Abstract
Both mast cells and IL-17 can contribute to host defense and pathology in part by orchestrating neutrophil recruitment, but the possible role of mast cells in IL-17–induced inflammation remains to be defined. We found that mast cells and IL-17, but neither IFN-γ nor FcRγ signaling, contributed significantly to the antigen (Ag)–dependent airway neutrophilia elicited in ovalbumin-specific T-cell receptor (TCR)–expressing C57BL/6-OTII mice, and that IFN-γ significantly suppressed IL-17–dependent airway neutrophilia in this setting. IL-18, IL-1β, and TNF each contributed significantly to the development of Ag- and T helper 17 (Th17 cell)–mediated airway neutrophilia. Moreover, IL-17 enhanced mast cell TNF production in vitro, and mast cell–associated TNF contributed significantly to Ag- and Th17 cell–mediated airway neutrophilia in vivo. By contrast, we detected no significant role for the candidate mediators histamine, PGD2, LTB4, CXCL10, or IL-16, each of which can be produced by mast cells and other cell types, in the neutrophil infiltration elicited in this model. These findings establish that mast cells and mast cell–derived TNF can significantly enhance, by FcRγ-independent mechanisms, the Ag- and Th17 cell–dependent development of a neutrophil-rich inflammatory response at a site of Ag challenge.
Introduction
Several proinflammatory cytokines, including TNF, IL-1, and IL-17, can directly induce neutrophil recruitment. For example, inhalation of recombinant TNF, IL-1, or IL-17 can provoke neutrophil infiltration into the airways.1–3 These cytokines can also play important roles in the pulmonary neutrophilia induced by infection with the Gram-negative bacteria Klebsiella pneumoniae, and can contribute to bacterial clearance.4–6
However, both the roles of these proinflammatory cytokines, and the most important cellular sources of their production, can vary according to the models of inflammation analyzed. For example, in mice, mast cell–derived TNF has been reported to contribute importantly to neutrophil recruitment and host defense against K. pneumoniae.7 By contrast, it has been reported that IL-17,8,9 but neither TNF nor IL-1,10 is important for the pulmonary neutrophilia induced by LPS, a component of Gram-negative bacteria.
To date, no reports have investigated the possible role of mast cells in the development of T helper 17 (Th17) cell–dependent, neutrophil-rich inflammatory responses, either in the airways or in other anatomical sites. To address this question, we decided to use a model of protein antigen (Ag) (ovalbumin [OVA])–induced neutrophil-predominant airway inflammation in OVA-specific T-cell receptor (TCR)–expressing mice. We took this approach because of reports indicating that OVA challenge can induce neutrophil-rich airway inflammation in OVA-specific TCR-expressing BALB/c-DO11.10 mice,11,12 and that IL-17 is required for the induction of airway hyperreactivity (AHR) in this model.13
However, we elected to use OVA-specific TCR-expressing C57BL/6-OTII mice,14 instead of BALB/c-DO11.10 mice, for the work reported herein. C57BL/6-OTII mice are on the same strain background as mice that are either profoundly deficient in mast cells, lack the FcRγ chain (that is required for the activation of mast cells via either the FcϵRI or FcγRIII that are expressed on their surface15,16 ), or lack IL-17 or any of several other specific cytokines or cytokine receptors. Therefore, by working with C57BL/6-OTII mice, we were able to use a genetic approach to investigate in detail the importance of each of these candidate elements in the airway neutrophil infiltration elicited by OVA challenge in this model.
Materials and methods
Experimental approach
We first established and characterized a model of OVA-induced neutrophil-rich airway inflammation in OVA-specific TCR-expressing C57BL/6-OTII mice. We then used a combination of pharmacologic and genetic approaches to investigate the potential roles of individual mediators, cytokines, and chemoattractants in the development of the neutrophil-rich airway inflammation induced by Ag challenge in this model. Finally, we used a genetic approach to investigate the roles of FcRγ, mast cells, and mast cell–derived TNF in this model of Ag- and Th17 cell–dependent, neutrophil-rich airway inflammation.
Mice
C57BL/6-OTII transgenic mice14 were provided by R. Schwartz (National Institute of Allergy and Infectious Diseases, National Institutes of Health) through Taconic Farms (Germantown, NY). Genetically mast cell–deficient C57BL/6-KitW-sh/KitW-sh mice were kindly provided by Dr Peter Besmer (Molecular Biology Program, Memorial Sloan-Kettering Cancer Center and Cornell University Graduate School of Medical Sciences, New York, NY). C57BL/6J-IL-17−/− mice,13 C57BL/6J-IL-16−/− mice,17 and C57BL/6J-TNF−/− mice18 were generated as described previously. WB/ReJ-KitW/+ mice, and C57BL/6J-wild-type, KitW-v/+, IFN-γ−/−, IL-18−/−, IL-1R1−/−, or Rag-1−/− mice were purchased from The Jackson Laboratories (Bar Harbor, ME). C57BL/6-FcRγ−/− mice were purchased from Taconic Farms. Genotyping of OTII mice was performed by fluorescence-activated cell-sorter (FACS) as described.14 All OTII mice used in our experiments were homozygous for the OTII transgene. To generate KitW/W-v OTII mice, we first produced WB/ReJ-KitW/+ OTII and C57BL/6J-KitW-v/+ OTII mice, which were allowed to mate to generate KitW/W-v OTII mice. All experiments used age-matched male and female mice that were at least 6 to 8 weeks old at the beginning of the experiments. All mice were housed at the animal care facilities at Stanford University Medical Center (Stanford, CA), and were kept under standard temperature, humidity, and timed lighting conditions, provided mouse chow and water ad libitum, and were treated in a humane manner, in compliance with the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press (revised 1996), and the Stanford Institutional Animal Care and Use Committee.
Induction of neutrophilic airway inflammation
For OVA-induced neutrophilic airway inflammation, mice were treated with 20 μL of 2.5 mg/mL OVA (grade V; Sigma-Aldrich, St Louis, MO), 2.5 mg/mL KLH (Sigma-Aldrich), or PBS intranasally for 3 days (once per day). For T-cell transfer, CD4+ T cells from the spleen and lymph nodes (LNs) of OTII, TNF−/− or IL-17−/− OTII mice were purified, then 2 × 107 cells were injected intravenously into Rag-1−/− mice, and these T cell → Rag-1−/− mice were treated with OVA as described immediately above. For LPS-induced airway neutrophilia, mice were treated intranasally with LPS (Escherichia coli serotype 055:B5, 10 μg/mouse; Sigma-Aldrich) as described.9
After the last OVA, KLH, or PBS inhalation (24 hours), bronchoalveolar lavage (BAL) cells were collected, cytospin specimens of the BAL cells were prepared, and the cells were identified after May-Grünwald-Giemsa staining as described.19 For IL-17 inhalation, mice were treated intranasally with 10 μg recombinant mouse IL-17 (rmIL-17; R&D Systems, Minneapolis MN) and BAL cells were collected 6 hours later. In some mice, airway responses to graded doses of methacholine were assessed 24 hours after the last intranasal challenge, either by a noninvasive approach (as described in Williams and Galli,19 with results reported as enhanced respiratory pause [Pen]) or by an invasive approach.20 Briefly, mice were deeply anesthetized with ketamine intramuscularly, and surgically intubated. Intubated mice were connected to plethysmograph chambers (PLY3111; Buxco Research Systems, Wilmington, NC) with a ventilator (type 845; Hugo Sachs Elektronik-Harvard Apparatus, March-Hugstetten, Germany) and mechanically ventilated. The ventilation was achieved at 150 breaths/minutes and a tidal volume of 0.2 mL. Aerosolized methacholine (20 μL) was administered for 5 seconds with a tidal volume of 0.2 mL. Up to 3 minutes after each aerosol challenge, the data for lung resistance (RL) and dynamic compliance (Cdyn; data not shown) were continuously monitored using BioSystem XA software (Buxco, Wilmington, NC).
Measurement of OVA-specific IgE and IgG1 levels in sera and cytokines enzyme-linked by immunosorbent assay (ELISA)
Sera were collected from “positive control” C57BL/6 mice that had been induced to express a mast cell/IgE–dependent form of airway inflammation, as described,19 as well as from OTII mice, at 24 hours after the last OVA or PBS inhalation. OVA-specific Ig levels were performed as described.13 Biotinylated anti-mouse IgG1 and IgE and HRP-conjugated streptavidin were obtained from BD PharMingen (San Diego, CA). Anti-OVA IgG1 (3B2E6) and IgE (TOS-2), kindly provided by Dr Mamoru Kiniwa (Pharmaco Bioregulation Research Laboratory, Taiho Pharmaceutical Co Ltd, Saitama, Japan), were used as standard antibodies. The TMB substrate was purchased from Sigma.
FACS analysis
For detection of BAL T-cell cytokine profiles, BAL cells were pooled from 20 OVA-treated OTII mice and incubated with anti-mouse CD16/CD32 (2.4G2; BD PharMingen) in a staining buffer (Hanks balanced salt solution [HBBS] containing 2% FCS and 0.1% sodium azide) on ice for 15 minutes. After FcR blocking, cells were incubated on ice for 40 minutes with APC anti-mouse CD4 (RM4-5; BD PharMingen) and biotinylated anti-mouse CD49b (DX5; eBioscience, San Diego, CA), and then incubated for 40 minutes on ice with PerCP-streptavidin (BD PharMingen). Cells were fixed in PBS containing 4% paraformaldehyde for 20 minutes at room temperature. After washing with a permeabilization buffer (0.1% saponin σ in the staining buffer), cells were incubated with nonlabeled or FITC- or PE-labeled rat IgG1 (BD PharMingen), anti-mouse IFN-γ (XMG1.2; BD PharMingen), IL-4 (11B11; BD PharMingen), or IL-17 (TC11-18H10.1; BD PharMingen).
Expression of intracellular molecules in CD49b−CD4+ T cells was analyzed on a FACS Calibur (Becton Dickinson, San Jose, CA) using Cell Quest software (Becton Dickinson).
Measurement of cytokine levels by ELISA
Broncheoalveolar lavage fluid (BALF) was collected as described above in “Induction of neutrophilic airway inflammation.” IL-4, IL-5, IL-17, IFN-γ, and TNF levels in BALF or in cell culture supernatants were examined by mouse IL-4, IL-5, IL-17, IFN-γ, and TNF BD OptEIA ELISA sets (BD PharMingen).
Preparation of BMCMCs, local mast cell engraftment of mast cell–deficient mice, and BMCMC culture
Bone marrow–derived cultured mast cells (BMCMCs) were obtained by culturing mouse femoral bone marrow cells in WEHI-3–conditioned medium (containing IL-3) for 6 to 12 weeks. For mast cell engraftment studies, BMCMCs were injected intravenously (1.0 × 107 cells) into 4- to 6-week-old KitW/W-v OTII mice or KitW-sh/W-sh OTII mice as described.21,22 The mice were used for experiments 8 weeks after transfer of BMCMCs. As previously reported,23 the numbers of mast cells counted in single-cell suspensions of lungs from KitW-sh/W-sh OTII mice which had received wild-type versus TNF−/− BMCMCs were not significantly different (data not shown). For measurement of TNF production, supernatants were obtained 6 hours after the culture of BMCMCs with or without various concentrations of rmIL-17 in the presence or absence of ionomycin.
Statistics
Unless otherwise specified, the unpaired Student t test, 2-tailed, was used for statistical evaluation of the results and all results are shown as means plus SD.
Results
A mouse model of Ag- and T-cell–dependent neutrophilic airway inflammation
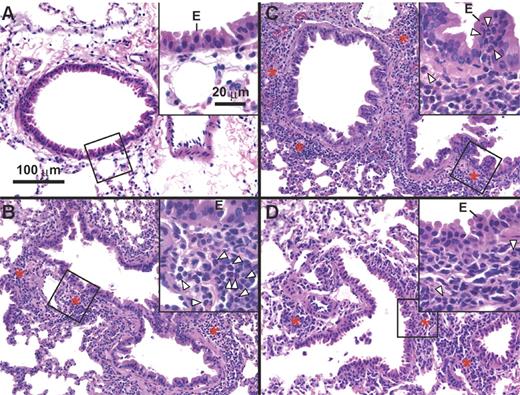
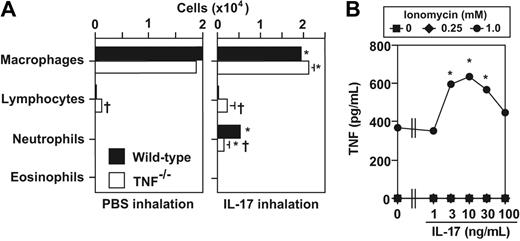
As in BALB/c-DO11.10 mice,11,12 OVA administration to OVA-specific TCR-expressing OTII transgenic mice induced lung inflammation in which neutrophils, rather than eosinophils, represent a prominent feature of the inflammatory response (Figure 1). As assessed 24 hours after the last administration of OVA, intranasal administration of 50 μg OVA daily for 3 days to naive OTII mice induced substantial peribronchial and perivascular inflammation, with a prominent component of neutrophils (Figure 1B,D), whereas this was not observed in naive wild-type (WT) mice challenged with PBS, OVA, or KLH or in PBS- or KLH-challenged naive OTII mice (Figure 1A; data not shown). In OVA-challenged OTII mice, neutrophils, but few or no eosinophils, occurred in peribronchial and perivascular inflammatory infiltrates (Figure 1B,D) and within bronchiolar epithelium (Figure 1C).
Antigen-induced neutrophilic airway inflammation in OTII mice. OTII mice were challenged with 50 μg of OVA (B-D) or PBS (A) intranasally for 3 days (once per day). Lung histology was performed in hematoxylin- and eosin-stained sections of formalin-fixed, paraffin-embedded tissues of mice that were killed 24 hours after the last OVA or PBS inhalation. Scale bars in photomicrographs and insets are 100 and 20 μm, respectively. Arrowheads indicate neutrophils; E, bronchiolar epithelium; asterisk, representative areas of peribronchiolar or perivascular inflammation; and area of section shown at higher magnification in the inset. Images were captured with a Nikon E1000M microscope (Nikon, Melville, NY) utilizing a 20×/0.75 NA objective and a 60×/0.95 objective for inserts (ocular eyepieces, 10×), a SPOT FLEX camera (Diagnostic Instruments, Sterling, MI) model 15.2, SPOT Advanced 4.5 software (Diagnostic Instruments), and Photoshop CS2 (Adobe Systems, San Jose, CA).
Antigen-induced neutrophilic airway inflammation in OTII mice. OTII mice were challenged with 50 μg of OVA (B-D) or PBS (A) intranasally for 3 days (once per day). Lung histology was performed in hematoxylin- and eosin-stained sections of formalin-fixed, paraffin-embedded tissues of mice that were killed 24 hours after the last OVA or PBS inhalation. Scale bars in photomicrographs and insets are 100 and 20 μm, respectively. Arrowheads indicate neutrophils; E, bronchiolar epithelium; asterisk, representative areas of peribronchiolar or perivascular inflammation; and area of section shown at higher magnification in the inset. Images were captured with a Nikon E1000M microscope (Nikon, Melville, NY) utilizing a 20×/0.75 NA objective and a 60×/0.95 objective for inserts (ocular eyepieces, 10×), a SPOT FLEX camera (Diagnostic Instruments, Sterling, MI) model 15.2, SPOT Advanced 4.5 software (Diagnostic Instruments), and Photoshop CS2 (Adobe Systems, San Jose, CA).
OTII mice treated with OVA daily for 3 days also exhibited many neutrophils in BALF at 24 hours after the last administration of OVA (Figure 2A), whereas only 1 or 2 OVA treatments resulted in few neutrophils in the BALF (data not shown). The response was OVA specific, as little or no inflammation was detected in PBS-, OVA-, or KLH-treated naive WT mice or in PBS- or KLH-treated naive OTII mice (Figure 2A). Consistent with findings in previous studies using BALB/c-DO11.10 mice,11 macrophages and lymphocytes, as well as neutrophils, were increased in BALF derived from OVA-challenged OTII mice, whereas few or no eosinophils were detected (Figure 2A). Neutrophil-dominant airway inflammation without significant numbers of eosinophils was detected 48, 72, 96, or 120 hours after the last OVA inhalation, although the numbers of neutrophils diminished with time (data not shown). Similar results were also observed when we tested lower (1, 5, 10, or 20 μg) or higher (100 or 200 μg) doses of Ag exposure (data not shown).
Antigen-induced neutrophilic airway inflammation in OTII mice. Wild-type (WT) or OTII mice were treated with 50 μg of OVA or KLH in PBS, or PBS alone intranasally for 3 days (once per day). Data in panels A-C, E are the average + SD of results pooled from 2 independent experiments, each of which gave similar results (n = 7-10). (A) Cells in BALF were quantified 24 hours after the last inhalation of OVA, KLH, or PBS. *P < .01; †P < .01 versus corresponding values for PBS-, OVA-, or KLH-treated wild-type mice and PBS- or KLH-treated OTII mice, respectively. (B) OVA-specific IgG1 and IgE in sera collected from mice in panel A 24 hours after the last OVA or PBS inhalation. (C) Airway responses (Penh and lung resistance [RL]) to methacholine were assessed 24 hours after the last inhalation of OVA, KLH, or PBS, as in panel A; BL indicates baseline. *P < .05 versus corresponding values for each of the other groups (by analysis of variance). (D) BALF cells pooled from 20 OTII mice 24 hours after the last OVA inhalation were stimulated with PMA plus ionomycin in the presence of monensin, and then the intracellular cytokine profiles of CD4+ T cells (% positive cells shown in the figure) were determined by FACS. Results shown are representative of those obtained in 2 independent experiments, each of which gave similar results. (E) Cytokine levels in BALF obtained 24 hours after the last OVA or PBS inhalation in mice shown in panel A. *P < .01; †P < .01 versus corresponding values for PBS- or OVA-treated wild-type mice and PBS-treated OTII mice, respectively.
Antigen-induced neutrophilic airway inflammation in OTII mice. Wild-type (WT) or OTII mice were treated with 50 μg of OVA or KLH in PBS, or PBS alone intranasally for 3 days (once per day). Data in panels A-C, E are the average + SD of results pooled from 2 independent experiments, each of which gave similar results (n = 7-10). (A) Cells in BALF were quantified 24 hours after the last inhalation of OVA, KLH, or PBS. *P < .01; †P < .01 versus corresponding values for PBS-, OVA-, or KLH-treated wild-type mice and PBS- or KLH-treated OTII mice, respectively. (B) OVA-specific IgG1 and IgE in sera collected from mice in panel A 24 hours after the last OVA or PBS inhalation. (C) Airway responses (Penh and lung resistance [RL]) to methacholine were assessed 24 hours after the last inhalation of OVA, KLH, or PBS, as in panel A; BL indicates baseline. *P < .05 versus corresponding values for each of the other groups (by analysis of variance). (D) BALF cells pooled from 20 OTII mice 24 hours after the last OVA inhalation were stimulated with PMA plus ionomycin in the presence of monensin, and then the intracellular cytokine profiles of CD4+ T cells (% positive cells shown in the figure) were determined by FACS. Results shown are representative of those obtained in 2 independent experiments, each of which gave similar results. (E) Cytokine levels in BALF obtained 24 hours after the last OVA or PBS inhalation in mice shown in panel A. *P < .01; †P < .01 versus corresponding values for PBS- or OVA-treated wild-type mice and PBS-treated OTII mice, respectively.
In certain models of Th2-associated airway inflammation, Ag-specific IgE and IgG1 production, and mast cell activation by Ig/Ag-FcRγ signaling (via FcϵRI and FcγRIII, which share a common γ chain), can contribute significantly to the development of eosinophil-rich airway inflammation.19,24 As expected, concentrations of OVA-specific IgG1 and IgE antibodies were markedly elevated in sera from WT mice induced to develop a mast cell/IgE-dependent model of airway inflammation (indicated as “Positive control” in Figure 2B). By contrast, as had been shown using BALB/c-DO11.10 mice,12 little or no OVA-specific IgE or IgG1 antibodies were detected in naive WT or OTII mice 24 h after 3 daily inhalations of OVA (Figure 2B).
This OVA-induced inflammation in OTII mice, like that in BALB/c-DO11.10 mice,13 was also associated with changes in airway function. Enhanced airway responses to aerosolized methacholine were detectable in the OVA-treated OTII mice tested with either noninvasive or invasive assays of airway function, but not in any of the other groups (Figure 2C).
Th2 cells were barely detectable among T cells in the BALF of OVA-challenged OTII mice, whereas substantial populations of both IFN-γ+ Th1 cells and IL-17+ Th17 cells were present (Figure 2D). Similarly, IL-4, IL-5, and IL-13 were below the limits of detection in BALF of OVA-challenged OTII mice, whereas IFN-γ and IL-17 were significantly increased (Figure 2E; data not shown).
Thus, all of our observations suggest that OVA-specific Th1 cells and/or Th17 cells, but not Th2 cells, contribute to the Ag-dependent neutrophilic airway inflammation observed in OVA-challenged OTII mice.
Evidence for opposing roles of IL-17 and IFN-γ in this model
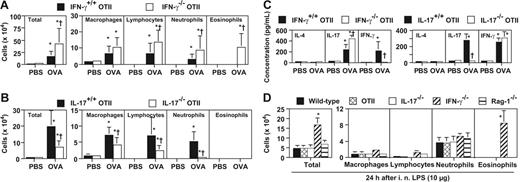
In some models, mice engrafted with DO11.10-derived Th1 cells exhibited neutrophilic airway inflammation upon Ag challenge, implicating IFN-γ in this process.25 However, in IFN-γ−/− OTII mice, we found that OVA inhalation induced significantly increased numbers of total inflammatory cells, lymphocytes, and neutrophils in BALF (Figure 3A). Eosinophils also were observed in the BALF of IFN-γ−/− OTII mice but not IFN-γ+/+ OTII mice (Figure 3A). These findings indicate that, in the OTII model, IFN-γ can suppress the development of large numbers of lymphocytes, neutrophils, and eosinophils in the airways.
IL-17 promotes, but IFN-γ suppresses, neutrophilic airway inflammation. Mice were treated with 50 μg of OVA or PBS intranasally for 3 days (once per day) (A-C) or 10 μg of LPS intranasally (D). Cells in BALF were quantified 24 hours after the last OVA, PBS, or LPS inhalation. (A) OVA or PBS-challenged IFN-γ+/+ OTII mice (PBS, n = 16; OVA, n = 21), and IFN-γ−/− OTII mice (PBS, n = 6; OVA, n = 14). (B) OVA- or PBS-challenged IL-17+/+ OTII mice (PBS, n = 12; OVA, n = 14), and IL-17−/− OTII mice (PBS, n = 7; OVA, n = 14). (C) Cytokine levels in BALF obtained 24 hours after the last OVA or PBS inhalation in mice shown in panels A and B. (D) LPS-challenged wild-type mice (n = 15), OTII mice (n = 7), IL-17−/− mice (n = 15), IFN-γ−/− mice (n = 12), and Rag-1−/− mice (n = 7). *P < .01 versus corresponding values for PBS-treated mice; †P < .01 versus corresponding values for OVA-treated IFN-γ+/+ OTII mice (A,C) or OVA-treated IL-17+/+ OTII mice (B,C). *P < .01 versus corresponding values for LPS-treated mice of any other genotype (D). Data are the average + SD of results pooled from 2 or 3 independent experiments, all of which gave similar results.
IL-17 promotes, but IFN-γ suppresses, neutrophilic airway inflammation. Mice were treated with 50 μg of OVA or PBS intranasally for 3 days (once per day) (A-C) or 10 μg of LPS intranasally (D). Cells in BALF were quantified 24 hours after the last OVA, PBS, or LPS inhalation. (A) OVA or PBS-challenged IFN-γ+/+ OTII mice (PBS, n = 16; OVA, n = 21), and IFN-γ−/− OTII mice (PBS, n = 6; OVA, n = 14). (B) OVA- or PBS-challenged IL-17+/+ OTII mice (PBS, n = 12; OVA, n = 14), and IL-17−/− OTII mice (PBS, n = 7; OVA, n = 14). (C) Cytokine levels in BALF obtained 24 hours after the last OVA or PBS inhalation in mice shown in panels A and B. (D) LPS-challenged wild-type mice (n = 15), OTII mice (n = 7), IL-17−/− mice (n = 15), IFN-γ−/− mice (n = 12), and Rag-1−/− mice (n = 7). *P < .01 versus corresponding values for PBS-treated mice; †P < .01 versus corresponding values for OVA-treated IFN-γ+/+ OTII mice (A,C) or OVA-treated IL-17+/+ OTII mice (B,C). *P < .01 versus corresponding values for LPS-treated mice of any other genotype (D). Data are the average + SD of results pooled from 2 or 3 independent experiments, all of which gave similar results.
In contrast to the results with the IFN-γ−/− OTII mice, OVA-challenged IL-17−/− OTII mice exhibited significantly reduced numbers of total inflammatory cells in the BALFs (64% reduced vs the corresponding levels in the IL-17+/+ OTII mice; Figure 3B). Neutrophil numbers in the BALF from the IL-17−/− OTII mice were profoundly reduced (Figure 3B), whereas the number of circulating blood neutrophils in naive and/or OVA-challenged mice was not significantly different between IL-17+/+ OTII and IL-17−/− OTII mice (data not shown). Numbers of macrophages and lymphocytes were also significantly reduced in the BALF of OVA-challenged IL-17−/− OTII mice versus the corresponding levels in the IL-17+/+ OTII mice, but not to the same extent as with neutrophils (Figure 3B).
Like IL-4, IFN-γ can suppress Th17 cell differentiation in vitro.26,27 IL-17 levels in BALF from OVA-treated IFN-γ −/− OTII mice were significantly increased compared with those from OVA-treated IFN-γ+/+ OTII mice (Figure 3C). However, the presence or absence of IL-17 had no detectable effect on IFN-γ levels in BALF from OVA-treated IL-17−/− versus IL-17+/+ OTII mice (Figure 3C), despite the lower numbers of lymphocytes in the BALF of IL-17−/− OTII mice (Figure 3B). IL-4 and IL-5 were below the limits of detection in BALF from any of the groups of mice (Figure 3C; data not shown).
IL-17 also has been implicated in the development of LPS-induced lung neutrophilia in mice.8,9 This is an important point in the context of an analysis of OVA-induced neutrophilic inflammation in OTII mice, since preparations of commercially obtained OVA can contain substantial amounts of LPS.28,29 However, we found that very similar numbers of neutrophils were detected in BALF obtained 24 hours after LPS inhalation in WT, OTII, IL-17−/−, or Rag-1−/− mice (Figure 3D). Similar numbers of BALF neutrophils also were observed in identically treated IFN-γ−/− mice, but these mice also exhibited large numbers of BALF eosinophils (Figure 3D). These findings indicate that neutrophilic airway inflammation, as assessed in BALF, can be induced by E coli–derived LPS in the absence of IL-17 or IFN-γ and in the absence of mature T cells, B cells, or immunoglobulins.
Taken together, our observations indicate that IL-17, rather than IFN-γ, has an important role in the development of Ag-specific neutrophilic airway inflammation in the OTII model. By contrast, neither IL-17 nor IFN-γ is essential for LPS-induced neutrophil recruitment to the lungs.
IL-18, IL-1R1, and TNF promote neutrophilic airway inflammation in this model
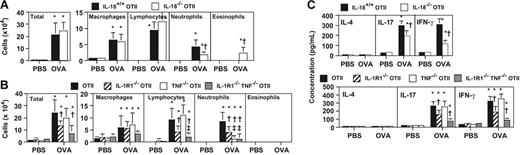
IL-18, IL-1, and TNF can promote IL-17 production.30 IL-18−/− OTII mice exhibited significantly decreased neutrophilia 24 hours after the last OVA challenge (approximately 50% reduction vs corresponding levels in IL-18+/+ OTII mice; Figure 4A), although the reduction was not as great as that observed in IL-17−/− OTII mice (Figure 3B). IL-18−/− OTII mice also exhibited increased numbers of BALF eosinophils (Figure 4A), as did IFN-γ−/− OTII mice (Figure 3A).
IL-18 and IL-1 contribute to the induction of Th17 cell–mediated neutrophilic airway inflammation. Mice were treated with 50 μg of OVA or PBS intranasally for 3 day (once per day). Cells in BALF were quantified 24 hours after the last OVA or PBS inhalation. (A) OVA- or PBS-challenged IL-18+/+ OTII mice (PBS, n = 6; OVA, n = 10), and IL-18−/− OTII mice (PBS, n = 6; OVA, n = 10). (B) OVA- or PBS-challenged wild-type OTII mice (OTII: PBS, n = 6; OVA, n = 10), IL-1R1−/− OTII mice (PBS, n = 6; OVA, n = 10), TNF−/− OTII mice (PBS, n = 6; OVA, n = 10), and IL-1R1−/− TNF−/− OTII mice (PBS, n = 6; OVA, n = 10). (C) Cytokine levels in BALF from mice in panels A and B. *P < .01 versus corresponding values for PBS-treated mice; †P < .05 versus corresponding values for OVA-treated IL-18+/+ OTII mice (A,C) or wild-type OTII mice (B,C). ‡P < .05 versus corresponding values for OVA-treated IL-1R1−/− OTII mice (B). Data are the average + SD of results pooled from 2 independent experiments, each of which gave similar results.
IL-18 and IL-1 contribute to the induction of Th17 cell–mediated neutrophilic airway inflammation. Mice were treated with 50 μg of OVA or PBS intranasally for 3 day (once per day). Cells in BALF were quantified 24 hours after the last OVA or PBS inhalation. (A) OVA- or PBS-challenged IL-18+/+ OTII mice (PBS, n = 6; OVA, n = 10), and IL-18−/− OTII mice (PBS, n = 6; OVA, n = 10). (B) OVA- or PBS-challenged wild-type OTII mice (OTII: PBS, n = 6; OVA, n = 10), IL-1R1−/− OTII mice (PBS, n = 6; OVA, n = 10), TNF−/− OTII mice (PBS, n = 6; OVA, n = 10), and IL-1R1−/− TNF−/− OTII mice (PBS, n = 6; OVA, n = 10). (C) Cytokine levels in BALF from mice in panels A and B. *P < .01 versus corresponding values for PBS-treated mice; †P < .05 versus corresponding values for OVA-treated IL-18+/+ OTII mice (A,C) or wild-type OTII mice (B,C). ‡P < .05 versus corresponding values for OVA-treated IL-1R1−/− OTII mice (B). Data are the average + SD of results pooled from 2 independent experiments, each of which gave similar results.
IL-1R1−/− OTII mice challenged with OVA exhibited significant reductions (of 36%-64% vs corresponding levels in IL-1R1+/+ OTII mice) in numbers of total inflammatory cells, lymphocytes, and neutrophils in BALF (Figure 4B). TNF−/− OTII mice also exhibited a significant reduction in levels of BALF neutrophils in this model (by approximately 75% vs reductions of approximately 50% in either IL-18−/− OTII mice [Figure 4A] or IL-1R1−/− OTII mice [Figure 4B]), while TNF−/− OTII mice had numbers of total inflammatory cells, macrophages, and lymphocytes in BALF that were statistically indistinguishable from those in control (TNF+/+) OTII mice (Figure 4B). IL-1R1−/− TNF−/− OTII mice exhibited the strongest reductions in numbers of all BAL leukocytes compared with other mice; however, the reduction in levels of neutrophils was comparable with that in TNF−/− OTII mice (Figure 4B).
These observations indicate that TNF is more important than IL-1 or IL-18 for Th17-mediated airway neutrophilia. By contrast, LPS-induced airway neutrophilia in IL-18−/− mice was comparable with that in wild-type mice (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). And, in contrast to the results in IFN-γ−/− mice, eosinophils did not appear in the BALF of LPS-treated IL-18−/− mice (Figure S1). As reported by others,10 LPS-induced airway neutrophilia was not influenced by the lack of IL-1R1, TNF, or mast cells (Figure S1).
IL-17 concentrations in BALF after OVA challenge were somewhat reduced in both IL-18−/− and IL-1R1−/− OTII mice (Figure 4C), consistent with the reduced OVA-induced neutrophilia and T-cell recruitment in such mice. This result suggests that IL-18 and/or IL-1 may function upstream of IL-17. Indeed, IL-1–mediated arthritis is known to be suppressed by IL-17 deficiency.31 On the other hand, consistent with the normal levels of OVA-induced lymphocyte recruitment observed in TNF−/− OTII mice (Figure 4B), IL-17 levels in BALF from TNF−/− OTII mice were similar to those from control OTII mice, even though neutrophil levels in BALF from TNF−/− OTII mice were markedly reduced (Figure 4B-C). These observations suggest that TNF may function downstream of IL-17 in the local inflammatory response.
In contrast to the evidence indicating that IL-18, IL-1/IL-1R1, and TNF contribute to the development of Ag-induced neutrophil infiltration in this model, pharmacologic or genetic evidence suggests that such Th17 cell–mediated neutrophilic airway inflammation can be elicited independently of histamine, PGD2, LTB4, or CXCL10, and with little or no contribution of IL-16 (Figure S2).
Requirement for mast cells but not FcRγ-dependent signaling
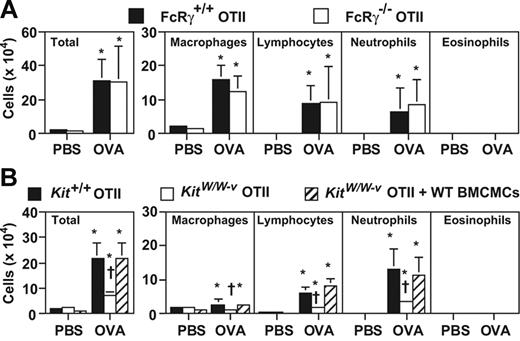
Mast cells, and IgE/Ag-FcϵRI–mediated mast cell activation, can enhance Th2 cell–associated eosinophil-dominant airway inflammation.19,24 Since no OVA-specific IgG1 or IgE was detected in sera from OTII mice 24 hours after the last OVA inhalation (Figure 2B), it is very unlikely that neutrophilic inflammation in this model requires IgE/Ag-FcϵRI or IgG1/Ag-FcγRIII signaling. Our findings showing essentially the same levels of OVA-induced leukocytes in the BALF of FcRγ−/− versus FcRγ+/+ OTII mice, in which mast cells and all other cell types lack FcRγ, also support this conclusion (Figure 5A).
Mast cells, but not FcRγ-dependent signaling, promote Th17-mediated neutrophilic airway inflammation. Mice were treated with 50 μg of OVA or PBS intranasally for 3 days (once per day). Cells in BALF were quantified 24 hours after the last OVA or PBS inhalation. (A) FcRγ+/+ OTII mice (PBS, n = 8; OVA, n = 14), and FcRγ−/− OTII mice (PBS, n = 7; OVA, n = 14). (B) Kit+/+ OTII mice (PBS, n = 18; OVA, n = 24), KitW/W-v OTII mice (PBS, n = 7; OVA, n = 14) and KitW/W-v OTII mice which were systemically engrafted with Kit+/+ BMCMCs (KitW/W-v OTII + WT BMCMCs) (PBS, n = 7; OVA, n = 14). *P < .01 versus corresponding values for PBS-treated mice; †P < .01 versus corresponding values for OVA-treated Kit+/+ OTII mice or OVA-treated KitW/W-v OTII mice plus WT BMCMCs (B). Data are the average + SD of results pooled from 3 independent experiments, all of which gave similar results.
Mast cells, but not FcRγ-dependent signaling, promote Th17-mediated neutrophilic airway inflammation. Mice were treated with 50 μg of OVA or PBS intranasally for 3 days (once per day). Cells in BALF were quantified 24 hours after the last OVA or PBS inhalation. (A) FcRγ+/+ OTII mice (PBS, n = 8; OVA, n = 14), and FcRγ−/− OTII mice (PBS, n = 7; OVA, n = 14). (B) Kit+/+ OTII mice (PBS, n = 18; OVA, n = 24), KitW/W-v OTII mice (PBS, n = 7; OVA, n = 14) and KitW/W-v OTII mice which were systemically engrafted with Kit+/+ BMCMCs (KitW/W-v OTII + WT BMCMCs) (PBS, n = 7; OVA, n = 14). *P < .01 versus corresponding values for PBS-treated mice; †P < .01 versus corresponding values for OVA-treated Kit+/+ OTII mice or OVA-treated KitW/W-v OTII mice plus WT BMCMCs (B). Data are the average + SD of results pooled from 3 independent experiments, all of which gave similar results.
However, compared with the congenic WT (WBxB6-Kit+/+) OTII mice, c-kit mutant, genetically mast cell–deficient WB × B6-KitW/W-v OTII mice exhibited significantly reduced levels of total inflammatory cells, macrophages, lymphocytes (69% reduction), and neutrophils in BALF at 24 hours after the last OVA challenge. These abnormalities specifically reflected the mast cell deficiency of the KitW/W-v OTII mice, rather than other consequences of the animals' c-kit mutations, since the reduced responses were restored to WT levels in KitW/W-v OTII mice that had been systemically and selectively engrafted with Kit+/+ BMCMCs (Figure 5B).
Thus, mast cells are required for the development of neutrophilic airway inflammation in OVA-challenged OTII mice, but this mast cell function can be expressed independently of the activation of the cells by FcRγ-dependent mechanisms.
Role of mast cell–derived versus T-cell–derived TNF
We specifically investigated whether mast cell–derived TNF might function downstream of IL-17 in this model. In confirmation of a study in rats showing that inhalation of recombinant human IL-17 induced neutrophil influx into the lungs,3 WT mice treated with rmIL-17 intranasally developed high levels of neutrophils in BALF (Figure 6A). This effect was significantly attenuated (by approximately 70% vs findings in WT mice) in TNF−/− mice (Figure 6A), indicating that the IL-17–mediated neutrophil influx observed in this setting is highly dependent on TNF.
TNF is required for IL-17–induced neutrophil influx in the lungs. (A) Cells in BALF were quantified 8 hours after intranasal treatment of mice with PBS or rmIL-17 (10 μg). Wild-type mice (PBS, n = 5; IL-17, n = 4) and TNF−/− mice (PBS, n = 5; IL-17, n = 4). Data are the average + SD of results pooled from 2 independent experiments, each of which gave similar results. *P < .01 versus corresponding values for PBS-treated mice; †P < .01 versus corresponding values for IL-17–treated wild-type mice. (B) TNF levels in culture supernatants of BMCMCs that were stimulated with ionomycin in the presence of various concentration of rmIL-17 for 6 hours. Data are the average ± SD of triplicate determinations in 1 of 3 independent experiments, all of which gave similar results (because the SDs were very small, the bars indicating the SDs are obscured by the symbols). *P < .01 versus corresponding values for the cells incubated without rmIL-17 (0 ng/mL).
TNF is required for IL-17–induced neutrophil influx in the lungs. (A) Cells in BALF were quantified 8 hours after intranasal treatment of mice with PBS or rmIL-17 (10 μg). Wild-type mice (PBS, n = 5; IL-17, n = 4) and TNF−/− mice (PBS, n = 5; IL-17, n = 4). Data are the average + SD of results pooled from 2 independent experiments, each of which gave similar results. *P < .01 versus corresponding values for PBS-treated mice; †P < .01 versus corresponding values for IL-17–treated wild-type mice. (B) TNF levels in culture supernatants of BMCMCs that were stimulated with ionomycin in the presence of various concentration of rmIL-17 for 6 hours. Data are the average ± SD of triplicate determinations in 1 of 3 independent experiments, all of which gave similar results (because the SDs were very small, the bars indicating the SDs are obscured by the symbols). *P < .01 versus corresponding values for the cells incubated without rmIL-17 (0 ng/mL).
IL-17 can induce TNF production by macrophages.32 At 3, 10, or 30 ng/mL, IL-17 significantly enhanced levels of TNF production by mouse BMCMCs in the presence of 1.0 μM ionomycin (Figure 6B), but IL-17 was without effect in the absence of ionomycin. These in vitro observations suggest that Th17 cell–derived IL-17 may enhance mast cell TNF production, but perhaps only if the mast cells have been primed or activated by an additional signal.
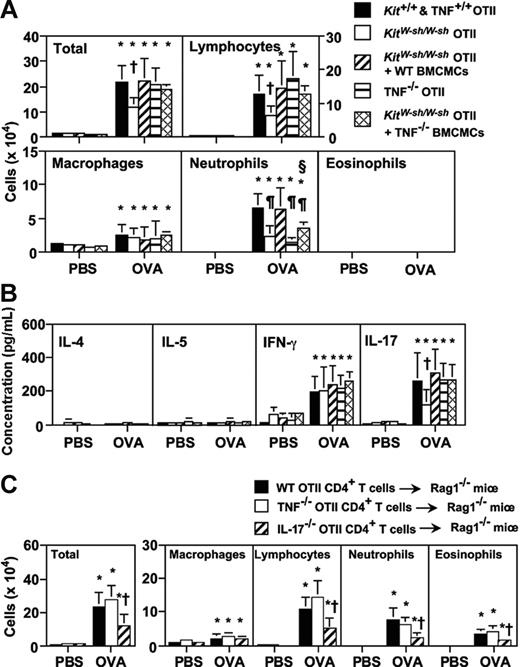
Like mast cell–deficient WB × B6-KitW/W-v OTII mice, at 24 hours after the last OVA inhalation, mast cell–deficient C57BL/6-KitW-sh/W-sh OTII mice exhibited significantly reduced concentrations of IL-17, as well as reduced numbers of total inflammatory cells (46% reduction vs the corresponding levels in the Kit+/+ OTII mice), lymphocytes (51% reduction), and neutrophils (64% reduction) in BALF (Figure 7A-B). Moreover, the reduced responses were restored to Kit+/+ OTII mouse levels in KitW-sh/W-sh OTII mice that had been systemically engrafted with either WT BMCMCs or IL-18−/− BMCMCs (Figure 7A-B; data not shown). Moreover, engraftment of KitW-sh/W-sh OTII mice with TNF−/− BMCMCs repaired to WT levels the total number of cells, macrophages, and lymphocytes in BALF 24 hours after the last OVA challenge, but only partially corrected the defect in neutrophil levels (Figure 7A).
Mast cell–derived TNF, but not T-cell–derived TNF, contributes to Th17 cell–mediated neutrophilic airway inflammation. (A-B) Mice were treated with 50 μg of OVA or PBS intranasally for 3 days (once per day). Cells (A) and cytokine levels (B) in BALF were quantified 24 hours after the last inhalation of OVA. Kit+/+ and wild-type OTII mice (PBS, n = 11; OVA, n = 15), KitW-sh/W-sh OTII mice (PBS, n = 13; OVA, n = 18), KitW-sh/W-sh OTII mice that were systemically engrafted with wild-type BMCMCs (KitW-sh/W-sh OTII + WT BMCMCs) (PBS, n = 5; OVA, n = 11), TNF−/− OTII mice (PBS, n = 9; OVA, n = 15) and KitW-sh/W-sh OTII mice that were systemically engrafted with TNF−/− BMCMCs (KitW-sh/W-sh OTII + TNF−/− BMCMCs) (PBS, n = 6; OVA, n = 11) (A-B). Data are the average + SD of results pooled from 3 independent experiments, all of which gave similar results. *P < .01 versus corresponding values for PBS-treated mice; †P < .05 versus corresponding values for each of the 3 other OVA-treated groups; ¶P < .01 versus corresponding values for OVA-treated Kit+/+ and wild-type OTII mice and KitW-sh/W-sh OTII mice plus WT BMCMCs; and §P < .05 versus corresponding values for OVA-treated TNF−/− OTII mice. (C) Rag-1−/− mice were injected intravenously with wild-type (WT), TNF−/−, or IL-17−/− OTII CD4+ T cells, and then the mice were treated with 50 μg of OVA or PBS intranasally for 3 days (once per day). Cells in BALF were quantified 24 hours after the last inhalation. WT OTII CD4+ T-cell–injected Rag-1−/− mice (PBS, n = 6; OVA, n = 10), TNF−/− OTII CD4+ T-cell–injected Rag-1−/− mice (PBS, n = 6; OVA, n = 10), and IL-17−/− OTII CD4+ T-cell–injected Rag-1−/− mice (PBS, n = 6; OVA, n = 8). Data are the average + SD of results pooled from 2 independent experiments, each of which gave similar results. *P < .01 versus corresponding values for PBS-treated mice; †P < .05 versus corresponding values for OVA-treated WT OTII CD4+ T-cell–injected Rag-1−/− mice or TNF−/− OTII CD4+ T-cell–injected Rag-1−/− mice.
Mast cell–derived TNF, but not T-cell–derived TNF, contributes to Th17 cell–mediated neutrophilic airway inflammation. (A-B) Mice were treated with 50 μg of OVA or PBS intranasally for 3 days (once per day). Cells (A) and cytokine levels (B) in BALF were quantified 24 hours after the last inhalation of OVA. Kit+/+ and wild-type OTII mice (PBS, n = 11; OVA, n = 15), KitW-sh/W-sh OTII mice (PBS, n = 13; OVA, n = 18), KitW-sh/W-sh OTII mice that were systemically engrafted with wild-type BMCMCs (KitW-sh/W-sh OTII + WT BMCMCs) (PBS, n = 5; OVA, n = 11), TNF−/− OTII mice (PBS, n = 9; OVA, n = 15) and KitW-sh/W-sh OTII mice that were systemically engrafted with TNF−/− BMCMCs (KitW-sh/W-sh OTII + TNF−/− BMCMCs) (PBS, n = 6; OVA, n = 11) (A-B). Data are the average + SD of results pooled from 3 independent experiments, all of which gave similar results. *P < .01 versus corresponding values for PBS-treated mice; †P < .05 versus corresponding values for each of the 3 other OVA-treated groups; ¶P < .01 versus corresponding values for OVA-treated Kit+/+ and wild-type OTII mice and KitW-sh/W-sh OTII mice plus WT BMCMCs; and §P < .05 versus corresponding values for OVA-treated TNF−/− OTII mice. (C) Rag-1−/− mice were injected intravenously with wild-type (WT), TNF−/−, or IL-17−/− OTII CD4+ T cells, and then the mice were treated with 50 μg of OVA or PBS intranasally for 3 days (once per day). Cells in BALF were quantified 24 hours after the last inhalation. WT OTII CD4+ T-cell–injected Rag-1−/− mice (PBS, n = 6; OVA, n = 10), TNF−/− OTII CD4+ T-cell–injected Rag-1−/− mice (PBS, n = 6; OVA, n = 10), and IL-17−/− OTII CD4+ T-cell–injected Rag-1−/− mice (PBS, n = 6; OVA, n = 8). Data are the average + SD of results pooled from 2 independent experiments, each of which gave similar results. *P < .01 versus corresponding values for PBS-treated mice; †P < .05 versus corresponding values for OVA-treated WT OTII CD4+ T-cell–injected Rag-1−/− mice or TNF−/− OTII CD4+ T-cell–injected Rag-1−/− mice.
These findings indicate that mast cell–derived TNF, but not mast cell–derived IL-18, is particularly important for the orchestration of Th17 cell–mediated neutrophilic airway inflammation in this model. On the other hand, neutrophil numbers in BALF after the last OVA inhalation were significantly less in TNF−/− OTII mice than in KitW-sh/W-sh OTII mice which had been systemically engrafted with TNF−/− BMCMCs (Figure 7A). This suggests that TNF produced by cell types in addition to mast cells also contributes to neutrophil influx in this setting.
Both Th1 cells and Th17 cells can produce TNF.33 We therefore investigated the possible role of T-cell–derived TNF in this model. CD4+ T cells from WT OTII or TNF−/− OTII mice were transferred to Rag-1−/− mice intravenously, and then the mice were treated with OVA or PBS intranasally for 3 days. After the last OVA inhalation (24 hours), neutrophil numbers in BALF were comparable in Rag-1−/− mice that had received WT or TNF−/− OTII CD4+ T cells (Figure 7C), whereas neutrophil numbers in BALF were significantly reduced in Rag-1−/− mice that had received IL-17−/− OTII CD4+ T cells (Figure 7C). However, in contrast with OTII mice that had been treated intranasally with OVA (in our usual “active” model of OVA-induced neutrophilic inflammation), the Rag-1−/− mice that had received OTII T cells adoptively (in the “passive” model) exhibited eosinophil infiltration after the last OVA inhalation (Figure 7C).
These findings suggest that at least some of the molecular mechanisms underlying the induction of airway inflammation in the “active” and “passive” models of OVA-induced airway inflammation used in this study probably differ. However, the results shown in Figure 7C clearly demonstrate, in the passive model of OVA-induced airway inflammation, that T-cell–derived IL-17, but not TNF, can contribute significantly to the OVA-dependent recruitment of neutrophils to the lungs in this setting.
Discussion
We used OVA-specific TCR-expressing C57BL/6-OTII mice, which have the advantage of containing a potentially very large population of Ag (OVA)–specific T cells, to develop a model of protein Ag-induced, T-cell–dependent airway neutrophilia, and to investigate the potential role of mast cells in this response. Our findings indicate that, in this model, neutrophil infiltration was mediated predominantly by IL-17–producing Th17 cells, not Th1 cells. By crossing the OTII mice with mice deficient in various cytokines, receptors, or mast cells, we showed that IL-17, IL-18, IL-1/IL-1R, TNF, mast cells and mast cell–associated TNF contributed significantly to the airway neutrophilia elicited in this model. Notably, we also found that IFN-γ can suppress the development of airway neutrophilia in OVA-challenged OTII mice, as has been reported in models of Th2-associated airway eosinophilia.34 Thus, our evidence also indicates that Th1 cells can negatively regulate aspects of Th17 cell and IL-17–dependent inflammation, at least in this model system. The results obtained in our analyses of the leukocytes present after OVA challenge in the BALF specimens derived from the many different types of cytokine-, receptor- or mast cell–deficient OVA-specific TCR-expressing OTII mice investigated in this model are summarized in Table S1.
This model is OVA dependent, as challenge of OTII mice with PBS or with an unrelated Ag (KLH) failed to elicit a robust neutrophil-rich inflammatory response. However, the pathogenesis of the OVA-induced, IL-17–associated neutrophil infiltration that is elicited in this model is probably complex. For example, certain cytokines (eg, IL-18, TNF, and IL-1β) can promote T cell–IL-17 production synergistically with IL-23,30,35,36 suggesting one mechanism that might contribute to OVA- and IL-17–dependent airway neutrophilia in vivo.
Our experiments suggest an additional mechanism that might also have a role in this setting. We found that IL-17 can enhance mouse mast cell TNF production in vitro (Figure 6B), and that mast cell–associated TNF in turn can contribute to Th17 cell–mediated airway neutrophilia in mice in vivo (Figure 7A). IL-17 levels in BALF from TNF−/− OTII mice were similar to those from TNF+/+ OTII mice, even though neutrophil levels in BALF from TNF−/− OTII mice were markedly reduced compared with those in TNF+/+ OTII mice (Figure 5B-C). These findings strongly suggest that TNF functions downstream of IL-17 in the development of this local inflammatory response.
The exact mechanism(s) of mast cell activation in this model remain(s) to be defined. However, it appears very unlikely that mast cells are being activated by OVA-specific IgE or IgG1 antibodies and specific Ag (OVA). The activation of mouse mast cells by either IgE plus Ag (via FcϵRI) or by IgG1 plus Ag-containing immune complexes (via FcγRIII) requires the FcRγ chain that is shared by FcϵRI and FcγRIII.15,16 The fact that deletion of the FcRγ chain did not influence the airway neutrophilia response to OVA in OTII mice (Figure 3A), together with the lack of detectable OVA-specific IgE or IgG1 antibodies in OTII mice after 3 days of OVA challenge (Figure 2B), indicates that mast cell function is recruited in this model independently of mast cell activation via either IgE plus Ag or OVA-IgG1 immune complexes.
Our findings thus establish that mast cells and mast cell–derived TNF can significantly enhance, by FcRγ-independent mechanisms, an Ag- and Th17 cell–dependent inflammatory response that is associated with significant neutrophil recruitment in the affected organ. Whether this observation pertains solely to conditions, such as those in the OTII mouse, in which very large populations of Ag-specific T cells are present, or is a more general phenomenon, is not yet clear. Similarly, more work will be required to characterize fully the conditions that can contribute to the elicitation of mast cell function in this OTII model. For example, many commercially obtained preparations of OVA contain LPS,28,29 mast cells can express TLR4 and other Toll-like receptors (TLRs), and stimulation of mast cells via TLR4 or other TLRs can elicit mast cell production of TNF and other cytokines.37,38
However, we found that the cell and cytokine requirements for the elicitation of LPS-induced airway neutrophilia in C57BL/6 mice differed markedly from those that contributed to the airway neutrophil infiltration induced by OVA challenge of C57BL/6-OTII mice. Even though other reports have indicated that both IL-1 and TNF can contribute to the airway neutrophilia induced by the instillation of E coli,39 we detected equivalent levels of neutrophils in the BALF of LPS (10 μg E coli–derived LPS, intranasally)–challenged WT C57BL/6 mice, C57BL/6-OTII mice, or C57BL/6 mice that lacked IL-17, IFN-γ, or T or B cells (Figure 3D), or that lacked IL-18, IL-1R1, TNF, or mast cells (Figure S1). By contrast, elicitation of optimal levels of Ag-induced airway neutrophilia in C57BL/6-OTII mice required IL-17 (Figure 3B), IL-18 (Figure 4A), IL-1R1 and TNF (Figure 4B), and mast cells and mast cell–derived TNF (Figure 7A).
These finding sclearly indicate that different mechanisms contribute to E coli–LPS–induced, as opposed to OVA-induced, neutrophil recruitment in the models tested. Nevertheless, LPSs derived from different species of bacteria can have distinct effects on immune responses.40 Accordingly, we cannot formally rule out the possibility that effects of LPS contaminating the OVA preparations used in our study may have influenced the results obtained in the OVA-challenged OTII mice, either through effects on mast cells and/or by other mechanisms.
In summary, this work identifies a previously unrecognized mechanism, namely, the enhancement of IL-17–dependent neutrophil infiltration by mast cell–derived TNF (and probably other mast cell–derived mediators), by which mast cells can significantly influence the expression of T-cell–associated immune responses. Our data were derived entirely from studies in mouse models, and primarily from analyses of OVA TCR-expressing C57BL/6-OTII mice. The extent to which such mast cell–dependent mechanisms may influence the expression of IL-17–dependent immune responses in other settings remains to be determined, and may well vary according to the particular biological response examined. However, it is worth mentioning here that work in human subjects both has suggested that neutrophils may be potentially important contributors to airway pathology in certain subsets of patients with asthma,41–48 and has identified IL-17 expression in the lungs of some subjects with this disorder.32,49–53 Therefore, it will be of interest to attempt to investigate whether the mechanisms that contribute to the mast cell–dependent enhancement of IL-17–dependent airway neutrophilia that have been revealed in our analyses of OTII mice have any bearing on the regulation of neutrophil recruitment that can occur in human asthma.
Authorship
Author contributions: S.N. and S.J.G. designed research; S.N., H.S., and G.J.B. performed research; S.N., H.S., G.J.B., and S.J.G analyzed data; and S.N. and S. J. G. wrote the paper.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Stephen J. Galli, Department of Pathology, L-235, Stanford University School of Medicine, 300 Pasteur Dr, Stanford, CA 94305-5324; e-mail: sgalli@stanford.edu; or Susumu Nakae, Department of Pathology, L-235, Stanford University School of Medicine, 300 Pasteur Dr, Stanford, CA 94305-5324; e-mail: nakae@stanford.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Drs Yoichiro Iwakura (Institute of Medical Science, University of Tokyo) for IL-17−/− mice, Jonathon D. Sedgwick (DNAX Research Inc. [Present address: Eli Lilly and Company]) for TNF−/− mice, Peter Besmer (Memorial Sloan-Kettering Cancer Center and Cornell University Graduate School of Medical Sciences) for KitW-sh/KitW-sh mice, R. Schwartz (National Institutes of Health) for OTII transgenic mice, Shosaku Narumi (School of Medicine, University of Tokyo) for anti-rat CXCL10 mAb, and Mamoru Kiniwa (Taiho Pharmaceutical Co Ltd) for OVA-specific Abs, and Glaxo Smith Kline for SB-209247. We also thank Dr Z.-S. Wang for excellent technical assistance, Dr Motoyasu Iikura for discussions regarding the histology, and all of the members of the Galli lab for helpful discussions.
Supported by United States Public Health Service grants (to S.J.G.) AI-23990, CA-72074, and HL-67674.

![Figure 2. Antigen-induced neutrophilic airway inflammation in OTII mice. Wild-type (WT) or OTII mice were treated with 50 μg of OVA or KLH in PBS, or PBS alone intranasally for 3 days (once per day). Data in panels A-C, E are the average + SD of results pooled from 2 independent experiments, each of which gave similar results (n = 7-10). (A) Cells in BALF were quantified 24 hours after the last inhalation of OVA, KLH, or PBS. *P < .01; †P < .01 versus corresponding values for PBS-, OVA-, or KLH-treated wild-type mice and PBS- or KLH-treated OTII mice, respectively. (B) OVA-specific IgG1 and IgE in sera collected from mice in panel A 24 hours after the last OVA or PBS inhalation. (C) Airway responses (Penh and lung resistance [RL]) to methacholine were assessed 24 hours after the last inhalation of OVA, KLH, or PBS, as in panel A; BL indicates baseline. *P < .05 versus corresponding values for each of the other groups (by analysis of variance). (D) BALF cells pooled from 20 OTII mice 24 hours after the last OVA inhalation were stimulated with PMA plus ionomycin in the presence of monensin, and then the intracellular cytokine profiles of CD4+ T cells (% positive cells shown in the figure) were determined by FACS. Results shown are representative of those obtained in 2 independent experiments, each of which gave similar results. (E) Cytokine levels in BALF obtained 24 hours after the last OVA or PBS inhalation in mice shown in panel A. *P < .01; †P < .01 versus corresponding values for PBS- or OVA-treated wild-type mice and PBS-treated OTII mice, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/9/10.1182_blood-2006-09-046128/4/m_zh80090700240002.jpeg?Expires=1769086991&Signature=qYyxSMBAppJZDdZ1-g9y~K-UOWJq8PUhmM8IaL6ZV3MVKtVixWUir2GjW3jz7WDwwQ9vPE~whzYTW3zEAzXkQS3ipeWaAdF3NxRjDzfJkZdIpgcLfxDQAolb6Jr2ISeSecn314PmWUSvxtEJnCRRi1OwDfrHsC-HoRk3CGcOHvge5KpGH7qmqEZFHuZu5hTMrGk10ZhHVzaXTB8Po8S7kAXpy0avbnDg57xf-gaNbhKJOGM8~ok-SMPfFnySQ5GLNOc5xiDEGMjBPE3L5e38YUhiU57ZKpamcowNVzllCzBR1a1TGP9cu8PT0M9bkC-D~pSVEX-dyDLKyWXSJWe2WQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal