-FITC.
Analysis of differentiation, activation, and migration markers on CD4+ and CD8+ T lymphocytes expressing CCR6. Differentiation markers CD27, CD45RA, CD28, CD127 (A-D), activation markers CD95, HLA-DR, CD38, and CD57 (B,E), and migration markers such as CCR7, CD62L, CCR5, and CXCR3 (C,F) were studied for their expression by CD4+ T cells (A-C) and CD8+ T cells (D-F). For flow cytometric analysis, 15 000 events were accumulated. Representative staining of PMBCs from 8 UI individuals is shown. Percentages of cells in each quadrant are indicated. Because antibody directed against CD62-L was conjugated to FITC fluorochrome, the CCR6 antibody used in the figure was conjugated to PE unlike all others stainings that used CCR6-FITC.
Because memory T cells are also subdivided into central TEM and effector TEM memory cells according to their homing properties, we studied chemokine receptors and selectin expression. Most CD4+CCR6+ lymphocytes expressed CCR7 (60% ± 9%) and CD62-L (68% ± 11%) and can thus be identified as central memory CD4+ lymphocytes. Moreover CD4+CCR6+ cells also exhibited the presence of CCR5 (47% ± 5%), and CXCR3 (76% ± 7%). In contrast, CD8+CCR6+ T cells were mostly positive for CCR5 and CXCR3 (> 90% cells) and very few for CCR7 or CD62-L, which define the so-called effector-memory CD8+ lymphocytes. These data indicate that CD4 and CD8 T cells expressing CCR6 have similar antigen-experienced T-cell phenotypes but distinct migratory potencies according to their specific pattern of chemokine receptor expression.
After TCR engagement, CCR6+CD4+ and CD8+ central memory T cells as well as CD4+ effector memory produced high levels of TNF-α and IL-2
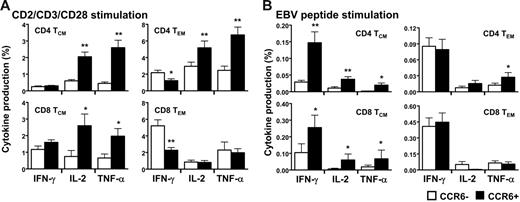
Functional capacities of the T-cell memory subpopulations expressing CCR6 were determined in vitro in freshly isolated peripheral blood. The CCR6+ T cells neither spontaneously produced cytokines nor did they proliferate; both findings indicate that these CCR6+ memory T cells were resting (data not shown). After a short period of activation with antibodies against CD2, CD3, and CD28, similar proportions of IFN-γ–producing CD4+ and CD8+ central memory expressing or not CCR6 were detected (Figure 2A left panels and Figure S1A-D for representative staining, available on the Blood website; see the Supplemental Materials link at the top of the online article). However, a smaller percentage of CCR6+ effector memory CD4+ and CD8+ T cells produced IFN-γ (Figure 2A, right panels). Striking differences were seen in IL-2 and TNF-α production. Indeed, a significantly higher proportion of the CCR6+ T cells produced IL-2 and TNF-α in both central and effector memory cells (Figure 2A upper panels). In addition, the subset of CCR6+CD8+ central memory T cells was also more likely to secrete IL-2 and TNF-α but not the CCR6+CD8+ effector memory subset (Figure 2A lower panels). About 30% to 50% of the IL-2 and the TNF-α produced by CD3/CD28-stimulated CD4+ T cells originated from CCR6+ cells, whereas only 10% to 15% of it were from the CCR7+. Furthermore, 80% of IL-2–producing CD4+CCR6+ T cells were CCR7+ (data not shown). Similar profiles of cytokine production with higher proportions of CCR6+ T cells producing IL-2 and TNF-α were observed after polyclonal stimulation with SEB (Figure S1E) and phytohemagglutinin (PHA) stimulation (data not shown). These analyses indicated that CD4+CCR6+ T cells were the major memory population producing IL-2 and TNF-α and that CCR6+, irrespective of CCR7, may represent the central memory phenotype.
In vitro functional assay of CD4+ and CD8+ T cells expressing CCR6. Freshly isolated peripheral blood lymphocytes (PBLs) of UI individuals were stimulated with anti-CD2, anti-CD3, and anti-CD28 antibodies (A) and 15mer EBV pool of peptides (B) for 12 hours in the presence of brefeldin A for cytokine production assay (see “Patients, materials, and methods”). CD4+ T cells and CD8+ T cells were then analyzed by flow cytometric analysis as a function of CCR6 expression for their production of IFN-γ, IL-2, and TNF-α in the 2 memory subsets defined as TCM (CD45RA−/CCR7+) and TEM (CD45RA−/CCR7−). Results are shown as the percentage of cytokine secreting CD4+ or CD8+ T cells expressing or not CCR6 and represent 5 independent experiments. *P < .05 **P < .01. Error bars represent the standard error of the mean.
In vitro functional assay of CD4+ and CD8+ T cells expressing CCR6. Freshly isolated peripheral blood lymphocytes (PBLs) of UI individuals were stimulated with anti-CD2, anti-CD3, and anti-CD28 antibodies (A) and 15mer EBV pool of peptides (B) for 12 hours in the presence of brefeldin A for cytokine production assay (see “Patients, materials, and methods”). CD4+ T cells and CD8+ T cells were then analyzed by flow cytometric analysis as a function of CCR6 expression for their production of IFN-γ, IL-2, and TNF-α in the 2 memory subsets defined as TCM (CD45RA−/CCR7+) and TEM (CD45RA−/CCR7−). Results are shown as the percentage of cytokine secreting CD4+ or CD8+ T cells expressing or not CCR6 and represent 5 independent experiments. *P < .05 **P < .01. Error bars represent the standard error of the mean.
CD8+CCR6+ T cells were antigen-experienced and stained positively for several antigen-specific tetramers, including HIV, CMV, influenza virus, and EBV (data not shown). We thus determined the EBV-specific response in memory T cells expressing or not CCR6 using pools of 15mer peptides covering BZLF1 and EBNA3 protein. Similarly to what has been observed in response to polyclonal stimulations, CCR6+ central memory T cells, that is, CD4+ TCM and CD8+ TCM, contained higher proportions of IL-2– and TNF-α–secreting cells (Figure 2B left panels). However, the proportion of IFN-γ–producing TCM cells was greater in the CCR6+ compared to CCR6− cells. In the effector memory subset, only TNF-α–secreting CCR6+CD4+ cells were in greater number, whereas the other cytokine-producing CCR6+ or CCR6− T cells were rather indistinguishable in proportion (Figure 2B right panels).
To further compare the functional potencies of CCR6+ T cells, subsets of memory T cells were sorted using the cell surface markers CD4, CD45RA, CCR7, and CCR6, and activated with polyclonal stimuli. Results in Table 1 indicated that the CD4+ TCM and CD4+ (TEM) cells secreting TNF-α were more likely to expressed CCR6. In addition, the phenotype of the different subsets of memory T cells remained unchanged after stimulation with only a slight shift to the CCR6− population (Figure S2) indicating that T-cell activation did not alter CCR6 surface expression, at least for the time of the stimulation. Overall, these results show that CCR6 characterizes the vast majority of TCM cells (both CD4+ and CD8+) and also CD4+ TEM cells producing IL-2 and TNF-α.
Both CCR6+ central memory and effector memory CD4+ T cells produced greater amounts of TNF-α than CCR6− T cells
| . | Central memory CD4 T cells . | Effector memory CD4 T cells . | ||
|---|---|---|---|---|
| CCR6− . | CCR6+ . | CCR6− . | CCR6+ . | |
| Anti-CD2/CD3/CD28 | 23 | 43 | 28 | 46 |
| SEB | 0.15 | 1.5 | 2 | 7 |
| . | Central memory CD4 T cells . | Effector memory CD4 T cells . | ||
|---|---|---|---|---|
| CCR6− . | CCR6+ . | CCR6− . | CCR6+ . | |
| Anti-CD2/CD3/CD28 | 23 | 43 | 28 | 46 |
| SEB | 0.15 | 1.5 | 2 | 7 |
T-cell memory subsets were sorted according to their expression of CD45RA, CCR7, and CCR6 and stimulated overnight with polyclonal stimulation in the presence of brefeldin A for cytokine production assay. Results are shown as the percentage of TNF-α-secreting CD4+ T cells expressing or not CCR6 and represent 2 independent experiments.
The proportion and number of CCR6+ resting memory T cells decreased in the blood of HIV-infected patients as HIV disease progressed
Because the subsets of memory cells might play a crucial role in the control of viral infection, we compared CCR6 expression on T lymphocytes in HIV-infected (HIV+) and uninfected (UI) adults (Table 2 and Figure S3 for representative staining). The proportion of CD4+CCR6+ cells was similar in both UI and HIV+ individuals, but their absolute number dropped to 106 ± 16 cells/mm3 in HIV+ subjects from 258 ± 22 cells/mm3 in UI subjects (P < .01). The proportion of CCR6+ T cells in the CD8 compartment was significantly lower in the HIV+ group. The absolute number of CD8+CCR6+ was 37 ± 5 cells/mm3 in the HIV+ group (P < .01) and 67 ± 7 cells/mm3 in the UI group. These data indicate that the number of CD4+CCR6+ and CD8+CCR6+ T cells declines in the blood of HIV-infected patients.
Proportion of CD4 and CD8 T lymphocytes expressing CCR6 chemokine receptors in UI and HIV-infected individuals
| . | UI . | HIV+ . | ||||
|---|---|---|---|---|---|---|
| % . | Cells/mm3 . | Range . | % . | Cells/mm3 . | Range . | |
| CD4+ | 44 ± 2 | 820 ± 88 | 601-1331 | 21 ± 2* | 388 ± 53† | 10-784 |
| CD8+ | 26 ± 3 | 540 ± 107 | 167-1056 | 51 ± 3* | 920 ± 96‡ | 156-1751 |
| CCR6+ among CD4+ | 31 ± 2 | 258 ± 22 | 137-395 | 26 ± 2 | 106 ± 16* | 3-194 |
| CCR6+ among CD8+ | 13 ± 1 | 67 ± 7 | 19-165 | 4 ± 1* | 37 ± 5† | 2-80 |
| . | UI . | HIV+ . | ||||
|---|---|---|---|---|---|---|
| % . | Cells/mm3 . | Range . | % . | Cells/mm3 . | Range . | |
| CD4+ | 44 ± 2 | 820 ± 88 | 601-1331 | 21 ± 2* | 388 ± 53† | 10-784 |
| CD8+ | 26 ± 3 | 540 ± 107 | 167-1056 | 51 ± 3* | 920 ± 96‡ | 156-1751 |
| CCR6+ among CD4+ | 31 ± 2 | 258 ± 22 | 137-395 | 26 ± 2 | 106 ± 16* | 3-194 |
| CCR6+ among CD8+ | 13 ± 1 | 67 ± 7 | 19-165 | 4 ± 1* | 37 ± 5† | 2-80 |
Proportions of CD4+ or CD8+ T cells and proportions of CD4+ and CD8+ T cells expressing chemokine receptor CCR6, reported as percentage ± standard error of the mean (SEM) and as number of cells/mm3 of peripheral blood ± SEM, with their ranges. Twenty-two HIV-infected patients and 27 UI individuals were sampled. The mean viral load of HIV-infected patients was 24 929 copies/mL, with a range of < 200 to 420 000 number of copies/mL.
*P < .001.
†P < .01.
‡P < .05.
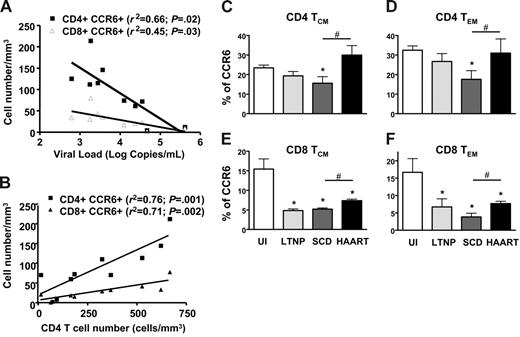
We investigated how this phenomenon might be related to the disease course. We first sought any correlations between the level of CCR6 expression on lymphocytes with standard indicators of HIV disease progress, such as viral load and CD4 cell counts (Figure 3A-B). Viral load was negatively correlated with the number of both CD4+CCR6+ and CD8+CCR6+ T cells (P = .02 and P = .03, respectively). The reduction in the proportion of CCR6+ T lymphocytes in the CD4 population was closely correlated with low CD4 T-cell counts, as were the CD4+CCR6+ and CD8+CCR6+ T-cell counts (P = .001 and P = .002, respectively). It was further revealed that more advanced patients had fewer CCR6+ T cells. Taken together, these results indicate that CCR6 expression may be a useful marker of HIV disease progression. Secondly, CCR6+ expression on TCM and TEM CD4+ and CD8+ T cells was compared in 4 groups of individuals (Figure 3C): UI individuals, HIV-infected LTNPs, HIV-infected patients with the disease following SCD, and patients who controlled their viral load after HAART. The loss of CCR6+ T cells in SCD patients altered all 4 subsets of memory cells, independently of their differentiation stage. In LTNPs and HAART patients, the proportions of CCR6+ were reduced in both CD8+ central and effector memory T cells compared to that of UI individuals (Figure 3E-F), but the CCR6+ T cells were more frequent in HAART patients than in SCD patients. The CCR6+ restoration observed in the HAART patients was even more pronounced in the CD4+ memory T cells than in the CD8+ memory T cells. These data confirmed that CCR6 expression on memory T cells was associated with HIV disease progression and partially with immune reconstitution after HAART.
Decreased expression of CCR6 on T cells in HIV-infected patients is associated with HIV disease progression and high capacity of CCR6 T cells in LTNPs to secrete IL-2 and TNF-α. Level of CCR6 expression on CD4+ T lymphocytes is analyzed with standard HIV disease progression parameters, viral load (A) and CD4+ T-cell number (B). Lymphocytes analyzed were obtained from 10 HIV-infected patients. Levels of CCR6 expression on both CD4 (C-D) and CD8 (E-F) T cells and in the 2 memory subsets defined as TCM (CD45RA−/CCR7+; C,E) and TEM (CD45RA−/CCR7−; D,F) in uninfected individuals (UI), in long-term nonprogressor HIV-infected patients (LTNP), in HIV-infected patients with standard course of disease (SCD), and in HIV-infected patients treated with HAART with controlled viremia (HAART). *P < .05, represents the significant difference between UI and SCD HIV-infected patients. #P < .05 represents the significant difference between HAART and SCD HIV-infected patients. Error bars represent SEM.
Decreased expression of CCR6 on T cells in HIV-infected patients is associated with HIV disease progression and high capacity of CCR6 T cells in LTNPs to secrete IL-2 and TNF-α. Level of CCR6 expression on CD4+ T lymphocytes is analyzed with standard HIV disease progression parameters, viral load (A) and CD4+ T-cell number (B). Lymphocytes analyzed were obtained from 10 HIV-infected patients. Levels of CCR6 expression on both CD4 (C-D) and CD8 (E-F) T cells and in the 2 memory subsets defined as TCM (CD45RA−/CCR7+; C,E) and TEM (CD45RA−/CCR7−; D,F) in uninfected individuals (UI), in long-term nonprogressor HIV-infected patients (LTNP), in HIV-infected patients with standard course of disease (SCD), and in HIV-infected patients treated with HAART with controlled viremia (HAART). *P < .05, represents the significant difference between UI and SCD HIV-infected patients. #P < .05 represents the significant difference between HAART and SCD HIV-infected patients. Error bars represent SEM.
In HIV LTNPs, CCR6+ T cells retained their high capacity to secrete IL-2 and TNF-α but standard HIV progressors lost this capacity
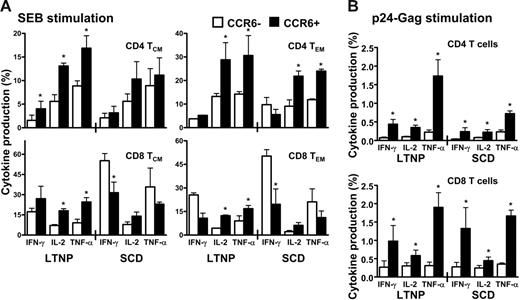
Because the CCR6+ T cells represented memory subpopulations directed against a broad range of antigens, we first investigated the cytokine production in HIV-infected LTNPs and SCD patients in response to a polyclonal stimulation using SEB (Figure 4A). In LTNPs, a higher proportion of IL-2– and TNF-α–producing CCR6+ T cells was detected in all the 4 subsets of memory T cells. In contrast, in SCD patients, the extent of CCR6+ producing these 2 cytokines was severely diminished in memory CD4+ T cells (Figure 4A upper panels) even if it remained significantly higher than that of CCR6− in the effector CD4 memory T cells (Figure 4A upper right panel). However, in CD8+ T cells from SCD patients, the proportion of CCR6+ T cells producing cytokines was only modestly altered compared to that of LTNPs, whereas, on the other hand, the secretions of IFN-γ and TNF-α were increased in CCR6− CD8 T cells from SCD patients compared to that of LTNPs. These findings provide further evidence for the role of CCR6 T cells in HIV disease progression.
High secretion of IL-2 and TNF-α by CCR6+ T cells in response to polyclonal or antigenic stimulation in LTNP HIV-infected patients in comparison to HIV-infected patients with SCD. Peripheral blood lymphocytes (PBLs) of LTNP or SCD HIV-infected patients were stimulated with SEB (A) or with a pool of HIV-Gag 15mer pool of peptides (B) for 12 hours in the presence of brefeldin A for cytokine production assay (see “Patients, materials, and methods”). CD4+ T cells and CD8+ T cells were then analyzed by flow cytometric analysis as a function of CCR6 expression for their production of IFN-γ, IL-2, and TNF-α in the 2 memory subsets defined as TCM (CD45RA−/CCR7+) and TEM (CD45RA−/CCR7−). Results are shown as the percentage of cytokine secreting CD4+ or CD8+ T cells expressing or not CCR6 and represent 5 independent experiments. Error bars represent SEM.
High secretion of IL-2 and TNF-α by CCR6+ T cells in response to polyclonal or antigenic stimulation in LTNP HIV-infected patients in comparison to HIV-infected patients with SCD. Peripheral blood lymphocytes (PBLs) of LTNP or SCD HIV-infected patients were stimulated with SEB (A) or with a pool of HIV-Gag 15mer pool of peptides (B) for 12 hours in the presence of brefeldin A for cytokine production assay (see “Patients, materials, and methods”). CD4+ T cells and CD8+ T cells were then analyzed by flow cytometric analysis as a function of CCR6 expression for their production of IFN-γ, IL-2, and TNF-α in the 2 memory subsets defined as TCM (CD45RA−/CCR7+) and TEM (CD45RA−/CCR7−). Results are shown as the percentage of cytokine secreting CD4+ or CD8+ T cells expressing or not CCR6 and represent 5 independent experiments. Error bars represent SEM.
We then compared cytokine secretions in LTNPs and SCD patients after HIV antigen-specific activation of T cells (Figure 4B). In LTNPs, high proportions of CD4+ CCR6+ T cells secreted cytokines with a ratio of TNF-α–secreting CD4+CCR6+ T cells to CD4+CCR6− T cells of 11.7 ± 2.3. For IL-2 and IFN-γ secretions, these ratios were of 3.8 ± 0.7 and 6.7 ± 1.7, respectively. In SCD patients, CD4+CCR6+ T cells no longer secreted TNF-α efficiently with a ratio of 3.7 ± 0.9 and IL-2 and IFN-γ secretions were modestly altered with ratios of 2.7 ± 0.7 and 5.6 ± 1.7, respectively. No significant differences could be measured in the CD8 T cells when comparing cytokine secretion in LTNPs and SCD patients. These data suggested that the role of CCR6+ T cells in SCD patients might be altered, reflecting skewed cytokine secretion and CCR6+ memory subsets reduction.
Only CCR6+ memory T cells were depleted from the peripheral blood
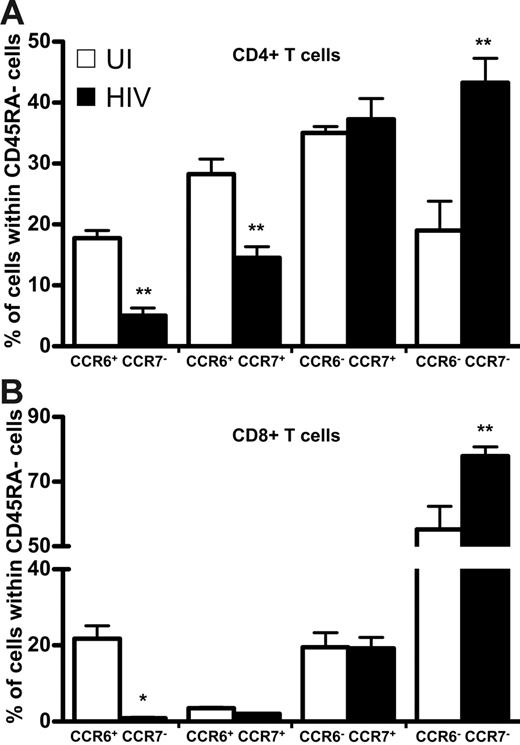
Because CCR7 defines T-cell memory subpopulations in CD45RA− cells,3 we compared the distribution among both HIV+ and UI individuals of CCR7 and CCR6 in CD45RA− memory T cells—both CD4+ (Figure 5A) and CD8+ (Figure 5B) T lymphocytes. The proportion of memory CD4+CCR6+ T cells expressing CCR7 was 2 times lower among patients than UI subjects, as was the population that was CCR7−, whereas the proportion of CCR7+CCR6− T cells was similar in both groups. Similar results were observed in the CD8+ T-cell population. The proportion of CCR7−CCR6− was strongly increased in HIV+ patients compared to that of UI individuals, in both CD4 and CD8 T cells.
Expression of CCR6 and CCR7 in CD4+ or CD8+ CD45RA− T cells in healthy and HIV-infected individuals. Results are expressed as a percentage of CD4+ (upper panel) and CD8+ (lower panel) CD45RA− T cells that do or do not express CCR6 coexpressed with CCR7; □, healthy individuals; ▪, HIV-infected patients. *P < .05; **P < .01. Error bars represent SEM.
Expression of CCR6 and CCR7 in CD4+ or CD8+ CD45RA− T cells in healthy and HIV-infected individuals. Results are expressed as a percentage of CD4+ (upper panel) and CD8+ (lower panel) CD45RA− T cells that do or do not express CCR6 coexpressed with CCR7; □, healthy individuals; ▪, HIV-infected patients. *P < .05; **P < .01. Error bars represent SEM.
Because CCR5 has been reported mainly on memory T cells,18 we analyzed its expression in both HIV+ and UI individuals. The proportion of CD4+ T cells expressing CCR5 was similar in both groups (26% ± 6% and 22% ± 2%, respectively) but the absolute number of CD4+CCR5+ T cells was significantly lower among HIV+ subjects than controls (111 ± 14 cells/mm3 compared with 168 ± 14 cells/mm3); this difference was nonetheless not as prominent as for CD4+CCR6+ T cells. In contrast to the decrease of CD8+CCR6+ T cells during disease, both the frequency and the absolute number of CD8+CCR5+ T cells were significantly higher in HIV+ patients (52%± 4%; 525 ± 114 cells/mm3) than in UI subjects (41% ± 3%; 208 ± 38 cells/mm3). These data indicate that this CCR6+ depletion affects only CCR6+ memory T cells, regardless of their CCR7 and CCR5 expression, and suggest the existence of a specific mechanism of memory T-cell depletion that depends on CCR6.
Neither CCL20 nor polyclonal activation of T cells induced the down-modulation of CCR6
Because every subset of CCR6+ T cells (unlike CCR5+ and CCR7+ T cells) was less frequent, both proportionally and in absolute numbers, in the blood of HIV+ individuals, we hypothesized that CCR6+ T cells disappeared in response to a CCR6-dependent mechanism by ligand-induced receptor internalization. We conducted polyclonal TCR stimulation of freshly isolated PMBCs with antibodies against CD3 and CD28 to determine the fate of CCR6. The percentage of cells expressing CCR6 did not change significantly after 5 days of culture in the presence or absence of polyclonal activators (data not shown). In addition, plasma CCL20 concentrations measured by ELISA in HIV+ patients and UI subjects neither exceeded 0.1 nM nor did they differ in the 2 groups (data not shown). Because neither TCR engagement nor systemic CCL20 altered CCR6 expression, we hypothesized that CCR6+ T cells may be sequestered in organs in response to locally produced CCL20.
CCR6+ resting memory T cells were sequestered and died in the spleen
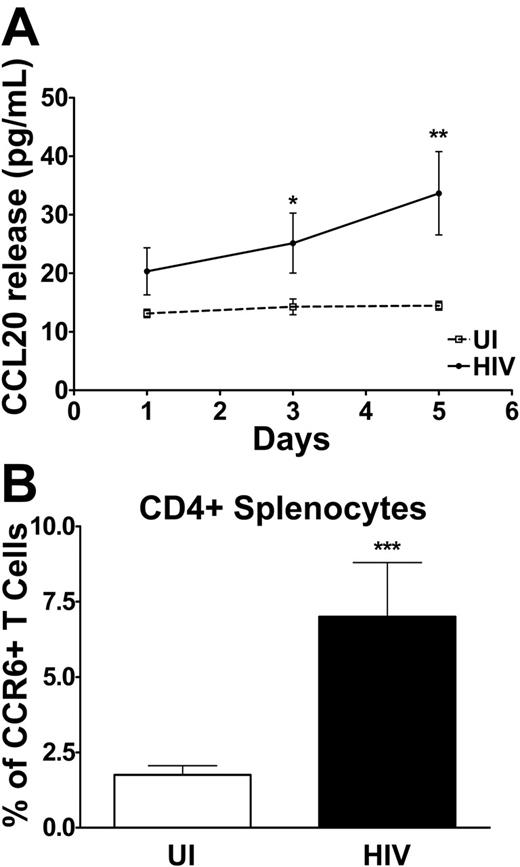
We compared the CCL20 production capacity of splenocytes of HIV-infected patients and of UI individuals following in vitro TCR engagement. As shown in Figure 6A, CCL20 production was barely, if at all, detectable in the supernatant of splenocyte cultures of UI subjects, even after 5 days. Splenocytes from HIV-infected patients, on the other hand, secreted low but clear levels of CCL20, up to 70 pg/mL, after 5 days of stimulation. Hypothesizing that CCL20 produced in the spleen of HIV patients might recruit CCR6+ T cells, we compared the proportions and numbers of CD4+CCR6+ (Figure 6B) and CD8+CCR6+ (data not shown) T lymphocytes in the spleen of HIV patients and healthy individuals; CD4+CCR6+ splenocytes were more frequent in HIV+ patients than in healthy individuals as were CD8+ T cells (4.1% ± 0.8% compared with 1.4% ± 0.2%, respectively, P < .001). In contrast, the frequencies of CCR7+ splenocytes were similar in HIV+ patients and in healthy individuals for CD4+and CD8+ T cells. These data indicated an accumulation of CCR6+ T cells in the spleen of HIV patients.
CCL20 released by splenocytes and surface expression of CCR6 on CD4+ splenocytes: comparison of uninfected and HIV-infected individuals. (A) CCL20 released by splenocytes at different times during culture was determined by ELISA and is expressed in picograms per milliliter for uninfected (dotted line) and HIV-infected (solid line) individuals. (B) Surface expression of CCR6 was determined by flow cytometry in CD4+ splenocytes isolated from the spleen of healthy individuals (□) and HIV-infected patients (▪) and presented as the percentage of CCR6+ splenocytes within CD4+. *P < .05 **P < .01 ***P < .001. Error bars represent SEM.
CCL20 released by splenocytes and surface expression of CCR6 on CD4+ splenocytes: comparison of uninfected and HIV-infected individuals. (A) CCL20 released by splenocytes at different times during culture was determined by ELISA and is expressed in picograms per milliliter for uninfected (dotted line) and HIV-infected (solid line) individuals. (B) Surface expression of CCR6 was determined by flow cytometry in CD4+ splenocytes isolated from the spleen of healthy individuals (□) and HIV-infected patients (▪) and presented as the percentage of CCR6+ splenocytes within CD4+. *P < .05 **P < .01 ***P < .001. Error bars represent SEM.
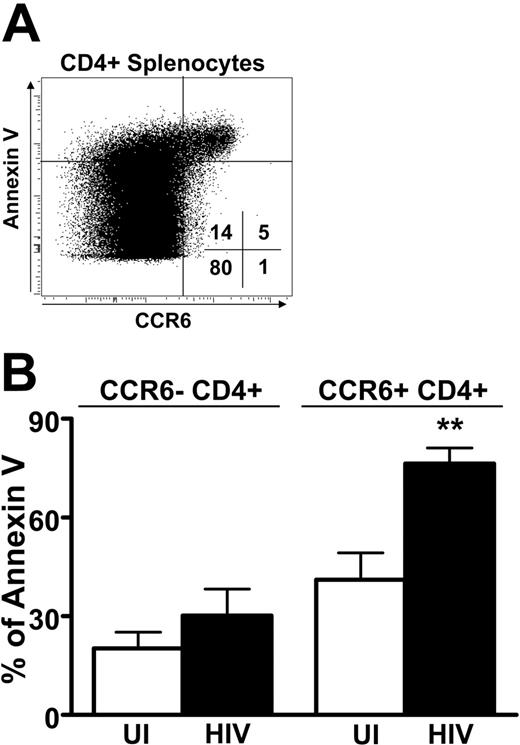
The phenotype of CCR6+ splenocytes was determined by flow cytometry in UI individuals and HIV patients (Table 3). Similarly to what was observed in the blood, the vast majority of CCR6+ T cells were CD27+ and CD28+, both in UI persons and in HIV patients. In addition, the proportion of CCR7+CCR6+ splenocytes was not significantly different between UI individuals and HIV patients even if it was reduced in the CD4+ T cells compared to that observed in the blood of UI individuals (Figure 1). Surprisingly, the CCR6+ CD4+ and CD8+ splenocytes from HIV patients were more likely to express CD95. Because nearly all CCR6+ splenocytes from HIV patients expressed CD95 and thus may be susceptible to apoptosis, we studied the fate of the splenocytes by analyzing their expression of annexin V, an early marker of apoptosis. Most CCR6+ T cells stained positively for annexin (Figure 7A). Interestingly, the frequency of CD4+CCR6+ annexin V+ splenocytes was higher in HIV+ patients than in healthy individuals (P = .006), although annexin V staining that did not differ in CD4+CCR6− T cells from HIV+ and UI subjects was similar (Figure 7B). Similar results were observed for CD8+ T cells (data not shown). These results indicate that CCR6 T cells, besides accumulating in the spleen, are more susceptible to apoptosis. Taken together these data suggest that CCR6 may participate in the redistribution of T-cell memory from the blood to secondary lymphoid organs and demonstrate that this mechanism may be altered during HIV infection.
Phenotypical characterization of CCR6+ CD4 and CD8 splenocytes in UI and HIV-infected individuals
| . | CCR7 . | CD27 . | CD28 . | CD95 . |
|---|---|---|---|---|
| CD4+CCR6+T cells | ||||
| UI | 32 ± 5.7 | 89.3 ± 2 | 95.8 ± 2.9 | 67.3 ± 5.5 |
| HIV | 30 ± 8.5 | 93.3 ± 2.7 | 92.7 ± 6.3 | 94 ± 1.5* |
| CD8+CCR6+T cells | ||||
| UI | 23.8 ± 5.3 | 74.8 ± 4.9 | 84 ± 4 | 48.5 ± 10.2 |
| HIV | 17.3 ± 3.9 | 85.3 ± 5.5 | 89 ± 3.2 | 88.7 ± 4.8* |
| . | CCR7 . | CD27 . | CD28 . | CD95 . |
|---|---|---|---|---|
| CD4+CCR6+T cells | ||||
| UI | 32 ± 5.7 | 89.3 ± 2 | 95.8 ± 2.9 | 67.3 ± 5.5 |
| HIV | 30 ± 8.5 | 93.3 ± 2.7 | 92.7 ± 6.3 | 94 ± 1.5* |
| CD8+CCR6+T cells | ||||
| UI | 23.8 ± 5.3 | 74.8 ± 4.9 | 84 ± 4 | 48.5 ± 10.2 |
| HIV | 17.3 ± 3.9 | 85.3 ± 5.5 | 89 ± 3.2 | 88.7 ± 4.8* |
Proportion of CCR6+ CD4+ or CD8+ T cells expressing differentiation markers CCR7, CD27, CD28, or CD95, reported as percentage ± SEM. Four frozen splenocytes suspensions from HIV-infected patients and 4 from UI individuals were sampled.
*P < .05.
Apoptosis of CCR6+ CD4 T cells: comparison of uninfected and HIV-infected individuals. (A) Sensitivity to apoptosis of CCR6-expressing splenocytes. Representative staining of splenocytes for expression of CCR6 and annexin V within CD8+ splenocytes. Percentages of cells in each quadrant are indicated. (B) The percentage of annexin V+ splenocytes was studied according to the expression of CCR6 within CD4+ splenocytes for 5 UI (□) and 5 HIV-infected (▪) individuals. **P < .01. Error bars represent SEM.
Apoptosis of CCR6+ CD4 T cells: comparison of uninfected and HIV-infected individuals. (A) Sensitivity to apoptosis of CCR6-expressing splenocytes. Representative staining of splenocytes for expression of CCR6 and annexin V within CD8+ splenocytes. Percentages of cells in each quadrant are indicated. (B) The percentage of annexin V+ splenocytes was studied according to the expression of CCR6 within CD4+ splenocytes for 5 UI (□) and 5 HIV-infected (▪) individuals. **P < .01. Error bars represent SEM.
Discussion
In this report, we showed that the chemokine receptor CCR6 identifies novel subsets of both central and effector lymphocytes. Their resting memory phenotypes, according to their differentiation and activation markers, are similar in both CD4+ and CD8+ T cells, and comprised a population of Th1-polarized memory cells secreting TNF-α and IL-2 after restimulation. These subsets of memory T cells are depleted in the blood of HIV patients independently of other markers such as CCR5 and CCR7. CCR6+ T cells seem to home to the spleen of HIV+ patients where they are more susceptible to cell death, than they are in UI subjects. The pathway for memory T-lymphocyte depletion observed here depends on both CCR6 and the homing pattern it creates and may participate in the immune dysfunction observed during HIV infection.
CCR6 has not been described as an HIV coreceptor, but it is expressed on CD4 T cells that express such HIV coreceptors as CCR5 and CXCR6.30 CD4+CCR6+ T cells may therefore be highly sensitive to HIV infection and thus prone to HIV-induced apoptosis. This should lead to a decline in CD4+CCR6+ T cells as CD4+ is depleted from the blood of HIV patients. Most CD4+CCR6+ T cells coexpress CCR7, but only CCR6-expressing CD4+ T cells decreased in HIV patients; the CD4+CCR7+ fraction was the same for patients and UI individuals. This finding suggests that only antigen-primed CD4 cells rather than naive CCR7+ cells disappear through this CCR6-dependent pathway.
Furthermore, we showed that there are fewer CD8+CCR6+ T cells in the blood of HIV patients, as well as fewer CD4+CCR6+ T cells. The number of CD8+CCR6+ T cells decreases during the course of HIV infection despite the concomitant increase in the overall CD8 T-cell count. The number of CD8+CCR5+ T cells also increases in the blood of HIV patients, as previously reported.15,31 Our results showed that the depletion of CD8+CCR6+ cells also concerns CD8+CCR5+ T cells. They also made clear that CCR6+ T cells specifically disappear from the blood of HIV patients regardless of other differentiation and migration cell markers and thus suggest that CCR6 plays a role in the depletion of active memory cells during anti-HIV immune response. A mechanism that includes antigen-induced T-cell activation and differentiation may therefore regulate CCR6 expression on the cell surface or regulate the redistribution of memory T cells. The peripheral extinction of T cells expressing CCR6 is also correlated with HIV disease parameters including viral load, CD4 depletion, and immune restoration after HAART. Our results might also suggest that CCR6+ cells would partially participate in the immune reconstitution after HAART. Longitudinal analysis of these memory populations of CD4 and CD8 cells would therefore be necessary to conclude on this point. The percentage of CCR6+ T cells may thus serve as a marker of disease progression.
We tested for and ruled out several possible mechanisms that might have reduced the CCR6 expression from T-cell surfaces, including high serum concentrations of CCL20 and hyperactivation of T cells. CCL20 serum concentrations were similar for HIV patients and UI subjects. As others have reported,23,32 T-cell activation does not alter CCR6 expression. Human β-defensin-2, another ligand for CCR6, may cause its internalization,33 but it is produced mainly in the epidermis and other epithelia,34,35 but not in the blood. We thus postulate that CCR6+ T cells are extracted from the blood by a CCR6-dependent pathway.
The primordial role of chemokines is to direct the migration of leukocytes throughout the body, under both physiologic and pathologic conditions. We suggest that CCR6+ memory cells migrate into lymphoid organs where CCL20 is produced after immune activation. This specific property of migration to the lymphoid organs was not associated with CCR7. A recent study reported the same CCR6 expression in the spleen during SIV infection but did not investigate its cause.36 The authors also showed that both CCL20 and CCR6 were up-regulated in the lymph nodes during acute SIV infection.36 Our report provides evidence that CCR6+ T cells may be trapped in secondary lymphoid organs because of increased local release of the CCR6 ligand, CCL20, during HIV infection. This phenomenon may actually be underestimated in spleen that produced limited amount of CCL20 in comparison to the gut mucosa and associated lymphoid organs where CCL20 is strongly produced.37 However, this hypothesis could not be tested because of the lack of such samples in HIV+ patients.
We also showed that after trapping, the CCR6 memory T cells undergo apoptosis. The cause of this cell death remains unknown. One hypothesis that may give a fair explanation is that migration of activated or cycling T cells to the spleen of HIV-infected patients, where inflammatory cytokines are produced, induces memory cell death. According to BrdU integration measures, about 30% of the CD4+CCR6+ and CD8+ T cells proliferated after 48 hours of CD3/CD28 stimulation (data not shown), a proliferative potency similar to that of CCR6− T cells (data not shown). These results also suggest that these cells are not terminally differentiated cells. Activated and cycling T cells are known to be sensitive to the death pathways mediated by Fas/fasL and TNF/TNFR.38–42 Because we observed no cell deaths in the presence of CCL20 (data not shown), we propose that CCR6+ memory cell death in the spleen may result from interaction between inflammatory conditions and HIV-induced immune hyperactivation.18,43
CCR6 has previously been described as a marker of resting memory phenotypes and functions.23,44 Here, we confirmed that CCR6 selectively labels CD45RA− T cells and characterizes distinct memory phenotypes for CD4+CCR6+ and CD8+CCR6+ T cells. These 2 lymphocyte populations share the same differentiation and activation markers but have distinct migratory properties. CD4+CCR6+ T cells have a central-memory phenotype, mainly expressing CCR7 and CD62L, whereas CD8+CCR6+ T cells are CCR7− and have an effector-memory phenotype. Our results describe new migratory properties for CCR7+ memory cells; even more, they emphasize their heterogeneity. CCR6 defines memory CD4 Th1-polarized T cells that produce concomitantly high levels of IL-2 and TNF-α. Although CCR6 is present on a subpopulation of CD8+ central memory T cells that produce IFN-γ, IL-2, and TNF-α, its expression did not further qualify subsets of CD8+ effector memory T cells. Our results indicate that CCR6 is a key marker for the memory T-cell subpopulation and may define memory cells better than CCR7, which are also present on naïve cells.
Previous studies show that HIV infection skews the maturation of memory HIV-specific CD8 T cells16 and specifically depletes memory CD4 T cells.18,43,45 Here, we observed another distortion of the HIV-induced immune response: depletion from the peripheral circulation of the CCR6+ memory T cells that represent about half of the so-called memory CD4 T cells. This depletion occurred with increased production of the CCR6 cognate ligand in the spleen of HIV patients. Further work should examine whether CCL20 secretion in secondary lymphoid organs is a natural response to viral infection or whether it is another HIV-mediated escape mechanism.
CCR6 may be used as a marker of disease progression during HIV infection. The loss of circulating CCR6+ T cells linked to HIV infection reported here may reflect a dramatic reduction in the capacity of the immune system to mount an appropriate response against mucosal pathogens because of the changes in the functional capacities of CCR6+ T cells and because of the ultimate death of CCR6+ T cells in the spleen of HIV-infected patients. In conclusion, the trapping and consequent death of central memory CD4 T cells and effector memory CD8 T cells in the spleen of HIV-infected patients may play a role in these patients' loss of immune response to infections.
Authorship
Contribution: C.L. designed and performed the research, analyzed data, and wrote the paper; B.C. designed the research and wrote the paper; E.M. performed the research and analyzed data; O.B. performed the research; A.S. and B.A. contributed vital reagents; P.D. designed the research; and C.C. designed and performed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
C.L. and B.C. contributed equally to this work.
Correspondence: Christophe Combadière, Laboratoire d'Immunologie Cellulaire, INSERM U543, Faculté de Médecine Pitié-Salpêtrière, 91, Boulevard de l'Hôpital, 75013 Paris, France; e-mail: combad@ccr.jussieu.fr.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Dr G. Carcelain and Dr E. Oksenhendler for the helpful discussion and the biopsy collection. We are greatly indebted to Claude Baillou and Catherine Blanc for their expert assistance with fluorescence-activated cell sorting.
C.L. has a fellowship from the French Ministry of National Education and Research.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal