Abstract
Dendritic cells (DCs) and chemokines are important mediators linking innate and adaptive immunity on activation by Toll-like receptor (TLR) agonists. We previously identified a kind of regulatory DC subset (diffDCs) that differentiated from mature DCs under splenic stroma and that inhibited T-cell proliferation. The responsiveness of such regulatory DCs to TLR agonists and their pattern of chemokine production remain to be determined. Here, we report that the regulatory DCs secrete a higher level of CXCR3 chemokine IFN-γ–induced protein-10 (IP-10) than immature DCs (imDCs), and more IP-10 is produced after stimulation with TLR-2, -4, -3, and -9 ligands. Blockade of IFN-α/β inhibits IP-10 production by TLR agonist-activated regulatory DCs. We show that the increased IRF-3 and IFN-β–induced STAT1 activation are responsible for the autocrine IFN-β–dependent preferential production of IP-10 by regulatory DCs. In addition, stimulation with recombinant mouse IFN-α/β induces more IP-10 production in regulatory DCs than that in imDCs. Moreover, the regulatory DCs selectively recruit more Th1 cells through IP-10 and inhibit Th1 proliferation. Our results demonstrate a new manner for regulatory DCs to down-regulate T-cell response by preferential IP-10 production and inhibition of recruited Th1 cell proliferation.
Introduction
Dendritic cells (DCs), the most potent professional antigen-presenting cells (APCs), are critical sentinels in antimicrobial immune responses with the unique ability to integrate a wide array of incoming signals and convey them to lymphocytes, directing the appropriate immune responses.1,2 It is well known that immature DCs (imDCs) undergo maturation on recognition of pathogenic components via Toll-like receptors (TLRs), which enable DCs to produce different profiles of cytokines and chemokines and control the development of Th1/Th2-type adaptive immune responses.3,4 In recent years, more and more studies have shown that DCs have the ability to down-regulate an immune response or induce immune tolerance. The ability of DCs to initiate an immune response or induce immune tolerance depends on their maturation state and different subsets.5,6 Some kinds of regulatory DCs with negative regulatory functions have been reported,7 and the regulatory DCs commonly used in most current work are prepared in vitro by culturing DC progenitors in the presence of immunosuppressive cytokines, such as IL-10 and TGF-β.8,9 However, how regulatory DCs will respond to TLR agonists and the roles of regulatory DCs triggered by TLR agonists in the control of Th1/Th2-type adaptive immune responses remain to be fully understood. Furthermore, the chemokine production in regulatory DCs has not yet been identified.
Mature DCs (maDCs) are usually considered to be terminally differentiated and undergo activation-induced apoptosis.10,11 However, our previous study demonstrated that spleen stromal cells, which mimic the secondary lymph organ microenvironment, can drive maDCs to proliferate and further differentiate into a unique form of regulatory DC subset (diffDCs), which express a higher level of IL-10 but minimal IL-12p70 and inhibit the proliferation of naïve CD4+ T cells in response to antigen presented by maDCs.12 Regulatory diffDCs can proliferate in response to antigen stimulation, thus exerting negative feedback control of the T-cell immune response in the late period of the immune response. In addition, endothelial splenic stroma may physiologically induce such regulatory DC differentiation from hematopoietic stem cells.13 Chemokines are important mediators in the activation of innate and adaptive immunity and also in the regulation of inflammation and immune responses.14 TLRs can sense microbial products and trigger maturation of DCs and chemokine production. Therefore, to better understand the functions of the regulatory DCs in inflammation and immune regulation, we investigated the chemokine expression pattern of the regulatory DCs with or without stimulation by TLR agonists, and then the sequence of the increased chemokine production by the TLR signaling-triggered regulatory DCs in the chemoattraction and regulation of T cells. We found that the regulatory DCs spontaneously secrete CXCR3 chemokine interferon-γ–inducible protein 10 (IP-10) and will produce much more IP-10 in response to TLR-2, -3, -4, and -9 agonists. Higher IP-10 production in the regulatory DCs depends on the increased autocrine production of IFN-β and enhanced activation of STAT1. Moreover, the regulatory DCs can chemoattract more Th1 cells through IP-10 and then inhibit the proliferation of Th1 cells, thus providing a new manner for negative feedback control of Th1 immune response and maintenance of immune homeostasis.
Materials and methods
Reagents and mice
Recombinant mouse GM-CSF and IL-4 were purchased from PeproTech (London, United Kingdom). RPMI 1640 medium and fetal bovine serum (FBS) were from Hyclone (Logan, UT). LPS (Escherichia coli, O26:B6) and LTA were purchased from Sigma (St Louis, MO) and nuclease-resistant phosphorothioate oligodeoxynucleotides, with no detectable endotoxins by Limulus assay, was supplied by Sangon (Shanghai, China). Fludarabine, an inhibitor of STAT1, was purchased from Sigma. Anti–mIP-10 antibody and recombinant mouse IP-10 were obtained from R&D Systems (Minneapolis, MN). The rabbit polyclonal anti–mouse IFN-β, IFN-α antibodies and recombinant mouse IFN-β and IFN-α A were purchased from PBL Biomedical Laboratories (New Brunswick, NJ). Anti–phospho-STAT1 monoclonal antibody (mAb), anti-STAT1 (pY701) mAb, anti-IRF3 mAb, actin mAb, and their respective horseradish peroxidase (HRP)–coupled secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). C57BL/6J mice at 5 to 6 weeks of age were obtained form Joint Ventures Sipper BK Experimental Animal Company (Shanghai, China). OVA (323-339)–specific TCR-transgenic mice (DO11.10) were obtained from the Jackson Laboratory (Bar Harbor, ME) and bred in specific pathogen-free conditions.
Culture of mouse imDCs and regulatory DCs
Mouse imDCs were prepared from bone marrow progenitors by culturing in 10 ng/mL rmGM-CSF and 1 ng/mL rmIL-4 as described previously.15 Nonadherent cells were gently washed out on the third day of culture; the remaining loosely adherent clusters were used on day 6 as imDCs. Regulatory DCs (diffDCs) derived from maDCs were generated by coculturing with splenic stroma as described previously.12
Reverse transcription-polymerase chain reaction
Total RNA was isolated from 1 × 106 resting and LPS-treated cells with TRIzol (Gibco BRL, Carlsbad, CA). cDNA was synthesized from 1 μg of the total RNA by extension with oligo (dT)18 primer and SuperScript II (200 U; Gibco BRL). The cDNA was amplified by polymerase chain reaction (PCR) with the following primers: TLR4 (forward, 5′-AGC AGA GGA GAA AGC ATC TAT GAT GC-3′, reverse, 5′-GGT TTA GGC CCC AGA GTT TTT CTC C -3′); IP-10 (forward, 5′-GGA CGG TCC GCT GCA ACT GCA TCC-3′; reverse, 5′-GCA GCC TGG GCA TGG CAC ATG GTG-3′); IFN-α (forward, 5′-TCT CTC CTG CCT GAA GGA C-3′; reverse, 5′-ACA CAG TGA TCC TGT GGA A-3′); IFN-β (forward, 5′-CAG CTC CAA GAA AGG ACG AA-3′; reverse, 5′-GTA GCT GTT GTA CTT CAT GAG-3′); GAPDH (forward, 5′-ACC ACA GTC CAT GCC ATC AC-3′; reverse, 5′-TCC ACC ACC CTG TTG CTG TA-3′). PCR amplification from cDNA was performed in a final volume of 50 μL containing 2.5 mM magnesium dichloride, 1.25 U ExTaq polymerase (TaKaRa, Dalian, China), and 1 μM specific primers. Cycling conditions were 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 60 seconds (geneAmp 9600 PCR system, Perkin Elmer, Wellesley, MA). All PCR products were resolved by 1% agarose gel electrophoresis and visualized by staining with ethidium bromide.
Cytokine assays
Cytokines in the culture supernatants collected at the indicated times were assayed by using mouse IP-10 (R&D Systems), IFN-α, and IFN-β enzyme-linked immunosorbent assay (ELISA) kits (PBL Biomedical Laboratories).
Western blot analysis of STAT1 activation and IRF-3 expression
STAT1 activation was detected as described previously.16 For the detection of IRF-3 expression level, nuclear and total cell extracts were prepared using NE-PER nuclear and cytoplasmic extraction reagents and M-TER mammalian protein extraction reagent (Pierce, Rockford, IL) containing protease inhibitor mixture (Calbiochem, San Diego, CA). Blots were probed for 1 hour with anti-STAT1, anti–phospho-STAT1, or IRF-3 (1:1000) and then were incubated with 1:5000 diluted HRP-conjugated secondary antibody for 1 hour at room temperature. Proteins were visualized using SuperSignalWest Femto Maximum Sensitivity Substrate, as instructed by the manufacturer (Pierce).
IRF-3 DNA-binding activity
IRF-3 DNA-binding activity was measured using the TransAMTM kit (Activemotif, Rixensart, Belgium) according to the manufacturer's protocol.
Preparation of Th1 and Th2 cells and in vitro chemotaxis assay
Preparation of Th1 and Th2 cells and their chemotaxis assay in vitro were described previously.17 Briefly, splenocytes from DO11.10 transgenic mice were purified using anti-CD4 (L3T4)–coated microbeads (Miltenyi Biotec, Auburn, CA). CD4+ T cells were then cocultured with DCs as APCs in the presence of 200 nM OVA323-339 peptide and either 400 ng/mL mIFN-γ plus 10 μg/mL anti–mIL-4 antibody or 10 ng/mL mIL-4 plus 10 μg/mL anti–mIFN-γ antibody to obtain Th1 or Th2 cells, respectively. After 4 days, cells were collected for use in the chemotaxis assay. Cell chemotaxis assay was performed in 3.0-μm pore size HD PET track-etched membrane cell culture inserts (BD Labware, Franklin Lakes, NJ). Cells 2 × 105 stained with CD4-FITC in 200 μL PRMI 1640 without FCS were added to the transwell insert. Then, 600 μL of 10-fold diluted DC-conditioned supernatant, which had been incubated with or without IP-10–neutralizing antibody for 30 minutes, was added to wells of a 24-well plate and transwell inserts placed into wells. After 2 hours of incubation at 37°C, cells that had migrated into wells were harvested and counted by FACSCalibur flow cytometry and analyzed by CellQuest software both from Becton Dickinson, Mountain View, CA.
Assays for proliferation and cytokine expression of antigen-specific Th1 and Th2 cells
The proliferation and intracellular cytokine expression of antigen-specific T cells was assayed as described previously.12 Briefly, purified Th1 cells from DO11.10 OVA323-339–specific TCR transgenic × C57BL/6 F1 hybrid mice were cocultured with DCs treated with or without LPS stimulation in the presence of OVA323-339 peptide at a 1:10 (DC/T) ratio in round-bottom 96-well plates (1 × 105 T cells/200 μL/well) for 5 days. Cells double stained with anti–CD4-FITC and 7-AAD were resuspended in exactly 200 μL PBS and cellular data acquired for 56 seconds using a flow cytometer. The number of CD4+7-AAD− live cells was calculated to represent the altitude of antigen-specific CD4 T-cell proliferation. Th1 cells were cocultured for 24 hours with DCs treated with or without LPS stimulation in the presence of peptides and brefeldin A and were stained intracellularly for IL-2 and IFN-γ expression detected by flow cytometry (cytokine and CD4 double staining).
Statistical analysis
Data are shown as mean ± SD for separate experiments. Statistical significance was determined by Student t test with a value of P below .05 considered as statistically significant.
Results
Regulatory diffDCs with or without TLR agonists stimulation preferentially secrete IP-10
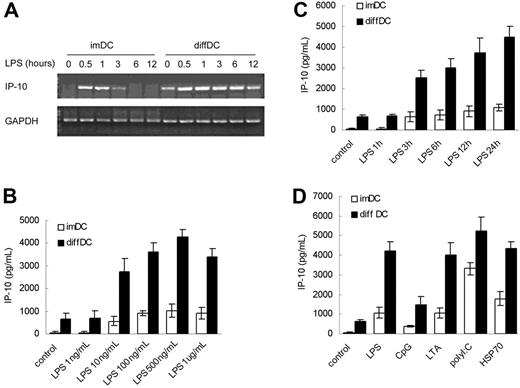
Our previous study demonstrated that regulatory diffDCs secrete a higher level of IL-10 but a lower level of IL-12p70 than imDCs and maDCs,12 and such regulatory DCs produce more IL-10 but minimal IL-12p70 in response to LPS stimulation. By reverse transcription-PCR (RT-PCR) screening of chemokine expression in regulatory diffDCs, we found there was no significant difference in expression of MCP-1 and MIP-1α in regulatory diffDCs and imDCs, even after stimulation with LPS (data not shown). Interestingly, diffDCs could spontaneously secrete a significantly higher level of IP-10 than imDCs (Figure 1A). LPS could stimulate diffDCs to produce more IP-10 protein than for imDCs in a dose- and time-dependent manner (Figure 1B–C). Furthermore, IP-10 production in diffDCs could be enhanced by stimulation with a variety of other TLR ligands, including CpG ODN (ligand of TLR9), LTA (ligand of TLR2), poly(I:C) (ligand of TLR3), or HSP70 (Figure 1D). These results suggested that the preferential IP-10 production is the steady and intrinsic characteristic of regulatory diffDCs, and TLR agonists can stimulate diffDCs to produce more IP-10.
TLR agonists stimulate regulatory diffDCs to produce a higher level of IP-10 than imDCs. (A) IP-10 mRNA expression in imDCs or diffDCs cultured in medium alone as a control (0) or stimulated with LPS at the indicated time. The transcript of mouse GAPDH gene was used as the amplification control. (B) IP-10 production by imDCs or diffDCs stimulated with various concentrations of LPS (1 ng to 1 μg/mL) for 24 hours was measured. imDCs and diffDCs cultured in medium (untreated DCs) were used as controls. (C) IP-10 production by imDCs or diffDCs following stimulation with 500 ng/mL LPS for various lengths of time was measured. (D) At 24 hours after stimulation of imDCs or diffDCs (5 × 105 cells/mL) with 500 ng/mL LPS, 1 μM CpG ODN, 5 ng/mL LTA, 1 μg/mL poly(I:C) or 50 ng/mL HSP70, IP-10 production was measured by ELISA. Values in panels B-D are expressed as means ± SD. Results are representative of 3 independent experiments.
TLR agonists stimulate regulatory diffDCs to produce a higher level of IP-10 than imDCs. (A) IP-10 mRNA expression in imDCs or diffDCs cultured in medium alone as a control (0) or stimulated with LPS at the indicated time. The transcript of mouse GAPDH gene was used as the amplification control. (B) IP-10 production by imDCs or diffDCs stimulated with various concentrations of LPS (1 ng to 1 μg/mL) for 24 hours was measured. imDCs and diffDCs cultured in medium (untreated DCs) were used as controls. (C) IP-10 production by imDCs or diffDCs following stimulation with 500 ng/mL LPS for various lengths of time was measured. (D) At 24 hours after stimulation of imDCs or diffDCs (5 × 105 cells/mL) with 500 ng/mL LPS, 1 μM CpG ODN, 5 ng/mL LTA, 1 μg/mL poly(I:C) or 50 ng/mL HSP70, IP-10 production was measured by ELISA. Values in panels B-D are expressed as means ± SD. Results are representative of 3 independent experiments.
Autocrine IFN-α/β secretion contributes to the preferential production of IP-10 by regulatory diffDCs in response to TLR agonists
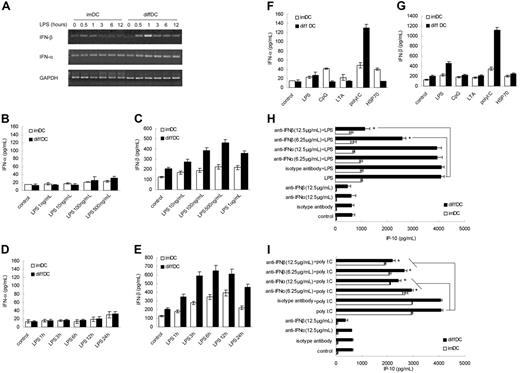
Next, we wanted to know why diffDCs spontaneously secrete a high level of IP-10 and produce more IP-10 in response to TLR agonists. It has been shown that type I IFN induced by TLR agonists can act in an autocrine fashion for IFN-inducible gene expression such as the chemokine IP-10.18 To investigate whether type I IFN is involved in the preferential production of IP-10 in diffDCs, IFN-α and IFN-β expression in imDCs and diffDCs at mRNA and protein levels was analyzed. The IFN-β mRNA expression in diffDCs was up-regulated more significantly than that in imDCs when stimulated with LPS. However, mRNA levels of IFN-α in imDCs and diffDCs were almost the same and remained unchanged even after LPS stimulation (Figure 2A). Consistently, LPS induced IFN-β production but not IFN-α production by diffDCs in a dose- and time-dependent manner (Figure 2B–E). In addition, LPS-induced IFN-β secretion (1 hour) is earlier than IP-10 production (3 hours). Furthermore, diffDCs secreted significantly more IFN-β than imDCs following poly(I:C) and LPS stimulation (Figure 1F–G). The early and high production of IFN-β by diffDCs is in accordance with the trends of IP-10 production by diffDCs, indicating that IFN-β in an autocrine manner may be responsible for the higher IP-10 production in diffDCs. To test this hypothesis, imDCs and diffDCs were incubated with neutralizing antibody to IFN-β or IFN-α for 30 minutes prior to LPS stimulation. As shown in Figure 2H, the preincubation with IFN-β–neutralizing antibody could dose dependently inhibit the IP-10 production of diffDCs stimulated with LPS. However, IFN-α blockade could not affect the IP-10 production of diffDCs. Therefore, the data demonstrate that preferential IP-10 production by diffDCs in response to LPS is dependent on IFN-β in an autocrine manner. However, the relevance of IFN-α in IP-10 production cannot be evaluated in the assay because LPS is a very poor inducer of IFN-α. Furthermore, it has been reported that IFN-α leads to an increase of IP-10 secretion in distinct cell types.19,20 To further confirm the involvement of IFN-α in the production of IP-10 in diffDCs, imDCs and diffDCs were incubated with neutralizing antibody to IFN-α or IFN-β for 30 minutes prior to stimulation with poly(I:C) (a strong inducer of IFN-α). Interestingly, anti–IFN-α or anti–IFN-β antibody could also dose dependently inhibit IP-10 production by diffDCs stimulated with poly(I:C) (Figure 2I). Our results indicated that TLR agonists-induced increased production of IP-10 in diffDCs is IFN-α/β dependent.
Autocrine IFN-α/β is responsible for the increased IP-10 production in diffDCs stimulated by TLR agonists. (A) Kinetics of mRNA expression of IFN-α and IFN-β in imDCs and diffDCs stimulated with or without LPS. (B-C) IFN-α and IFN-β production by imDCs and diffDCs (1 × 106 cells/mL) after stimulation with various concentrations of LPS for 24 hours. (D-E) IFN-α and IFN-β production by imDCs and diffDCs (1 × 106 cells/mL) after stimulation with 500 ng/mL LPS for indicated time. (F-G) IFN-α and IFN-β production was measured by ELISA in culture supernatants of imDCs and diffDCs (1 × 106 cells/mL) stimulated with different TLR agonists as in Figure 1D for 24 hours. Data are shown as means ± SD of 3 independent experiments. (H-I) imDCs and diffDCs were pretreated with 6.25 μg/mL and 12.5 μg/mL neutralizing antibody of mIFN-α or mIFN-β or isotype antibody, respectively, for 30 minutes and then stimulated with 500 ng/mL LPS or poly(I:C) for 24 hours. The level of IP-10 in supernatants was determined by ELISA. imDCs and diffDCs cultured in medium (untreated DCs) were used as controls. *P < .05.
Autocrine IFN-α/β is responsible for the increased IP-10 production in diffDCs stimulated by TLR agonists. (A) Kinetics of mRNA expression of IFN-α and IFN-β in imDCs and diffDCs stimulated with or without LPS. (B-C) IFN-α and IFN-β production by imDCs and diffDCs (1 × 106 cells/mL) after stimulation with various concentrations of LPS for 24 hours. (D-E) IFN-α and IFN-β production by imDCs and diffDCs (1 × 106 cells/mL) after stimulation with 500 ng/mL LPS for indicated time. (F-G) IFN-α and IFN-β production was measured by ELISA in culture supernatants of imDCs and diffDCs (1 × 106 cells/mL) stimulated with different TLR agonists as in Figure 1D for 24 hours. Data are shown as means ± SD of 3 independent experiments. (H-I) imDCs and diffDCs were pretreated with 6.25 μg/mL and 12.5 μg/mL neutralizing antibody of mIFN-α or mIFN-β or isotype antibody, respectively, for 30 minutes and then stimulated with 500 ng/mL LPS or poly(I:C) for 24 hours. The level of IP-10 in supernatants was determined by ELISA. imDCs and diffDCs cultured in medium (untreated DCs) were used as controls. *P < .05.
Increased IRF-3 and IFN-β–induced STAT1 phosphorylation are responsible for the preferential IP-10 production by diffDCs
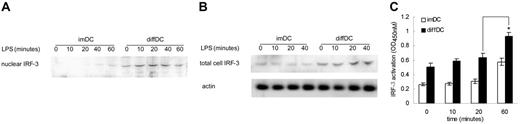
Then, we went further to investigate the mechanisms for autocrine IFN-β to induce higher production of IP-10 by diffDCs. It is well known that the induction of IFN-inducible genes is thought to be regulated by the transcription factor IRF-3, a member of the regulatory factor (IRF) family.21,22 Thus we tested whether there was difference in IRF-3 expression between diffDCs and imDCs with or without LPS stimulation. A higher level of IRF-3 could be detected in nuclear and total cell extracts of diffDCs than imDCs (Figure 3A–B). Although LPS stimulation could induce IRF-3 nuclear localization early at 20 minutes in imDCs, the amounts of IRF-3 in the nuclei of diffDCs was not affected on LPS stimulation. Considering that the function of IRF-3 depends both on its protein level as well as its activation state, we detected the activation of IRF-3 by assaying its DNA-binding activity in imDCs and diffDCs. As shown in Figure 3C, DNA-binding activity of IRF-3 in the nucleus of diffDCs was increased gradually and reached a maximum 1 hour after stimulation with LPS. Although IRF-3 was also activated in imDCs after LPS stimulation, the level of IRF-3 activation in imDCs was always much lower than that in diffDCs. These data indicated that IRF-3 expression and activation are higher in diffDCs than imDCs, especially after stimulation with LPS, suggesting the increased IRF-3 expression and activation might be involved in higher IP-10 production in diffDCs.
Increased expression and activation of IRF-3 in diffDCs. (A) Nuclear extracts from imDCs and diffDCs (1 × 106 cells/mL) cultured in medium alone or stimulated with 500 ng/mL LPS for 10, 20, 40, or 60 minutes were prepared, blotted, and probed with IRF-3–specific antibody. (B) imDCs and diffDCs were cultured in medium alone as control or stimulated with 500 ng/mL LPS for the indicated times. Total cell extracts were probed for IRF-3 and actin as a loading control. (C) Quantification of activated IRF-3 in imDCs and diffDCs after LPS stimulation by assaying IRF-3 DNA-binding activity. Data are shown as means ± SD of 3 independent experiments. *P < .05.
Increased expression and activation of IRF-3 in diffDCs. (A) Nuclear extracts from imDCs and diffDCs (1 × 106 cells/mL) cultured in medium alone or stimulated with 500 ng/mL LPS for 10, 20, 40, or 60 minutes were prepared, blotted, and probed with IRF-3–specific antibody. (B) imDCs and diffDCs were cultured in medium alone as control or stimulated with 500 ng/mL LPS for the indicated times. Total cell extracts were probed for IRF-3 and actin as a loading control. (C) Quantification of activated IRF-3 in imDCs and diffDCs after LPS stimulation by assaying IRF-3 DNA-binding activity. Data are shown as means ± SD of 3 independent experiments. *P < .05.
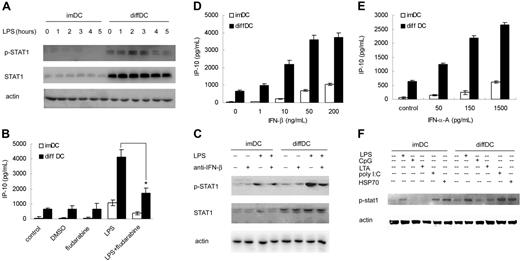
LPS-induced IP-10 expression is STAT1-dependent.23 Thus, we also examined the phosphorylation of STAT1 in diffDCs. As expected, diffDCs expressed sharply higher STAT-1 in the resting state, and STAT1 was more significantly phosphorylated following LPS stimulation in comparison with that in imDCs (Figure 4A). To confirm the role of STAT1 in the increased production of IP-10 in diffDCs, we assayed IP-10 production by diffDCs and imDCs in the presence of 50 μM of the STAT1 inhibitor fludarabine and found that LPS-induced IP-10 production in diffDCs was significantly decreased (Figure 4B). These results demonstrate that the increased STAT1 activation in diffDCs contributes to high production of IP-10 by diffDCs in response to LPS stimulation.
IFN-β–induced STAT1 activation is responsible for the increased IP-10 production in diffDCs. (A) imDCs and diffDCs were stimulated with or without 500 ng/mL LPS for the indicated time. Total cell lysates were then electrophoresed and probed with phospho-STAT1 and STAT1, respectively. Actin expression was used as control to confirm that equal amounts of protein lysates were in each lane. (B) imDCs and diffDCs were pretreated with 50 μM fludarabine (STAT1 inhibitor) for 30 minutes, then stimulated with 500 ng/mL LPS for 24 hours. An equal amount of DMSO contained in medium was used as a negative control. The levels of IP-10 in the supernatants were determined by ELISA. *P < .05. (C) Effect of neutralizing antibody against mouse IFN-β on the phosphorylation of STAT1 in imDCs and diffDCs stimulated with or without LPS. imDCs and diffDCs were pretreated with 12.5 μg/mL neutralizing antibody of IFN-β for 30 minutes, then stimulated with 500 ng/mL LPS for 2 hours. The activation of STAT1 was analyzed by Western blotting. imDCs and diffDCs in medium alone and treated with IFN-β antibody were used as controls. (D-E) DCs were stimulated with various doses of recombinant mIFN-β or IFN-α for 24 hours. Then the level of IP-10 in the supernatants was determined by ELISA. Data are shown as means ± SD of 3 independent experiments. Each experiment was preformed at least 3 times. (F) imDCs and diffDCs were stimulated for 2 hours with or without 100 ng/mL LPS, CpG ODN, LTA, poly(I:C), or HSP70. Phosphorylation of STAT1 in cell lysates was analyzed by Western blotting. Actin expression was used as control to confirm that equal amounts of protein lysates were in each lane.
IFN-β–induced STAT1 activation is responsible for the increased IP-10 production in diffDCs. (A) imDCs and diffDCs were stimulated with or without 500 ng/mL LPS for the indicated time. Total cell lysates were then electrophoresed and probed with phospho-STAT1 and STAT1, respectively. Actin expression was used as control to confirm that equal amounts of protein lysates were in each lane. (B) imDCs and diffDCs were pretreated with 50 μM fludarabine (STAT1 inhibitor) for 30 minutes, then stimulated with 500 ng/mL LPS for 24 hours. An equal amount of DMSO contained in medium was used as a negative control. The levels of IP-10 in the supernatants were determined by ELISA. *P < .05. (C) Effect of neutralizing antibody against mouse IFN-β on the phosphorylation of STAT1 in imDCs and diffDCs stimulated with or without LPS. imDCs and diffDCs were pretreated with 12.5 μg/mL neutralizing antibody of IFN-β for 30 minutes, then stimulated with 500 ng/mL LPS for 2 hours. The activation of STAT1 was analyzed by Western blotting. imDCs and diffDCs in medium alone and treated with IFN-β antibody were used as controls. (D-E) DCs were stimulated with various doses of recombinant mIFN-β or IFN-α for 24 hours. Then the level of IP-10 in the supernatants was determined by ELISA. Data are shown as means ± SD of 3 independent experiments. Each experiment was preformed at least 3 times. (F) imDCs and diffDCs were stimulated for 2 hours with or without 100 ng/mL LPS, CpG ODN, LTA, poly(I:C), or HSP70. Phosphorylation of STAT1 in cell lysates was analyzed by Western blotting. Actin expression was used as control to confirm that equal amounts of protein lysates were in each lane.
Because IFN-β plays an important role in the phosphorylation of STAT1, we tested whether the neutralizing antibody against IFN-β could affect the STAT1 activation in diffDCs. We assayed STAT1 activation in diffDCs after 500 ng/mL LPS stimulation in the presence of 12.5 μg/mL neutralizing antibody against mouse IFN-β and found that STAT1 phosphorylation in diffDCs was obstructed by IFN-β–neutralizing antibody (Figure 4C). These data showed that IFN-β secretion is responsible for phosphorylation of STAT1 in diffDCs. In addition, stimulation of diffDCs and imDCs with an equal amount of recombinant mouse IFN-β or IFN-α A induced higher production of IP-10 in diffDCs than that in imDCs, further confirming that elevated signaling pathway activity in diffDCs also contributes to the higher IP-10 production besides the preferential endogenous IFN-β or poly(I:C)-induced IFN-α/β secretion (Figure 4D–E). Furthermore, a lower dose of LPS (100 ng/mL), poly(I:C), and HSP70, but not CpG ODN and LTA, induced the increased phosphorylation of STAT1 in diffDCs more than that in imDCs (Figure 4F). Taken together, increased IRF-3 and IFN-β–induced STAT1 phosphorylation are responsible for the preferential IP-10 production by diffDCs in response to LPS stimulation.
diffDCs preferentially attract Th1 cells through the increased IP-10 and inhibit Th1 cell proliferation
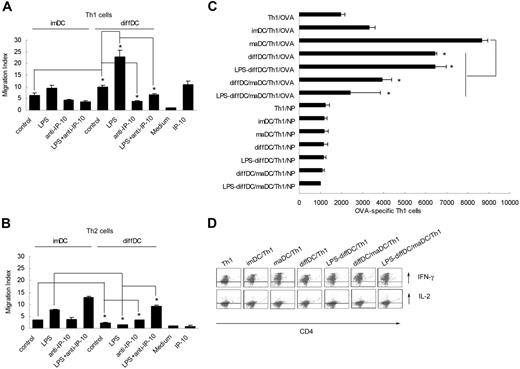
Finally, we investigated the biological significance of preferential IP-10 production by diffDCs in the immune regulation as exerted by diffDCs. Because IP-10 has opposing effects on CXCR3-bearing cells (Th1 cells) versus CCR3-bearing cells (Th2 cells) and can enhance the polarization of Th1 cell recruitment to sites of inflammation, we wondered whether diffDCs can preferentially chemoattract Th1 cells through IP-10.24,25 We incubated IP-10–neutralizing antibody with conditioned supernatant of LPS-stimulated diffDCs and imDCs for 30 minutes. Then, we used 10-fold diluted supernatant to chemoattract the purified Th1 or Th2 cells. As shown in Figure 5A–B, diffDCs could allure more Th1 but fewer Th2 cells. Moreover, neutralizing IP-10 resulted in a significant decrease in the number of chemoattracted Th1 cells but an increase in the number of chemoattracted Th2 cells, indicating that IP-10 in the supernatant of diffDCs is indispensable in chemoattracting Th1 cells and acts as an antagonist that inhibits CCR3-mediated chemotaxis.
diffDCs preferentially chemoattract Th1 via autocrine IP-10 in vitro. imDCs and diffDCs (1 × 106 cells/mL) cultured in medium alone or stimulated with 500 ng/mL LPS for 24 hours. Then IP-10–neutralizing antibody was incubated with the conditioned supernatant of diffDCs for 30 minutes. The supernatant was 10-fold diluted and used to chemoattract the Th1 or Th2 cells stained with CD4-FITC in 3.0-μm pore size transwell insert at 37°C. After 2 hours, Th1 (A) or Th2 (B) cells that had migrated into wells were harvested and counted by FACSCalibur flow cytometer and analyzed by CellQuest software. The results are expressed as migration index (number of cells that migrated in response to IP-10/number of cells that migrated in response to medium alone). *P < .05. (C) diffDCs inhibit Th1 cell proliferation. Purified CD4+ Th1 cells were cocultured with diffDCs as indicated in the presence of OVA323-339 peptide at a 1:10 (DC/T) ratio in round-bottom 96-well plates for 5 days. Then cells were collected and double stained with anti–CD4-FITC and 7-AAD and counted by FACS. Data are shown as mean ± SD of 3 independent experiments. (D) diffDCs sustain the intracellular expression of IL-2 and IFN-γ in Th1 cells. Purified CD4+ Th1 cells were cocultured with maDCs or diffDCs (LPS treated or not) in the presence of OVA peptides and brefeldin A for 24 hours. Intracellular expression of IL-2 and IFN-γ in Th1 cells was detected by flow cytometry (cytokine and CD4 double staining).
diffDCs preferentially chemoattract Th1 via autocrine IP-10 in vitro. imDCs and diffDCs (1 × 106 cells/mL) cultured in medium alone or stimulated with 500 ng/mL LPS for 24 hours. Then IP-10–neutralizing antibody was incubated with the conditioned supernatant of diffDCs for 30 minutes. The supernatant was 10-fold diluted and used to chemoattract the Th1 or Th2 cells stained with CD4-FITC in 3.0-μm pore size transwell insert at 37°C. After 2 hours, Th1 (A) or Th2 (B) cells that had migrated into wells were harvested and counted by FACSCalibur flow cytometer and analyzed by CellQuest software. The results are expressed as migration index (number of cells that migrated in response to IP-10/number of cells that migrated in response to medium alone). *P < .05. (C) diffDCs inhibit Th1 cell proliferation. Purified CD4+ Th1 cells were cocultured with diffDCs as indicated in the presence of OVA323-339 peptide at a 1:10 (DC/T) ratio in round-bottom 96-well plates for 5 days. Then cells were collected and double stained with anti–CD4-FITC and 7-AAD and counted by FACS. Data are shown as mean ± SD of 3 independent experiments. (D) diffDCs sustain the intracellular expression of IL-2 and IFN-γ in Th1 cells. Purified CD4+ Th1 cells were cocultured with maDCs or diffDCs (LPS treated or not) in the presence of OVA peptides and brefeldin A for 24 hours. Intracellular expression of IL-2 and IFN-γ in Th1 cells was detected by flow cytometry (cytokine and CD4 double staining).
Given the fact that diffDCs can preferentially chemoattract Th1 cells, we investigated whether diffDCs could affect Th1 function. We treated diffDCs and imDCs as indicated and then incubated with the purified CD4+ Th1 cells and assayed activation and proliferation of CD4+ Th1 cells. diffDCs strongly inhibited mature DC-induced proliferation of activated DO11.10 Th1 cells (Figure 5C), but did not interfere with intracellular IL-2 and IFN-γ expression elicited by mature DCs (Figure 5D), the same effects of diffDCs on the CD4+ T cells as observed previously.12 These results suggest that diffDCs can preferentially chemoattract Th1 cells through their increased IP-10 production, thus favoring to negatively regulate Th1 response by inhibiting Th1 cell proliferation.
Discussion
There are several kinds of regulatory DCs reported by different groups, which have similar functions but different phenotype. Most of regulatory DCs investigated now were induced by a high-dose immunosuppressive cytokine cocktail in vitro. Inhibition of T-cell proliferation is the functional hallmark of regulatory DCs. There are at least 3 mechanisms that may account for their regulatory properties, including lack of costimulating signal, deletion of antigen-reactive T cells by Fas/FasL system or tryptophan metabolites, and induction of Treg or T-cell anergy.26,27 However, we demonstrate that the inhibitory function of regulatory DCs (diffDCs) derived from maDCs under splenic stroma is mediated by NO, not mediated by inducing the production of Treg or T-cell anergy.12 In this study, we went further to understand the manner of regulatory diffDCs to negatively regulate T-cell response by the findings that regulatory diffDCs can selectively chemoattract Th1 cells through their increased preferential IP-10 production and then inhibit Th1 cell proliferation.
Following antigenic stimulation and multiple rounds of proliferation, CD4+ T cells can differentiate into Th1 and Th2 effectors. The selective recruitment of the Th1/Th2 subsets to an inflammatory site determines the character of the immune response. Although the mechanism of this selective recruitment remains to be established, recent work has shown that the pattern of chemokine plays a critical role in the selective recruitment of Th1 or Th2 cells.28 IP-10 binds and activates the 7 transmembrane G protein-coupled receptor CXCR3, which is a CXC chemokine receptor expressed in T cells (especially activated Th1 cells), B cells, NK cells, and DCs.14,29-31 The wide range of cells expressing CXCR3 suggests that IP-10 may play an important role in T-cell lymphopoiesis and the development of allergic and type 1 inflammatory responses. IP-10–deficient mice also had a defect in the generation and trafficking of effector T cells in vivo, indicating that IP-10 might also be essential to T-cell responses.24 In addition to CXCR3, IP-10 also binds CCR3, a CC chemokine receptor preferentially expressed on Th2 cells, and IP-10 acted as an antagonist in this case that inhibits CCR3-mediated chemotaxis.25 Increasing data support that IP-10 was dysregulated in many Th1-type inflammatory diseases, such as type 1 diabetes and atherosclerosis,32,33 and in autoimmune diseases, such as multiple sclerosis and myasthenia gravis.34,35 Tissue IP-10 levels correlate with the tissue infiltration of T lymphocytes in these diseases, suggesting that IP-10/CXCR3 signaling plays a potential role in the recruitment of T cells to the site of inflammation and autoimmune attack.
For the first time, we found that, compared with imDCs, regulatory diffDCs preferentially chemoattract Th1 cells by secreting a high level of IP-10. More interestingly, diffDCs could inhibit the proliferation of Th1 cells in the presence or absence of LPS stimulation. It is well known that migration is the basis of the infiltration of immune cells to the inflammatory tissue and lymphocyte recirculation. Thus, our results provide a better understanding of the manner of regulatory diffDCs for negative regulation of T-cell responses.
As to the mechanisms for preferential IP-10 production by regulatory diffDCs and more IP-10 production by diffDCs in response to TLR agonists, we show that the increased IRF-3 activity and autocrine IFN-β–induced STAT1 phosphorylation are responsible for the preferential IP-10 production by diffDCs in response to LPS. It has been shown that activation of TLR4 and TLR3 by LPS and poly(I:C) results in the induction of a set of IFN-α/β–induced genes. These gene inductions are regulated in a MyD88-independent (TRIF-dependent) manner and are activated by the transcription factor IRF-3.21,36-38 We showed that LPS, poly(I:C), and HSP70, but not CpG ODN and LTA, induced the increased phosphorylation of STAT1, indicating distinct TLR agonists play different roles in activation of STAT1 in diffDCs, which was in agreement with previous studies on activation of STAT1 by TLR agonists.23,39 Our present work shows that in contrast to imDCs, diffDCs produce more IFN-β at both protein and mRNA levels in response to LPS stimulation. Blockade of IFN-β before LPS stimulation by using a specific neutralizing antibody significantly reduces IP-10 production in diffDCs; however, IFN-α blockade has no effect on LPS-induced preferential IP-10 production by diffDCs. Moreover, poly(I:C) could induce more IFN-β and IFN-α production in diffDCs, and blockade of IFN-β or IFN-α could partially reduce poly(I:C)-mediated IP-10 production in diffDCs. Thus, it is likely that TLR agonists-induced autocrine IFN-α/β are responsible for the preferential IP-10 production in diffDCs. In resting cells, IRF-3 is located in cytoplasm. On stimulation with TLR agonists, IRF-3 is phosphorylated at multiple serine residues to form a homodimer that translocates into the nucleus and regulates the expression of IFN-β or other target genes.40-42 IRF3-defecient mice are defective in LPS-mediated induction of IFN-β and IFN-inducible genes but are intact in the production of inflammatory cytokines.22,42 diffDCs express a higher level of IRF-3 protein in nuclear extract and even more active IRF-3 after LPS stimulation than imDCs. These results suggest that the increased IP-10 production in diffDCs is dependent on IRF-3 and IFN-β. However, why coculture of maDCs with splenic stromal cells generates regulatory diffDCs with high IRF-3 expression needs further investigation.
LPS-induced IFN-β binds to type I IFN receptor in both an autocrine and paracrine manner and activates the classical JAK-STAT1 signaling pathway, which positively regulates feedback in LPS-induced IFN-β and can in turn induce other cytokines and chemokines including IP-10.23 Our data demonstrate that regulatory diffDCs express sharply higher STAT1 activity at the resting state as compared with imDCs, and STAT1 in diffDCs tends to be more significantly activated in an IFN-β-dependent manner after LPS treatment. STAT1 inhibitor fludarabine43 could markedly decrease the IP-10 production by diffDCs. Therefore, these results strongly confirm that LPS induces regulatory diffDCs to produce more IP-10 by activating IRF-3 and STAT1.
In conclusion, we demonstrate that splenic stromal cells can drive maDCs to differentiate to regulatory diffDCs characterized by preferential IP-10 secretion, contributing to negative regulation of the T-cell response by selectively chemoattracting Th1 cells and then inhibiting Th1 cell proliferation. The increased IRF-3 activity and autocrine IFN-β–induced STAT1 phosphorylation are responsible for the increased IP-10 production by diffDCs in response to TLR agonists.
Authorship
Contribution: X.C. and C.Q. designed the research project, analyzed, data and wrote the paper; and C.Q., H.A., Y.Y., and S.L. performed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xuetao Cao, Institute of Immunology and National Key Laboratory of Medical Immunology, Second Military Medical University, 800 Xiangyin Rd, Shanghai, 200433, People's Republic of China; e-mail: caoxt@public3.sta.net.cn.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (30418017, 30490240, 30121002), National Key Basic Research Program of China (2003CB515503, 2001CB510002), and Shanghai Committee of Science and Technology.
We thank Dr Zhenhong Guo and Dr Hongmei Xu for helpful discussion and Mrs X. Ma and S. Li for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal