Abstract
Imatinib mesylate (Gleevec) is effective therapy against Philadelphia chromosome–positive leukemia, but resistance develops in all phases of the disease. Bcr/Abl point mutations and other alterations reduce the kinase inhibitory activity of imatinib mesylate; thus, agents that target Bcr/Abl through unique mechanisms may be needed. Here we describe the activity of WP1130, a small molecule that specifically and rapidly down-regulates both wild-type and mutant Bcr/Abl protein without affecting bcr/abl gene expression in chronic myelogenous leukemia (CML) cells. Loss of Bcr/Abl protein correlated with the onset of apoptosis and reduced phosphorylation of Bcr/Abl substrates. WP1130 did not affect Hsp90/Hsp70 ratios within the cells and did not require the participation of the proteasomal pathway for loss of Bcr/Abl protein. WP1130 was more effective in reducing leukemic versus normal hematopoietic colony formation and strongly inhibited colony formation of cells derived from patients with T315I mutant Bcr/Abl–expressing CML in blast crisis. WP1130 suppressed the growth of K562 heterotransplanted tumors as well as both wild-type Bcr/Abl and T315I mutant Bcr/Abl–expressing BaF/3 cells transplanted into nude mice. Collectively, our results demonstrate that WP1130 reduces wild-type and T315I mutant Bcr/Abl protein levels in CML cells through a unique mechanism and may be useful in treating CML.
Introduction
Chronic myelogenous leukemia (CML) is a consequence of reciprocal translocation between chromosomes 9 and 22 resulting in the so-called Philadelphia chromosome,1,2 in which the first exon of the c-Abl gene is replaced with sequences of the breakpoint cluster region (bcr) gene3,4 to create the Bcr/Abl oncogene. The constitutively active kinase activity of Bcr/Abl in the cytosol contributes to its transforming function5 and drug resistance through activation of several key survival pathways, including the mitogen-activated protein kinase/extracellular signal-regulating kinase cascade, nuclear factor κB, and the signal transducer and activator of transcription (Stat) family.6-8
Previous studies showed that reducing intracellular levels of Bcr/Abl mRNA or protein led to inhibition of proliferation and clonogenic survival of Bcr/Abl-expressing leukemia cells.9,10 The introduction of imatinib mesylate (Gleevec; STI-571; CGP57148B; Novartis, East Hanover, NJ) revolutionized the treatment of CML, because it selectively inhibits the kinase activity of Bcr/Abl11 without adversely affecting normal cells. Imatinib mesylate is currently used as frontline therapy for CML and is effective in most cases. However, although imatinib mesylate produces treatment responses at both the hematologic and cytogenetic levels, a growing number of patients in blast crisis eventually experience relapse despite continued treatment with imatinib mesylate.12-14 Mutations within the kinase domain of Abl that interfere with the binding of the drug constitute a primary cause of resistance,15-22 although other mechanisms have been proposed. Many different approaches to overcome clinical resistance to imatinib mesylate have been described. Farnesyltransferase inhibitors such as SCH66336 and the proteasome inhibitor bortezomib (Velcade) were shown to have growth inhibitory effects on certain imatinib mesylate–resistant leukemias.23 The pyrido-pyrimidine–type kinase inhibitors PD166326 and SKI-60624 are active against common kinase-domain mutants of Bcr/Abl that cause resistance to imatinib mesylate. However, these agents do not affect the kinase activity of the T315I mutant, which sterically reduces drug/kinase affinity and prevents direct contact of these agents with the Bcr/Abl protein. Other kinase inhibitors such as PD180970 and CGP76030 show similar restrictions in affinity for the T315I mutant.25 Recently, second-generation compounds such as nilotinib (AMN107), with higher affinity for abl, or dasatinib (BMS-354825), with high affinity for both abl and src kinases, have been tested in phase 1 and 2 trials. Despite their effectiveness against many Bcr/Abl mutants in imatinib mesylate–resistant disease, these agents cannot suppress the kinase activity of T315 mutants, suggesting that the emergence of the T315 mutation in patients with CML will severely reduce the benefit of these kinase inhibitors.26,27 The novel compound ONO12380 was recently reported to inhibit Bcr/Abl kinase activity through a distinct mechanism in cell lines expressing the T315I mutation.28 However, this agent has not been tested in clinical studies. Thus, continuing to provide effective therapy for CML requires the development and testing of novel anti-Bcr/Abl agents that target Bcr/Abl through unique mechanisms that are not affected by mutations in the kinase domain.
One candidate agent, WP1130, is a second-generation tyrphostin derivative (degrasyn) discovered during screens for AG490-like molecules that suppress IL-6 and IL-3 activation of Stat molecules. WP1130 is thought to reduce cytokine-stimulated Stat activation through the rapid down-regulation of upstream Jak kinases (N.J.D., unpublished data, April 2005). We report here that in CML cells, WP1130 induced down-regulation of Bcr/Abl protein without affecting bcr-abl mRNA levels; it also caused rapid down-regulation (within 1 hour) of T315I Bcr/Abl protein but did not affect the stability of the Bcr or c-Abl proteins in CML cells. Down-regulation of Bcr/Abl by WP1130 also resulted in the loss of phosphorylation of Stat5 and the src kinase Hck, both downstream targets of Bcr/Abl, without affecting their expression levels. WP1130-induced down-regulation of Bcr/Abl was accompanied by apoptosis of CML cells. Finally, WP1130 suppressed colony formation by cells from patients with imatinib mesylate–responsive or –resistant disease with the T315I Bcr/Abl mutation and suppressed the growth of implanted K562 cells and BaF/3 cells expressing wild-type or T315I mutant Bcr/Abl in nude mice. Together, these results suggest that WP1130 may be useful in the treatment of Bcr/Abl-driven leukemias.
Materials and methods
Cell lines, antibodies, and drugs
BV173 (derived from a patient with CML in lymphoblastic crisis), BV173R (a clonal imatinib mesylate–resistant variant of BV173 expressing the T315I mutant Bcr/Abl; N.J.D., unpublished data, May 2004), and K562 (derived from a patient with CML in erythroid blast crisis) cells were grown in RPMI 1640 containing 10% heat-inactivated fetal bovine serum (FBS) and 2 mM glutamine. BaF/3 murine lymphoid cells (from Charles L. Sawyers, UCLA) and variants of BaF/3 stably expressing Bcr/Abl or the T315I, L364I, or E355G mutants of Bcr/Abl were grown in RPMI 1640 containing 10% heat-inactivated FBS and 2 mM glutamine with or without IL-3 (1 ng/mL). Primary antibodies used were antiphosphotyrosine (clone 4G10) (Upstate Biotechnology, Lake Placid, NY); anti-Stat5, anti-CrkL, anti-pCrkL, anti-Hsp90, anti-Hsp70, and anti-bcr (Cell Signaling Technologies, Danvers, MA); anti-pStat5 A/B (Tyr 694/699), antiactin (Sigma, St Louis, MO), anti-Hck, and anti-pHck (Santa Cruz Biotechnology, Santa Cruz, CA); and anti-Abl [8E-9 (PharMingen, San Diego, CA)]. Secondary antibodies were peroxidase-conjugated affiniPure Goat Anti-Mouse and Anti-Rabbit antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). WP1130 and imatinib mesylate were synthesized at M. D. Anderson Cancer Center. Dasatinib was kindly provided by Dr Francis Lee (Bristol-Myers Squibb, Princeton, NJ). Inhibitors were N-acetyl-L-leucyl-leucyl-L-norleucinal (LLnL; 100 μM), MG-132 (40 μM), ammonium chloride (2.5 mM), antipain (20 μM), and zVAD (40 μM).
Generation of stable Ba/F3 Bcr/Abl T315I mutant–expressing cells
BaF/3 cells growing in RPMI containing 10% FBS and 1 ng/mL IL-3 were harvested and washed in 1 × phosphate-buffered saline (PBS); 2 × 106 cells were transfected by electroporation (AMAXA Biosystems, Gaithersburg, MD) with 5 μg cDNA representing wild-type Bcr/Abl (pSG-Bcr/Abl; kindly provided by Dr Ralph Arlinghaus, Department of Molecular Pathology, M. D. Anderson) or the Bcr/Abl T315I mutant (introduced by site-directed mutagenesis [Stratagene Quickchange II XL kit; Stratagene, La Jolla, CA] and confirmed by direct sequencing). Transfected cells were incubated in 2 mL RPMI/10% FBS and 1 ng/mL IL-3 in a 6-well dish for 24 hours, washed in PBS, and further incubated in RPMI medium supplemented with 10% FBS but lacking IL-3. Viable colonies that had been cultured in IL-3–negative medium for 4 weeks were screened for the expression of Bcr/Abl by Western blotting. Expression of the T315I mutant was confirmed by loss of imatinib mesylate–mediated apoptosis and Bcr/Abl kinase inhibition (assessed by immunoblotting) in the transfectants.
Isolation of cellular fractions from clinical specimens
Mononuclear cells were isolated from peripheral blood or bone marrow of patients with imatinib mesylate–resistant CML after informed consent was obtained (as approved by the institutional review board of M. D. Anderson). Cells were purified on Ficoll-Hypaque gradients as previously described.29
Quantification of apoptosis
Hypodiploidy was measured in treated and control cells by propidium iodide staining and fluorescence-activated cell sorting (FACS). K562 cells were seeded in 6-well plates (2 × 106 cells/well) for 1 day before being treated with 5 μM WP1130. Cells were harvested at 0, 24, 48, and 72 hours after treatment; washed twice with PBS; resuspended in 420 μL PBS, and then 980 μL cold 100% ethanol was added drop-wise into each tube while the tubes were being vortexed at slow speed. Ethanol-fixed cells were stored at −20°C until needed. Fixed cells were centrifuged between 10 000 g and 12 000 g for 5 minutes, and the pellet was resuspended in 500 μL PBS/RNase (final concentration, 0.1 mg/mL), incubated at 37°C for 15 minutes, mixed with 500 μL PBS containing propidium iodide (final concentration 25 μg/mL), and analyzed with a FACScan cytofluorometer (Becton Dickinson, San Jose, CA).
MTT (viability) assay
CML cells were seeded in 96-well plates (2 × 104 cells/well) and WP1130, imatinib mesylate, or dasatinib (0.08-10 μM) were added in a final volume of 100 μL medium. Plates were incubated at 37°C for 72 hours, after which 20 μL of 3-4,5-dimethylthiazolyl-2-2, 5-diphenyltetrazolium bromide (MTT) reagent (Sigma; stock 5 mg/mL) was added, and the plates were incubated at 37°C for another 2 hours. Cells were lysed with 100 μL lysis buffer (20% sodium dodecyl sulfate [SDS] in 50% N, N-dimethylformamide adjusted to pH 4.7 with 80% acetic acid and 1 M hydrochloric acid; final concentration of acetic acid was 2.5% and hydrochloric acid was 2.5%) and incubated for 6 hours. The optical density of each sample at 570 nm was determined with a SPECTRA MAX M2 plate reader (Molecular Devices, Sunnyvale, CA).
Western blot analysis
Cell pellets were resuspended in modified RIPA lysis buffer (10 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-hydrochloric acid [pH 7.5]) with inhibitors (20 μg/mL aprotinin, 1 mM sodium fluoride, 2 mM sodium orthovanadate, 0.5 mM phenylmethanesulfonyl fluoride, and 250 mg/mL benzamidine) in ice for 30 minutes and centrifuged at 15 000 g for 30 minutes to collect whole cell lysates. Whole cell lysates (50-60 μg) were run on 10% SDS–polyacrylamide gel electrophoresis (PAGE) gels and transferred to a polyvinylidene difluoride membrane. Western blotting was done with specific primary antibodies and peroxidase-conjugated affiniPure anti-Mouse and anti-Rabbit secondary antibodies (Jackson ImmunoResearch Laboratories). Proteins were visualized with ECL Plus enhanced chemiluminescence reagents (Amersham Biosciences, Piscataway, NJ).
siRNA transfection
K562 cells (2.5 × 106) were transfected with siBcr/Abl (GCAGAGUUCAAAAGCCCUUUU [b3a2-1]) or a scrambled siRNA duplex (siControl) at a final siRNA concentration of 100 nM by electroporation (AMAXA Biosystems). Transfected cells were incubated in 2 mL RPMI medium supplemented with 10% FBS in 6-well culture plates and harvested at 24, 48, 72, or 96 hours after transfection; Bcr/Abl protein levels were determined by Western blotting. The b3a2-1 oligonucleotide30 covers the fusion sequence of the b3a2 variant of the bcr-abl gene.
Real-time quantitative reverse transcriptase–polymerase chain reaction (PCR) analysis of bcr/abl mRNA
Total RNA was isolated by lysing 5 to 10 × 106 cells with 1 mL TRIzol reagent (Invitrogen, Carlsbad, CA) for 5 minutes at room temperature. Chloroform (200 μL) was mixed with the cell lysate for 30 seconds, and the mixture was centrifuged at 15 000 g for 15 minutes. The supernatant was transferred into a fresh tube, and the RNA was precipitated by adding 500 μL isopropanol. After 5 minutes of mixing at room temperature and a 15-minute centrifugation at 15 000 g, the pellet was washed in 75% alcohol in diethylpyrocarbonate-treated water and centrifuged at 15 000 g for 15 minutes. The washed pellet was air-dried and resuspended in 30 to 50 μL RNase-free water. cDNA was synthesized from 1 μg RNA with the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. Real-time PCR was done with an iCycler iQ thermocycler (Bio-Rad). Ubiquitin forward primer (5′-ACCTGACCAGCAGCGTCTGATATT-3′), reverse primer (5′-TCGCAGTTGTATTTCTGG GCAAGC-3′) and a probe as well as the p210 bcr/abl (b3a2-1) forward primer (5′-CTGGCCCAACGATGGCGA-3′), reverse primer (5′-CACTCAGACCCTG AGCCTCAA-3′), and a probe were used in the reactions. All primers were synthesized by Sigma; probes were synthesized by Bio-Rad.
AML/ALL blast colony assays
Acute myeloblastic leukemia (AML) and acute lymphoblastic leukemia (ALL) blast colony assays were done with cells from patients with CML in myeloid or lymphoid blast crisis. For the AML blast colony assay,31,32 2 × 105 nonadherent T-cell–depleted marrow cells were plated in 0.8% methylcellulose in medium supplemented with 10% FBS and 50 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (Immunex Corp, Seattle, WA). WP1130 was diluted in isotonic 5% dextrose solution and added at the initiation of the cultures at 0.5 to 5 μM. The ALL blast colony assay, for cells obtained from patients with CML in lymphoid blast crisis, is a modification of a previous technique.33 Briefly, 1 × 105 nonadherent T-cell–depleted marrow cells were plated in 0.8% methylcellulose in α-medium supplemented with 30% FBS and irradiated (70 Gy). Normal donor peripheral blood low-density cells were used as feeder cells. WP1130 was dissolved in dimethylsulfoxide (DMSO) and added at the initiation of the cultures at 0.5 to 5 μM. Cultures for both assays were incubated in 35-mm Petri dishes in triplicate for 7 days at 37°C in a humidified, 5% CO2 atmosphere. Blast colonies were microscopically evaluated on day 7. A blast colony was defined as a cluster of 20 or more cells. Individual colonies were plucked, smeared on glass slides, and stained to confirm their leukemic cell composition.
Bone marrow colony assay
Normal bone marrow was voluntarily donated by bone marrow transplant donors. The granulocyte-erythrocyte-megakaryocyte-macrophage colony-forming unit assay was performed as previously described.34 CD34+ cells were positively selected with anti-CD34 magnetic beads in a magnetic-activated cell sorter system (Miltenyi Biotec, Auburn, CA). WP1130 was added to the medium, and cultures were evaluated at 14 days for the number of cell colonies.
Immune-complex kinase assay
Immune-complex kinase assays were done as previously described29 with slight modification. Bcr/Abl was immunoprecipitated from K562 cell lysate (3 mg total protein) with polyclonal anti-abl (Santa Cruz Biotechnology) and Protein A-Sepharose (Amersham). Immune complexes were washed 3 times in lysis buffer, divided into 5 equal aliquots, and each resuspended in 45 μL kinase buffer consisting of 20 mM HEPES (pH 7.5), 5 mM MgCl2, 5 mM MnCl2, and 0.1 mM sodium vanadate. Imatinib mesylate, WP1130, or 0.5 μL DMSO was then added. Kinase activity was initiated by the addition of 0.1 mM ATP, and samples (50 μL total volume) were incubated for 30 minutes at 30°C. Kinase activity was quenched by adding boiling SDS sample buffer, and after 5 minutes at 100°C proteins were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and immunoblotted with anti-pY. Phosphorylated BCR-ABL was detected with secondary antibody and enhanced chemiluminescence reagent as described in “Western blot analysis.”
Antitumor studies
All animal procedures were approved by the institutional review board at M.D. Anderson. Two models were used to test the in vivo efficacy of WP1130 against CML cells or BaF/3 cells that had been transformed by Bcr/Abl. Swiss Nu/Nu mice (5-7 weeks old) were obtained from the breeding facility at the Department of Experimental Radiation Oncology at M. D. Anderson. For the first study, K562 tumor cells were heterotransplanted into nude mice on day 0 as follows. Cells were suspended at 5 × 106 cells/mL in Matrigel (Becton Dickinson); 1 volume of cells was mixed with 1 volume of cold Matrigel, and 200 μL of this suspension was injected subcutaneously into the midline dorsal region. Mice were randomly assigned to 1 of 3 groups (5 mice per group), and on day 10 WP1130 or imatinib mesylate, in a 0.1-mL suspension of 1:1 dimethylsulfoxide to polyethylene glycol 300, was injected intraperitoneally into tumor-bearing mice. Mice were treated for 1.5 weeks as follows: WP1130 (30 mg/kg), every other day for 5 injections; imatinib mesylate (50 mg/kg), every day for a total of 9 injections; or vehicle alone (0.1-mL suspension of 1:1 dimethylsulfoxide:polyethylene glycol 300), every other day for 5 injections. Tumor volumes were measured with calipers (Cel Associates, Houston, TX) every other day.
To assess activity against T315I Bcr/Abl mutant cells, BaF/3 cells transfected with wild-type Bcr/Abl or the T315I mutant were transplanted into female Swiss nude mice (5-6 weeks old). BaF/3wt cells were suspended (5 × 107 cells/mL) in RPMI medium, and 0.1 mL of this suspension was injected intravenously into 10 mice. The same procedure was used to inoculate 10 mice with BaF/3/T315I cells. One day after tumor cell inoculation, mice were randomly assigned to 1 of 3 groups: one group received WP1130 (40 mg/kg, intraperitoneally) in 50 μL DMSO/PEG300 (vehicle) every other day (7 injections); another received imatinib mesylate (100 mg/kg, intraperitoneally) in vehicle daily (14 injections); and the third received vehicle alone daily (14 injections). Spleens from each mouse were harvested, photographed, and weighed 15 days after tumor cell inoculation.
Results
The low-molecular-weight compound WP1130 (Figure 1A) was originally identified during a screen of a small chemical library for agents that inhibit IL-6–mediated Stat3 phosphorylation (N.J.D., unpublished data, April 2001). Structure-activity studies led to the chemical design and synthesis of WP1130, also called degrasyn (a synthetic inducer of protein degradation), to define its major mechanism of action. Investigations of the specificity of WP1130 revealed that it induced rapid down-regulation of Bcr/Abl without affecting Bcr or c-Abl. Studies in other tumor cell types demonstrated that WP1130 also regulates the stability of Jak2 and c-Myc without affecting other kinases (HER1, HER2, c-kit, Fak, Erk1, Erk2, Akt, Btk, Src, and Src-related kinases) or transcription factors (wild-type p53, Stat1, Stat3, Stat5, c-Jun, NF-κB, Max). In addition, the kinase regulatory pathway that leads to reduction of Bcr/Abl and Jak2 protein levels in WP1130-treated myeloid and lymphoid cells seems similar but is distinct from its effects on c-Myc. Cytotoxicity studies demonstrated that WP1130 is more effective in inducing apoptosis of myeloid and lymphoid tumor cells (IC50 ∼ 0.5 to 2.5 μM) than normal CD34+ hematopoietic precursors, dermal fibroblasts, or endothelial cells (IC50 ∼ 5 to 10 μM) (data not shown). Here, we compared the antileukemic and anti-Bcr/Abl activity of WP1130 with that of the Bcr/Abl kinase inhibitor imatinib mesylate. Our results suggest that WP1130 reduces Bcr/Abl protein levels through a unique mechanism that is not affected by mutations that interfere with imatinib mesylate binding.
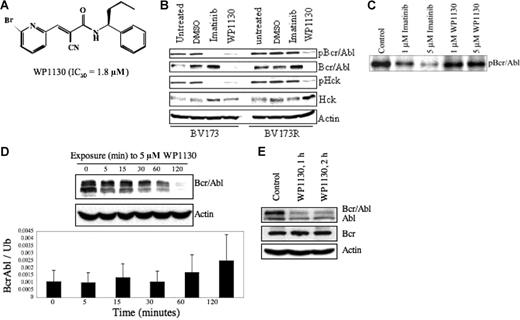
WP1130 inhibits the autoactivation of Bcr/Abl by inducing its rapid down-regulation. (A) Chemical structure of WP1130. The IC50 of Bcr/Abl-expressing cell lines (K562, BV-173) is also noted. (B) The BV173 (wild-type Bcr/Abl) and BV173R (T315I Bcr/Abl mutant) CML cell lines (1 × 106 cells/mL each) were treated with 5 μM imatinib mesylate or WP1130 for 1 hour. Cells were harvested, and the expression of Bcr/Abl, pY-Bcr/Abl, Hck, and pY396-Hck were determined by Western blotting. Actin was blotted as a protein-loading control. Controls were cells exposed to a comparable volume of solvent (DMSO) for 1 hour. (C) Immune-complex kinase assays were performed with Bcr/Abl isolated from K562 cells and incubated with the indicated agent for 30 minutes at 30°C in the presence of unlabeled ATP. Bcr/Abl autophosphorylation was determined by immunoblotting the resolved proteins for phosphotyrosine. (D) K562 CML cells (1 × 106 cells/mL) were treated with 5 μM WP1130 for the indicated times and subjected to Western blotting to assess Bcr/Abl protein expression levels over the 120-minute treatment period (top). Actin was used as a protein-loading control. Real-time PCR analysis of mRNA extracted from the harvested cells (bottom) was carried out to examine the effect of WP1130 on transcription of the bcr/abl gene. Results are expressed as mean ± standard error of 3 independent experiments. (E) K562 CML cells (1 × 106 cells/mL) were treated with 5 mM WP1130 for 1 hour or 2 hours, and the expression of Bcr/Abl, c-Abl, and Bcr proteins in treated and untreated cells was determined by Western blotting. Actin was used as a protein-loading control.
WP1130 inhibits the autoactivation of Bcr/Abl by inducing its rapid down-regulation. (A) Chemical structure of WP1130. The IC50 of Bcr/Abl-expressing cell lines (K562, BV-173) is also noted. (B) The BV173 (wild-type Bcr/Abl) and BV173R (T315I Bcr/Abl mutant) CML cell lines (1 × 106 cells/mL each) were treated with 5 μM imatinib mesylate or WP1130 for 1 hour. Cells were harvested, and the expression of Bcr/Abl, pY-Bcr/Abl, Hck, and pY396-Hck were determined by Western blotting. Actin was blotted as a protein-loading control. Controls were cells exposed to a comparable volume of solvent (DMSO) for 1 hour. (C) Immune-complex kinase assays were performed with Bcr/Abl isolated from K562 cells and incubated with the indicated agent for 30 minutes at 30°C in the presence of unlabeled ATP. Bcr/Abl autophosphorylation was determined by immunoblotting the resolved proteins for phosphotyrosine. (D) K562 CML cells (1 × 106 cells/mL) were treated with 5 μM WP1130 for the indicated times and subjected to Western blotting to assess Bcr/Abl protein expression levels over the 120-minute treatment period (top). Actin was used as a protein-loading control. Real-time PCR analysis of mRNA extracted from the harvested cells (bottom) was carried out to examine the effect of WP1130 on transcription of the bcr/abl gene. Results are expressed as mean ± standard error of 3 independent experiments. (E) K562 CML cells (1 × 106 cells/mL) were treated with 5 mM WP1130 for 1 hour or 2 hours, and the expression of Bcr/Abl, c-Abl, and Bcr proteins in treated and untreated cells was determined by Western blotting. Actin was used as a protein-loading control.
WP1130 reduces kinase activity of both wild-type and T315I mutant Bcr/Abl by inducing rapid down-regulation of Bcr/Abl protein
First, we examined the activity of WP1130 against Bcr/Abl and the role of tyrosine kinase inhibition in its mechanism of action in cells that express either wild-type (BV173) or T35I mutant Bcr/Abl (BV173R). Cells were treated with 5 μM imatinib mesylate or 5 μM WP1130, and cellular extracts were analyzed for phosphotyrosine content and activation of Bcr/Abl and Hck, a src-related kinase regulated by Bcr/Abl in CML cells.35,36 WP1130 inhibited phosphorylation of both the wild-type and the T315I mutant Bcr/Abl proteins, as demonstrated by the rapid disappearance (within 1 hour) of phosphotyrosyl-Bcr/Abl in both BV173 and BV173R cells (Figure 1B). Direct assessment of Bcr/Abl protein levels demonstrated that the reduction in tyrosine phosphorylation was due to the down-regulation of Bcr/Abl protein. The corresponding loss of HCK phosphorylation suggested that WP1130-induced Bcr/Abl down-regulation inhibits Bcr/Abl signaling (Figure 1B). As expected, imatinib mesylate inhibited the phosphorylation of wild-type Bcr/Abl and Hck in BV173 cells but did not affect Bcr/Abl or Hck activation in the T3151 mutant BV173R cells (Figure 1B). These results suggest that WP1130 induces rapid down-regulation of Bcr/Abl and inhibits Bcr/Abl signaling in cells expressing either wild-type or the T315I mutant form of Bcr/Abl.
Because WP1130 is chemically and structurally similar to the Jak2 kinase inhibitor AG490, we assessed the direct effects of WP1130 on Bcr/Abl kinase activity in an immune-complex kinase assay. WP1130, at concentrations necessary to reduce Bcr/Abl levels in intact cells (1-5 μM), did not significantly inhibit Bcr/Abl autophosphorylation, whereas imatinib mesylate did (Figure 1C). We conclude that WP1130 does not reduce Bcr/Abl protein levels by directly interfering with its kinase activity.
WP1130 does not affect bcr/abl transcription or promote proteasomal degradation of Bcr/Abl protein
To investigate the mechanism by which WP1130 affects Bcr/Abl protein levels, we assessed the effects of WP1130 on bcr-abl gene expression and the role of proteasome activity in Bcr/Abl down-regulation. To determine the effects of WP1130 on bcr-abl gene expression, we harvested K562 cells after 0, 5, 15, 30, 60, or 120 minutes of treatment with 5 μM WP1130, measured the ratios of bcr/abl to ubiquitin mRNA transcript levels by real-time PCR, and, in parallel, tested Bcr/Abl protein expression levels by Western blotting (Figure 1D). WP1130 induced down-regulation of the Bcr/Abl protein but was not accompanied by a corresponding decrease in expression of the Bcr/Abl transcript. Nimmanapalli et al37 showed that a 48-hour exposure to arsenic trioxide (Trisenox) induced down-regulation of Bcr/Abl through translational inhibition of Bcr/Abl. Because the half-life of Bcr/Abl protein in CML cells is approximately 40 hours, it seems unlikely that WP1130 acts by translational inhibition of Bcr/Abl in K562 cells; rather, WP1130 may have a direct role in inducing down-regulation of the Bcr/Abl protein. Moreover, WP1130 had no effect on Bcr protein levels and had only limited effects on c-Abl protein levels (Figure 1E), suggesting that the WP1130-induced down-regulation of Bcr/Abl is mediated by a novel mechanism that recognizes a post-translational modification of the Bcr/Abl fusion protein or is targeted by WP1130 because of its subcellular distribution.
Mechanism of WP1130-induced Bcr/Abl down-regulation
To determine whether WP1130-induced down-regulation of Bcr/Abl is proteasome dependent, we treated BV173 and BV173R cells with a proteasomal inhibitor (MG-132; 40 μM) 2 hours before adding WP1130. Inhibition of proteasomal activity did not inhibit WP1130-induced down-regulation of Bcr/Abl or protect against loss of activation of Bcr/Abl downstream targets Stat5 and Hck (Figure 2A). However, inhibition of proteasomal activity protected c-Myc against WP1130-induced degradation in MM-1 multiple myeloma cells (Figure 2B). These results indicate that Bcr/Abl down-regulation by WP1130 is proteasome independent and distinct from the activity of WP1130 against c-Myc.
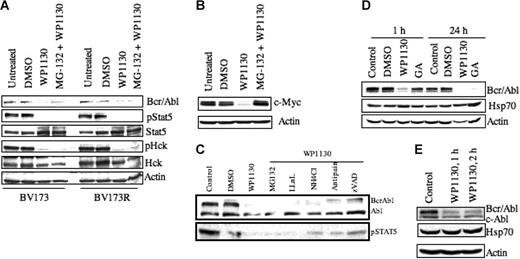
Down-regulation of Bcr/Abl protein by WP1130 is not associated with changes in Hsp70 and is not suppressed by protease inhibition. (A) BV173 CML cells (wild-type Bcr/Abl) and BV173R cells (T315I Bcr/Abl mutant) (1 × 106 cells/mL) were treated with 5 μM WP1130 for 1 hour or 40 μM of the proteasomal inhibitor MG-132 for 2 hours before treatment with WP1130 (5 μM) for 1 hour. Cells were harvested, and the expression of Bcr/Abl, pStat5, Stat5, pHck, and Hck was determined by Western blotting. Actin was used as a protein-loading control. (B) MM-1 multiple myeloma cells were treated with 5 μM WP1130 for 1 hour or pretreated with 40 μM MG-132 for 2 hours before treatment with WP1130 for 1 hour. Untreated and vehicle-treated (DMSO) cells served as controls. Cell lysates were immunoblotted for c-Myc. Actin was used as a protein-loading control. (C) K562 cells (1 × 106) were treated with 5 μM WP1130 for 1 hour or pretreated with protease inhibitors (40 μM MG-132, 100 μM LLnL, 2500 μM NH4Cl, 20 μM antipain, 40 μM zVAD) for 2 hours before incubation with WP1130 for 1 hour. Lysates were immunoblotted with anti-Abl to detect Bcr/Abl and c-Abl. pStat5 was also immunoblotted to determine the effect of inhibitors on Bcr/Abl signaling. (D) K562 cells (1 × 106) were treated with 5 μM WP1130 or 5 μM geldanamycin (GA) for 1 hour or 24 hours before analyzing lysates for expression of Bcr/Abl and Hsp70 protein by Western blotting. Actin was used as a protein-loading control. (E) K562 cells (1 × 106) were treated with 5 μM WP1130 for 1 hour or 2 hours, and the expression of Bcr/Abl and Hsp70 proteins in the treated and untreated cells was determined by Western blotting. Actin was used as a protein-loading control.
Down-regulation of Bcr/Abl protein by WP1130 is not associated with changes in Hsp70 and is not suppressed by protease inhibition. (A) BV173 CML cells (wild-type Bcr/Abl) and BV173R cells (T315I Bcr/Abl mutant) (1 × 106 cells/mL) were treated with 5 μM WP1130 for 1 hour or 40 μM of the proteasomal inhibitor MG-132 for 2 hours before treatment with WP1130 (5 μM) for 1 hour. Cells were harvested, and the expression of Bcr/Abl, pStat5, Stat5, pHck, and Hck was determined by Western blotting. Actin was used as a protein-loading control. (B) MM-1 multiple myeloma cells were treated with 5 μM WP1130 for 1 hour or pretreated with 40 μM MG-132 for 2 hours before treatment with WP1130 for 1 hour. Untreated and vehicle-treated (DMSO) cells served as controls. Cell lysates were immunoblotted for c-Myc. Actin was used as a protein-loading control. (C) K562 cells (1 × 106) were treated with 5 μM WP1130 for 1 hour or pretreated with protease inhibitors (40 μM MG-132, 100 μM LLnL, 2500 μM NH4Cl, 20 μM antipain, 40 μM zVAD) for 2 hours before incubation with WP1130 for 1 hour. Lysates were immunoblotted with anti-Abl to detect Bcr/Abl and c-Abl. pStat5 was also immunoblotted to determine the effect of inhibitors on Bcr/Abl signaling. (D) K562 cells (1 × 106) were treated with 5 μM WP1130 or 5 μM geldanamycin (GA) for 1 hour or 24 hours before analyzing lysates for expression of Bcr/Abl and Hsp70 protein by Western blotting. Actin was used as a protein-loading control. (E) K562 cells (1 × 106) were treated with 5 μM WP1130 for 1 hour or 2 hours, and the expression of Bcr/Abl and Hsp70 proteins in the treated and untreated cells was determined by Western blotting. Actin was used as a protein-loading control.
Pretreatment of K562 cells with a variety of protease inhibitors, including LLnL, ammonium chloride, antipain, or the pan-caspase inhibitor zVAD before incubation with WP1130 failed to rescue WP1130-induced Bcr/Abl down-regulation (Figure 2C). These results suggest that WP1130 induces Bcr/Abl down-regulation by activating a novel regulatory pathway.
Bcr/Abl protein stability is regulated by its association with the chaperone heat shock protein Hsp90. The benzoquinone ansamycin antibiotic geldanamycin binds strongly to Hsp90 and competes for ATP binding, disrupting its chaperone activity.38 Inactivation of Hsp90 is followed by an increase in Hsp70 expression and a shift in the binding of Bcr/Abl from Hsp90 to Hsp70,39 which reduces the stability of Bcr/Abl. To assess the possible involvement of an Hsp90/Hsp70 mechanism in the action of WP1130, we compared the effects of WP1130 and geldanamycin on Bcr/Abl down-regulation. K562 cells were treated with 5 μM WP1130 or 5 μM geldanamycin (a concentration that reduces Bcr/Abl levels in K562 cells39 ). After 1 hour of treatment, WP1130, but not geldanamycin, reduced Bcr/Abl protein levels (Figure 2D). After 24 hours, Bcr/Abl down-regulation was observed in cells treated with either compound. As described previously,39 geldanamycin-induced degradation of Bcr/Abl at 24 hours was accompanied by an increase in Hsp70 (Figure 2D), whereas WP1130-induced Bcr/Abl degradation was not (Figure 2D–E). These results suggest that the WP1130-induced rapid down-regulation of Bcr/Abl is distinct from the effects of geldanamycin and is not associated with Hsp70 induction.
WP1130 suppresses growth of wild-type or imatinib mesylate–resistant BaF/3 cells and colony formation of primary CML cells from patients with imatinib mesylate–resistant disease
Next, we compared the antileukemic activity of WP1130 with that of imatinib mesylate in BaF/3 cells expressing either wild-type Bcr/Abl or the T315I, L364I, or E355G Bcr/Abl kinase mutants. BaF/3 cells expressing wild-type Bcr/Abl were sensitive to imatinib mesylate, but BaF/3 cells expressing Bcr/Abl kinase mutations expressed various levels of resistance to imatinib mesylate (Figure 3A). BaF/3 cells expressing either wild-type or mutated Bcr/Abl were equally sensitive to WP1130, indicating that Bcr/Abl kinase mutations, including T315I, do not affect cellular sensitivity to this agent (Figure 3A). Analysis of the effects of WP1130 on IL-3–dependent parental BaF/3 cells indicated that WP1130 blocks Stat5 activation by rapidly (2 hours) down-regulating Jak2 protein (Figure 3B; V.K., manuscript in preparation). Because parental BaF/3 cells depend on IL-3 and Jak2 activation for survival, growth inhibition of parental BaF/3 cells by WP1130 is likely due to WP1130-mediated inactivation of the IL-3/Jak2/Stat5 signal transduction cascade.
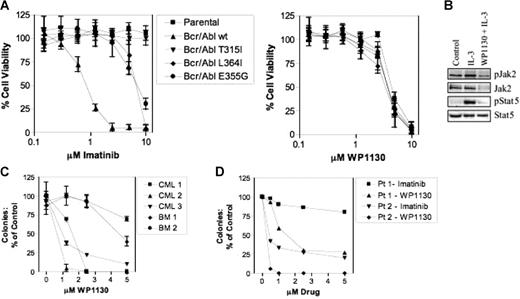
WP1130 reduces the viability of cells expressing wild-type and mutant Bcr/Abl. (A) BaF/3 cells (1 × 104) expressing wild-type or mutant Bcr/Abl (T315I, L364I, E355G) were treated with the indicated concentration of WP1130 or imatinib mesylate for 72 hours before analysis of cell viability (MTT staining). IL-3–dependent BaF/3 cells (parental) were also screened for drug sensitivity. The results represent the average ± SEM of 4 assays. (B) BaF/3 (2 × 106) (parental) cells were treated with IL-3 (1 ng/mL) alone or pretreated for 2 hours with 5 μM WP1130 before IL-3 incubation for 15 minutes. Cell lysates were prepared, and Jak2 was immunoprecipitated and immunoblotted with anti-pY (top) followed by anti-Jak2 (bottom). Total cell lysates were also blotted for pStat5 and Stat5. (C) CD34+ cells from 2 bone marrow donors (BM) or 3 patients with newly diagnosed CML were cultured with WP1130 at the concentration indicated before analysis of effects on colony formation as described in “Materials and methods.” Results are reported as the percentage of colonies in control samples (vehicle alone). Analysis was performed in triplicate, and the results are presented as mean ± SEM. (D) Fractionated bone marrow cells obtained from patients in CML myeloid and lymphoid blast crisis were cultured in AML and ALL colony culture assays, respectively. Data are reported as the percentage of control of duplicate cultures. Patient 1 was in lymphoid blast crisis and patient 2 was in myeloid blast crisis.
WP1130 reduces the viability of cells expressing wild-type and mutant Bcr/Abl. (A) BaF/3 cells (1 × 104) expressing wild-type or mutant Bcr/Abl (T315I, L364I, E355G) were treated with the indicated concentration of WP1130 or imatinib mesylate for 72 hours before analysis of cell viability (MTT staining). IL-3–dependent BaF/3 cells (parental) were also screened for drug sensitivity. The results represent the average ± SEM of 4 assays. (B) BaF/3 (2 × 106) (parental) cells were treated with IL-3 (1 ng/mL) alone or pretreated for 2 hours with 5 μM WP1130 before IL-3 incubation for 15 minutes. Cell lysates were prepared, and Jak2 was immunoprecipitated and immunoblotted with anti-pY (top) followed by anti-Jak2 (bottom). Total cell lysates were also blotted for pStat5 and Stat5. (C) CD34+ cells from 2 bone marrow donors (BM) or 3 patients with newly diagnosed CML were cultured with WP1130 at the concentration indicated before analysis of effects on colony formation as described in “Materials and methods.” Results are reported as the percentage of colonies in control samples (vehicle alone). Analysis was performed in triplicate, and the results are presented as mean ± SEM. (D) Fractionated bone marrow cells obtained from patients in CML myeloid and lymphoid blast crisis were cultured in AML and ALL colony culture assays, respectively. Data are reported as the percentage of control of duplicate cultures. Patient 1 was in lymphoid blast crisis and patient 2 was in myeloid blast crisis.
Because Jak2 participates in normal hematopoiesis, the dose-dependent effects of WP1130 on colony formation by normal CD34+ cells (from bone marrow donors) were compared with those of leukemic cells (from patients with newly diagnosed CML) (Figure 3C). WP1130 was more effective in reducing leukemic cell colony formation (effective inhibition at 1.25-2.5 μM); higher concentrations were required to reduce colony numbers from normal progenitor cells. The suppressive activity of WP1130 on normal hematopoietic progenitor cells may be mediated through inhibition of Jak2/Stat5 signaling (as described in IL-3–dependent BaF/3 cells; Figure 3B). However, these results suggest that the differential activity of WP1130 against Bcr/Abl–dependent leukemic cells may be exploited for CML therapy.
To determine whether WP1130 is effective against primary leukemic cells harboring the T315I mutation, we tested the clonogenic capacity of primary cells isolated from patients with CML in lymphoid (Pt 1) or myeloid blast crisis (Pt 2) with imatinib mesylate–resistant disease owing to expression of the T315I mutant form of Bcr/Abl. Primary cells were exposed to imatinib mesylate or WP1130 at various concentrations and subjected to AML or ALL blast colony assays (Figure 3D). Colony formation was significantly inhibited by WP1130 at concentrations as low as 0.5 μM. In contrast, cells grown in the presence of imatinib mesylate showed 25% to 75% inhibition of colony growth at 5 μM. These results provide further evidence that human CML cells expressing the Bcr/Abl T315I mutation are considerably more sensitive to WP1130 than imatinib mesylate. However, we cannot rule out the possibility that WP1130 affects both Bcr/Abl and other signaling modules (eg, Jak2, c-Myc) in leukemic cells to suppress their growth and survival.
Antileukemic effect of WP1130 is due to the induction of apoptosis
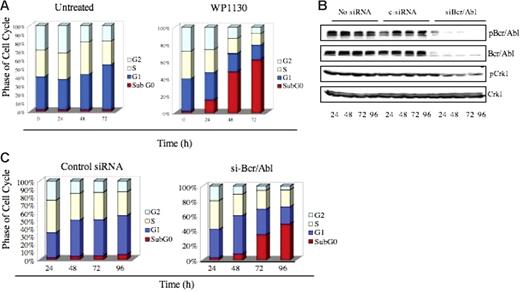
We observed a rapid induction of apoptosis 24 to 48 hours after WP1130 (5 μM) treatment of K562 cells, evident as an increase in the subG0 population (Figure 4A). To determine whether loss of the Bcr/Abl protein correlates with the induction of apoptosis, we used siRNA to reduce Bcr/Abl expression in K562 cells and compared its effects to WP1130. Cells transfected with siRNA designed to specifically target the p210 form of Bcr/Abl30 showed reduced expression of Bcr/Abl protein at 24 hours that was sustained 96 hours after transfection (Figure 4B). Bcr/Abl expression and apoptosis were not altered in untreated or control siRNA–treated K562 cells, whereas Bcr/Abl siRNA treatment reduced both Bcr/Abl and cell survival (Figure 4C). siRNA-mediated Bcr/Abl down-regulation and induction of apoptosis were similar to the temporal effects of WP1130 in K562 cells (compare Figure 4A and 4C), suggesting that WP1130-mediated down-regulation of Bcr/Abl is one of the activities associated with the onset of apoptosis in WP1130-treated CML cells.
WP1130-induced apoptosis results from down-regulation of Bcr/Abl. (A) K562 cells (1 × 106) were incubated in medium with or without WP1130 (5 μM) for the indicated times, and cell-cycle distribution was determined by FACS analysis. (B). K562 cells were electroporated with either 100 nM scrambled siRNA (control) or 100 nM siRNA specifically targeting Bcr/Abl for the indicated times, and expression of Bcr/Abl, pBcr/Abl, pCrk1, and Crk1 was determined by Western blotting. The phosphorylation of CrkL was used as an indicator of Bcr/Abl activation. (C) FACS analysis of K562 cells electroporated with either 100 nM scrambled siRNA (control) or 100 nM siRNA specifically targeting Bcr/Abl for the indicated times.
WP1130-induced apoptosis results from down-regulation of Bcr/Abl. (A) K562 cells (1 × 106) were incubated in medium with or without WP1130 (5 μM) for the indicated times, and cell-cycle distribution was determined by FACS analysis. (B). K562 cells were electroporated with either 100 nM scrambled siRNA (control) or 100 nM siRNA specifically targeting Bcr/Abl for the indicated times, and expression of Bcr/Abl, pBcr/Abl, pCrk1, and Crk1 was determined by Western blotting. The phosphorylation of CrkL was used as an indicator of Bcr/Abl activation. (C) FACS analysis of K562 cells electroporated with either 100 nM scrambled siRNA (control) or 100 nM siRNA specifically targeting Bcr/Abl for the indicated times.
WP1130 suppresses CML tumor growth in nude mice
Our in vitro analysis suggested that WP1130 affects Bcr/Abl protein levels, signaling, and apoptosis in CML cells through a mechanism distinct from that of imatinib mesylate and geldanamycin. To assess the possible therapeutic activity of WP1130 against CML, nude mice received a subcutaneous transplant with K562 cells and were treated with 30 mg/kg WP1130 every other day for 9 days after palpable tumor formation (10 days). This dose and schedule are well tolerated and effective in other tumor models (N.J.D., unpublished observation, September 2006). Antitumor activity was compared with that after daily imatinib mesylate treatment (50 mg/kg). Nineteen days after tumor inoculation, tumors in the control group reached maximum allowable dimension, and the effect of therapy was assessed. WP1130 treatment suppressed K562 tumor growth to an extent comparable to that observed in imatinib mesylate–treated animals (Figure 5A), suggesting that WP1130 is active in reducing the growth of established K562 tumors.
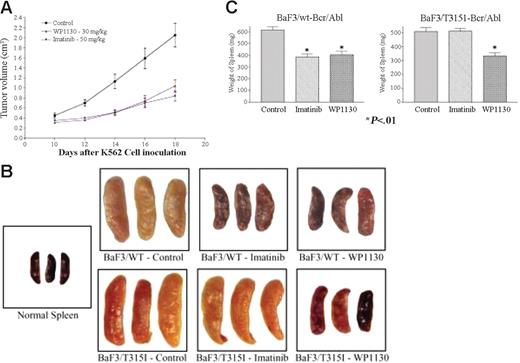
WP1130 reduces the growth of CML tumors and Bcr/Abl-expressing cells in nude mice. K562 cells were inoculated subcutaneously into nude mice as described. Initial tumor volumes were measured 10 days later, and the mice were randomly assigned to 3 treatment groups (5 animals/group). Animals were treated with WP1130 (30 mg/kg every other day for 9 days, 5 injections) or imatinib mesylate (50 mg/kg every day for 9 days, 9 injections). Tumors were measured with electronic calipers, and volumes were calculated in cubic millimeters by determining the length × breadth × height of each tumor. Results are expressed as mean ± standard error of the mean of the measurements from all 5 animals per group during the treatment. Animals were weighed when the tumors were measured, and no difference in body weight was noted between treated or control animals (data not shown). (B) BaF/3/WT- or BaF/3/T315I Bcr/Abl-expressing cells were injected (intravenously) into nude mice as described in “Materials and methods.” One day later, animals were randomly separated into 3 groups. One group received vehicle alone (control), the second group received imatinib mesylate daily, and the third group was treated with WP1130 every other day. Fifteen days after tumor cell inoculation, animals were killed, and their spleens were removed, weighed, and photographed. Spleens from non–tumor-bearing animals (normal spleen) were also photographed for comparison. (C) The average ± SEM spleen weight from each treatment group is shown. P < .01 from Student t test.
WP1130 reduces the growth of CML tumors and Bcr/Abl-expressing cells in nude mice. K562 cells were inoculated subcutaneously into nude mice as described. Initial tumor volumes were measured 10 days later, and the mice were randomly assigned to 3 treatment groups (5 animals/group). Animals were treated with WP1130 (30 mg/kg every other day for 9 days, 5 injections) or imatinib mesylate (50 mg/kg every day for 9 days, 9 injections). Tumors were measured with electronic calipers, and volumes were calculated in cubic millimeters by determining the length × breadth × height of each tumor. Results are expressed as mean ± standard error of the mean of the measurements from all 5 animals per group during the treatment. Animals were weighed when the tumors were measured, and no difference in body weight was noted between treated or control animals (data not shown). (B) BaF/3/WT- or BaF/3/T315I Bcr/Abl-expressing cells were injected (intravenously) into nude mice as described in “Materials and methods.” One day later, animals were randomly separated into 3 groups. One group received vehicle alone (control), the second group received imatinib mesylate daily, and the third group was treated with WP1130 every other day. Fifteen days after tumor cell inoculation, animals were killed, and their spleens were removed, weighed, and photographed. Spleens from non–tumor-bearing animals (normal spleen) were also photographed for comparison. (C) The average ± SEM spleen weight from each treatment group is shown. P < .01 from Student t test.
To determine whether WP1130 also affects cells transformed by mutant Bcr/Abl, BaF/3 cells expressing wild-type or T315I mutant Bcr/Abl were injected intravenously into nude mice (as recently described in Naito et al40 ), and 1 day later animals were treated with 40 mg/kg WP1130 (every other day) or 100 mg/kg imatinib mesylate daily. BaF/3 cells expressing Bcr/Abl traffic to the liver and spleen, and tumor infiltration into these organs can be used to gauge the therapeutic response.41 To determine whether WP1130 affects deposition of Bcr/Abl-expressing cells, spleens from control and treated animals were harvested, photographed, and weighed to quantify the effect of therapy on disease advancement. The BaF/3-Bcr/Abl–expressing cells did traffic to the spleen (Figure 5B), altering its appearance and increasing its weight by approximately 8-fold compared with non–tumor-bearing animals (normal spleens). Treatment of animals inoculated with BaF/3 cells expressing wild-type Bcr/Abl with WP1130 or imatinib mesylate reduced tumor cell splenic deposition, as determined by reductions in spleen size, color, and weight. Tumor cell content in animals inoculated with T315I Bcr/Abl–expressing BaF/3 cells was not affected by imatinib mesylate but was significantly reduced by WP1130 (Figure 5C). Although additional studies are under way to determine the most effective dose and schedule, these preliminary results suggest that WP1130 is active in reducing Bcr/Abl-expressing tumor cell burden, and its activity is not affected by mutations that block the imatinib mesylate response.
Discussion
Imatinib mesylate, currently the most effective drug used for the treatment of CML, binds to the kinase domain of Abl and prevents the auto-activation of Bcr/Abl, a prerequisite for the onset of the disease. Over time, disease in some patients fails to respond to imatinib mesylate, in part because of mutations in the kinase domain of Abl. Dasatinib (BMS-354825), a dual Abl and Src kinase inhibitor synthesized by Bristol-Myers Squibb, was shown to inhibit the growth of CML cells, including most cells with mutations common in CML-resistant disease.26 Compounds tested in preclinical studies, including small-molecule kinase inhibitors, such as pyrido-pyrimidine derivatives24 and nilotinib,27 are also active against mutants of Bcr/Abl that do not respond to imatinib mesylate. However, until now, the T315I mutation has remained resistant to all described Src and Bcr/Abl kinase inhibitors that block ATP binding.
Here, we show that treating CML cells with WP1130 induced rapid (within 1 hour) down-regulation of Bcr/Abl, without attenuating its mRNA levels or engaging the proteasomal degradation pathway. The antileukemic mechanism seems to be distinct from that of previously described mediators of Bcr/Abl down-regulation. WP1130-induced degradation of Bcr/Abl is independent of the Hsp90/Hsp70 pathway, which engages a proteasomal-dependent mechanism for Bcr/Abl down-regulation by geldanamycin.38,39 By targeting the Bcr/Abl protein through a distinct mechanism, WP1130 has an advantage over imatinib mesylate in that its activity is not inhibited by a variety of Abl kinase mutations, including T315I (Figure 3A). We found that WP1130 strongly inhibited the growth of cell lines expressing either wild-type or the T315I mutant form of Bcr/Abl as well as leukemic blast progenitor cells from patients who had developed imatinib mesylate–resistant CML blast crisis (Figure 3D).
We are not the first to identify a compound that can reduce Bcr/Abl protein levels in CML cells expressing the wild-type or T315I mutant Bcr/Abl. Like WP1130, adaphostin, an analog of the tyrphostin AG957, has been shown to down-regulate Bcr/Abl10,42 and was recently shown to be effective against CML cells expressing T315I-Bcr/Abl through a proteasome-independent pathway that has not been defined but seems to be distinct from its ability to activate reactive oxygen species.43 Because the major antiproliferative effect of adaphostin is mediated by apoptosis induced by activation of reactive oxygen species,44 whether the down-regulation of Bcr/Abl by adaphostin significantly contributes to its apoptotic effects remains to be determined. In another study, treatment with Trisenox37 inhibited the translation of Bcr/Abl, resulting in the down-regulation of both wild-type and T315I mutant protein. However, this mechanism required 48 hours of treatment. Unlike adaphostin and Trisenox, WP1130 induced down-regulation of Bcr/Abl within 60 minutes. Here, we show that down-regulation of Bcr/Abl by siRNA30 or WP1130 induces apoptosis, supporting an association between WP1130 activity against Bcr/Abl and the induction of apoptosis. However, other activities may also contribute to the observed effects of WP1130 on CML cell survival.
Rapid reduction of Bcr/Abl protein may have advantages over kinase inactivation or inhibition for treating CML because of the multiple structural elements of Bcr/Abl that mediate multiple protein interactions that engage diverse signaling pathways responsible for cell proliferation, adhesion, and inhibition of apoptosis.45-49 Thus, destruction of Bcr/Abl might lead to the dispersal of these interacting proteins, resulting in a rapid and more complete collapse of cell signaling and survival. In support of this hypothesis, we observed that WP1130 induced more rapid and complete apoptosis of CML cells expressing wild-type Bcr/Abl than did imatinib mesylate. However, imatinib mesylate is more target-selective than WP1130, because it has no Jak2 kinase inhibitory activity. Previous studies demonstrate that the dual Src/Abl kinase inhibitor, pyrido[2,3d]pyrimidine derivative PD180970, failed to inhibit growth or elicit apoptosis in Bcr/Abl-negative cells,50 suggesting that, although these inhibitors target several molecules, their primary target is Bcr/Abl. Studies in our laboratory have shown that cells of B-cell lineage are the most sensitive to WP1130, whereas epithelial cells are more resistant to the antiproliferative/apoptotic activity of this compound. Therapeutic assessments in animal models suggest that WP1130 has antileukemic activity and little toxicity. Collectively, our results suggest that WP1130 may be useful in the treatment of CML and other diseases that express target proteins recognized by the protein down-regulatory pathway activated by WP1130.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicholas J. Donato, University of Michigan Comprehensive Cancer Center, Division of Hematology/Oncology, Rm CCGC 4306, 1500 East Medical Center Dr, Ann Arbor, MI 48109; e-mail: ndonato@med.umich.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Christine Wogan of the Department of Scientific Publications at M. D. Anderson Cancer Center for editing this manuscript.
This work was supported by the National Cancer Institute (grant P01-CA46939) and the Leukemia Lymphoma Society (grant CF01-313, 6118).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal