Abstract
The tumor suppressor PTEN is mutated in many human cancers. We previously used the Cre-loxP system to generate mice (LckCrePten mice) with a Pten mutation in T-lineage cells. Here we describe the phenotype of Pten-deficient Vα14iNKT cells. A failure in the development of Vα14iNKT cells occurs in the LckCrePten thymus between stage 2 (CD44highNK1.1−) and stage 3 (CD44highNK1.1+), resulting in decreased numbers of peripheral Vα14iNKT cells. In vitro, Pten-deficient Vα14iNKT cells show reduced proliferation and cytokine secretion in response to αGalCer stimulation but enhanced inhibitory Ly49 receptor expression. Following interaction with dendritic cells (DCs) loaded with αGalCer, Pten-deficient Vα14iNKT cells demonstrate activation of PI3K. Indeed, the effects of the Pten mutation require intact function of the PI3K subunits p110γ and p110δ. In vivo, LckCrePten mice display reduced serum IFNγ after αGalCer administration. Importantly, Vα14iNKT cell–mediated protection against the metastasis of melanoma cells to the lung was impaired in the absence of Pten. Thus, the Pten/PI3K pathway is indispensable for the homeostasis and antitumor surveillance function of Vα14iNKT cells.
Introduction
Vα14iNKT cells, originally defined as NK1.1+ T cells,1-3 are a unique lymphoid lineage characterized by the expression of several receptors that distinguish them from T, B, and natural killer (NK) cells. These receptors include an invariant rearranged T-cell receptor α (TCRα) chain, NK1.1, the IL-2/IL-15Rβ chain (CD122), and Ly49. The TCRα chain expressed by Vα14iNKT cells is encoded by the Vα14 and Jα18 gene segments and is usually associated with a TCRβ chain containing the Vβ8 gene segment, a combination not expressed by conventional T cells.4 Vα14iNKT cells constitute more than half of the NK1.1+ T-cell population in the murine spleen and most NK1.1+ T cells in the thymus and liver.5 Vα14iNKT cells respond to glycolipid antigens such as the marine sponge–derived glycolipid α-galactosylceramide (αGalCer). αGalCer is presented to NKT cells by CD1d, an antigen presentation molecule that structurally resembles major histocompatibility complex (MHC) class I. Because of their restricted specificity, Vα14iNKT cells can be readily identified by staining with CD1d tetramers or dimers loaded with αGalCer.6,7
During their development in the thymus, Vα14iNKT precursors at the CD4+CD8+ (double positive [DP]) stage are positively selected by CD1d-mediated presentation of self-glycolipids (such as iGb3) by CD4+CD8+ thymocytes.8,9 Vα14iNKT precursors (CD44lowNK1.1−; stage 1) then develop sequentially into immature NKT cells (CD44highNK1.1−; stage 2) and finally into mature Vα14iNKT cells (CD44highNK1.1+; stage 3).10,11 Vα14iNKT cells apparently also undergo negative selection such that NKT cell maturation is severely reduced in situations in which activation signals for developing cells are enhanced to supraphysiological levels either in vitro or in vivo.12-16
In response to TCR ligation, mature peripheral Vα14iNKT cells promptly produce large amounts of Th1 cytokines (IFNγ, TNF) as well as Th2 cytokines (IL-4, IL-10, IL-13).17,18 Vα14iNKT cells therefore play important roles in a variety of innate and adaptive immune responses, including those mediating autoimmunity or combating microbial infections or tumor metastasis.5,19-21 Indeed, mice deficient in Vα14iNKT cells show impaired tumor rejection.21 This observation is consistent with the antitumor effect of αGalCer administration on various tumor types, including melanomas and lung, colon, and hematopoietic cancers.22 Despite the importance of Vα14iNKT cells, little is known about their intracellular signaling pathways or how these pathways might be regulated.
PTEN is a tumor suppressor gene23 that is mutated in many human sporadic cancers and in hereditary tumor susceptibility disorders such as Cowden disease.24 PTEN is a multifunctional phosphatase whose major substrate is phosphatidylinositol-3,4,5-triphosphate (PIP3),25 a lipid second messenger molecule. PIP3 is generated by the action of phosphoinositide-3-kinases (PI3Ks). Four distinct class I PI3K isotypes exist in mammals: PI3Kα, β, δ, and γ.26 The function of the PIP3 generated by PI3K is to activate numerous downstream targets, including the serine-threonine kinase PKB/Akt that is involved in antiapoptosis, proliferation, and oncogenesis. By using its lipid phosphatase activity to dephosphorylate PIP3, PTEN negatively regulates the PI3K-PKB/Akt pathway and thereby exerts tumor suppression.27 Thus, murine T cells lacking Pten show enhanced proliferation and activation in vitro, and T cell–specific Pten-deficient mice develop T-cell lymphomas/leukemias and are prone to autoimmunity.28 Conversely, mice deficient for the PI3K subunit p110γ show impaired T-cell development and activation,29 and T cells from animals with a kinase-inactivating mutation (D910A) of p110δ have a defect in TCR-stimulated proliferation.30 Analyses of these and other genetically modified mice have begun to provide insights into the pathways and molecules mediating intracellular signaling in Vα14iNKT cells. However, the specific functions of PI3K and Pten in these cells are totally unknown.
In this study, we demonstrate that mutation of Pten in murine Vα14iNKT cells results in developmental failure leading to increased numbers of immature Vα14iNKT cells and decreased numbers of mature Vα14iNKT cells of impaired functionality. Moreover, these defects depend on PI3K function. Our results indicate that the Pten/PI3K pathway is important for the homeostasis of Vα14iNKT cells and their antitumor surveillance function.
Materials and methods
Mice
LckCrePtenflox/flox,28 Pten+/−,31 PI3K p110γ−/−,29 PI3K p110δD910A/D910A,30 Jα18−/−,21 and AktPH-GFPtg/tg transgenic32 mice (all C57BL6/J background, H-2b) have been previously described. Because LckCrePtenflox/flox mice begin to develop T-cell lymphomas at age 3 months, all experiments were performed using lymphoma-free mice younger than 8 weeks of age. LckCrePten+/+ and Ptenflox/flox mice28 were indistinguishable in pilot experiments examining flow cytometry, cell proliferation, and cytokine production (data not shown), so that Ptenflox/flox mice were used as representative wild-type (WT) controls in this study. The Institutional Review Board of the Akita University School of Medicine approved all animal experiments.
Reagents and melanoma cell line
A synthetic form of αGalCer (KRN7000) was provided by the Kirin Brewery (Gumma, Japan). Antibodies to TCRβ-FITC (H57-597), NK1.1-FITC or -PE (PK136), CD24/HSA-biotin (30-F1), and PE-Cy5-streptavidin were purchased from eBioscience (San Diego, CA). Antibodies to TCRβ-APC (H57-597), CD45R/B220-APC (RA3-6B2), Ly49C/I-biotin (5E6), Ly49A-biotin (A1), CD44-FITC (IM7), mouse IgG1–biotin (A85-1), and mouse CD1d dimmer–Ig fusion protein were purchased from BD PharMingen (San Diego, CA). The B16F10 mouse melanoma cell line was supplied by the Cell Resource Center for Biomedical Research, Tohoku University, Japan.
Flow cytometry and cell sorting
Mononuclear cells (MNCs) from the thymus, liver, and spleen were obtained as previously described.33 For routine staining of Vα14iNKT cells, MNCs were first preincubated with CD16/32 mAb to prevent nonspecific binding of antibodies to FcγR. The cells were then incubated with CD1d dimmer–Ig (αGalCer-CD1d)7 followed by anti–mouse IgG1–biotin, PE-Cy5-streptavidin, and anti-TCRβ–APC. To analyze NKT cell development, cells were also stained with anti-NK1.1–PE and anti-CD44–FITC. To detect Ly49 expression, cells were stained with anti-NK1.1–FITC, anti-TCRβ–APC, CD1d dimmer–Ig (αGalCer-CD1d), anti–mouse IgG1–PE, anti-Ly49C/I–biotin (or anti-Ly49A–biotin), and streptavidin-PE-Cy5. All flow cytometry and sorting experiments were performed using MoFlo and Summit software (Dako Cytomation, Glostrup, Denmark).
PCR and Western blotting
For analyzing the deletion frequency of the Pten gene at the DNA level, genomic DNA samples from sorted TCRβ+NK1.1+HSAlow thymocytes of 8-week-old Ptenflox/flox and LckCrePtenflox/flox mice were amplified by polymerase chain reaction (PCR) as described.34 Sense primer (5′-GTGAAAGTGCCCCAACATAAGG-3′) and antisense primer (5′-CTCCCACCAATGAACAAACAGT-3′) were used to detect the WT and floxed Pten alleles; sense primer (5′-GGCCTAGGACTCACTAGATAGC-3′) and antisense primer (5′-CTCCCACCAATGAACAAACAGT-3′) were used to detect the deleted PtenΔ allele. Amplified fragments of 514 bp (Ptenflox allele) and 705 bp (PtenΔ allele) were obtained.
Total lysates (10 μg) of sorted TCRβ+NK1.1+HSAlow thymocytes from 8-week-old Ptenflox/flox and LckCrePtenflox/flox mice were analyzed by Western blotting using antibodies directed against Pten (Cascade Bioscience, Winchester, MA) or actin (Santa Cruz Biotechnology, Santa Cruz, CA).
In vitro and in vivo activation of NKT cells
For in vitro activation of NKT cells, thymocytes were isolated from 8-week-old LckCrePtenflox.flox or Ptenflox.flox mice, and NK1.1+TCRβ+HSAlow NKT cells were purified by cell sorting. αGalCer-loaded dendritic cells (DCs) were prepared from spleens as described.35 Briefly, spleens from WT mice were digested using collagenase and erythrocytes, and MNCs were collected by step centrifugation using 28% bovine serum albumin and serumfree RPMI medium. Isolated MNCs were cultured for 2 hours, and adherent cells were further cultured overnight with or without 100 ng/mL αGalCer. Nonadherent CD11c+ cells were plated at 2 × 104 per well in a 96-well plate and γ-irradiated (30 Gy). These cells were used as the αGalCer-loaded DCs. Purified NKT cells (1 × 104 per well) were incubated with αGalCer-loaded DCs in 96-well plates in RPMI 1640 medium containing 10% FCS. The αGalCer-stimulated NKT cells were harvested on day 2, and their proliferation was assayed by pulsing for 12 hours with 0.5 μCi (0.0185 MBq) [3H]-thymidine (Amersham, Arlington Heights, IL) per well and measuring [3H]-thymidine incorporation by standard methods. Culture supernatants were assayed for the production of IFNγ and IL-4 by ELISA (Pierce Endogen, Rockford, IL).
For in vivo activation of NKT cells, αGalCer (2 μg) was intraperitoneally injected into 8-week-old Ptenflox/flox, Pten+/−, and LckCrePtenflox/flox mice. Serum IL-4 and IFNγ levels were examined by enzyme-linked immunosorbent assay (ELISA) at 2 hours and 12 hours after αGalCer injection, respectively.
NKT cell maturation in FTOC
Fetal thymus organ culture (FTOC) was performed as described11 with some modifications. Fetal thymus lobes from embryonic day 14.5 Jα18−/− fetal mice were cultured for 3 days on Nucleopore membrane (Whatman, Middlesex, United Kingdom) in RPMI medium supplemented with 10% FCS, nonessential amino acids, pyruvate, and β-mercaptoethanol (all from Invitrogen, Carlsbad, CA). Purified CD44highNK1.1−αGalCer-CD1d+TCRβ+ immature Vα14iNKT cells (1 × 105) isolated from the thymus of a 3-week-old LckCrePtenflox/flox or Ptenflox/flox mouse were microinjected into each thymic lobe of a Jα18−/− embryo and cultured for additional 9 days. The maturation of the injected NKT cells was evaluated by flow cytometry as described.
Tumor immunity
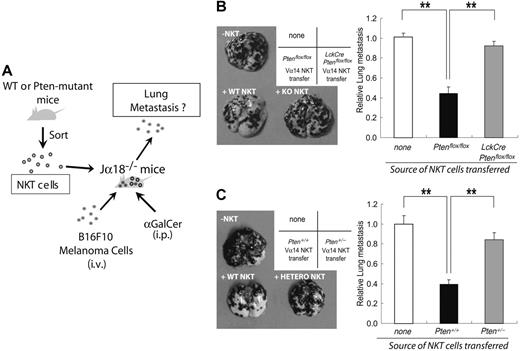
The B16F10 melanoma cell line was maintained in the same culture medium as that used for FTOC. Jα18−/− mice (8 weeks old) were intravenously inoculated with B16F10 cells (4 × 105). After 3 hours, these mice were intravenously injected with sorted αGalCer-CD1d+TCRβ+ thymocytes (1 × 106) from 8-week-old Ptenflox/flox, LckCrePtenflox/+, or LckCrePtenflox/flox mice. After 30 minutes, 2 μg αGalCer was intraperitoneally injected into the mice. On days 4 and 8 after cell transfer, the mice received an additional 2 μg αGalCer. The mice were killed on day 14, and the number of surface lung metastases was evaluated with aid of a dissecting microscope. The data were recorded as the mean number of metastases per lung ± SEM.
Confocal video microscopy
TCRβ+NK1.1+HSAlow NKT cells (1 × 104) purified from thymocytes of 8-week-old AktPH-GFPtg/tg mice and DCs (1 × 105) either loaded or not with αGalCer were coplated in fibronectin-coated glass-base dishes for 10 minutes. In some experiments, the PI3K inhibitor wortmannin (10 nM; Sigma, St Louis, MO) was added before the cells were mixed. Time-lapse studies to detect PIP3 identified by the AktPH-GFP bioprobe32 were performed at 37°C using a heating device (Harvard Apparatus, Holliston, MA) adapted to a DM IRE2 Leica (Wetzlar, Germany) microscope fitted with a confocal imaging system (Yokogawa, Tokyo, Japan) and equipped with a Leica HCX APO 100×/1.30 numerical aperture objective. Confocal fluorescent or differential interface contrast (Nomarski optics) images were acquired using an Orca-ER cooled charge-coupled-device (CCD) camera (Hamamatsu Photonics, Hamamatsu, Japan) driven by IPLab software version 3.0.4 (BD Bioscience).
Statistical methods
Statistical differences were determined using the Student t test.
Results
Pten deficiency in NKT cells of LckCrePtenflox/flox mice
We previously reported a conditional Pten knockout mouse in which the Pten gene was deleted by Lck, a T cell–specific promoter (LckCrePtenflox/flox mice).28 Genomic PCR examination of DNA from purified TCRβ+NK1.1+HSAlow thymocytes from 8-week-old Ptenflox/flox and LckCrePtenflox/flox mice showed that highly efficient Cre-mediated recombination had occurred not only in conventional T cells but also in NKT cells (Figure 1A). The deletion of Pten was confirmed at the protein level by Western blotting (Figure 1B).
Pten gene deletion in NKT cells in LckCrePtenflox/flox mice. (A) Genomic DNA from TCRβ+NK1.1+HSAlow thymocytes of the indicated genotype was analyzed by PCR. In LckCrePtenflox/flox mice, most TCRβ+NK1.1+HSAlow thymocytes contained the deleted Pten allele (Δ) rather than the WT Pten allele (flox). (B) Western blot analysis of Pten protein expression by the thymocytes in panel A. Actin loading control.
Pten gene deletion in NKT cells in LckCrePtenflox/flox mice. (A) Genomic DNA from TCRβ+NK1.1+HSAlow thymocytes of the indicated genotype was analyzed by PCR. In LckCrePtenflox/flox mice, most TCRβ+NK1.1+HSAlow thymocytes contained the deleted Pten allele (Δ) rather than the WT Pten allele (flox). (B) Western blot analysis of Pten protein expression by the thymocytes in panel A. Actin loading control.
Impaired development of NKT cells in Pten-deficient mice
To examine the role of Pten in NKT cell development, we analyzed the NK1.1+TCRβ+ NKT population in the thymus, spleen, and liver of 7- to 8-week-old LckCrePtenflox/flox and Ptenflox/flox mice. LckCrePtenflox/flox mice showed reductions not only in the percentages of NK1.1+TCRβ+ cells present in all 3 tissues (Figure 2A) but also in absolute numbers of these NKT cells in the thymus (4.5-fold decrease), spleen (2.1-fold), and liver (4.3-fold) (Figure 2B).
Altered NKT cell populations in LckCrePtenflox/flox mice. (A-B) Decreased numbers of NK1.1+TCRβ+ cells in LckCrePtenflox/flox mice. (A) MNCs were isolated from the thymus, spleen, and liver of mice of the indicated genotypes, and NK1.1+TCRβ+ cells were analyzed by flow cytometry. Numbers within the panels indicate the relative percentages of NK1.1+TCRβ+ cells in total lymphocyte populations. (B) Absolute numbers of the NK1.1+TCRβ+ cells in panel A. (C-E) Altered maturation and numbers of Vα14iNKT cells. (C, left panel, and D) The percentage of Vα14iNKT cells in total MNCs is normal in the thymus of LckCrePtenflox/flox mice but decreased in spleen and liver. (C, right panel, and E) Stage 2 (CD44+NK1.1−) Vα14iNKT cells were increased, but stage 3 (CD44+NK1.1+) cells were decreased in the thymus of LckCrePtenflox/flox mice. The percentages of NKT subsets in αGalCer-CD1d+TCRβ+-gated thymocytes (C, right panel) and in total MNCs in thymus (E) are shown. For panels B, D, and E, results are expressed as the mean ± SEM for 3 mice per group. **P < .01.
Altered NKT cell populations in LckCrePtenflox/flox mice. (A-B) Decreased numbers of NK1.1+TCRβ+ cells in LckCrePtenflox/flox mice. (A) MNCs were isolated from the thymus, spleen, and liver of mice of the indicated genotypes, and NK1.1+TCRβ+ cells were analyzed by flow cytometry. Numbers within the panels indicate the relative percentages of NK1.1+TCRβ+ cells in total lymphocyte populations. (B) Absolute numbers of the NK1.1+TCRβ+ cells in panel A. (C-E) Altered maturation and numbers of Vα14iNKT cells. (C, left panel, and D) The percentage of Vα14iNKT cells in total MNCs is normal in the thymus of LckCrePtenflox/flox mice but decreased in spleen and liver. (C, right panel, and E) Stage 2 (CD44+NK1.1−) Vα14iNKT cells were increased, but stage 3 (CD44+NK1.1+) cells were decreased in the thymus of LckCrePtenflox/flox mice. The percentages of NKT subsets in αGalCer-CD1d+TCRβ+-gated thymocytes (C, right panel) and in total MNCs in thymus (E) are shown. For panels B, D, and E, results are expressed as the mean ± SEM for 3 mice per group. **P < .01.
We next investigated numbers of Vα14iNKT cells, a major subset of NKT cells, using the reagent CD1d dimer–Ig (αGalCer-CD1d).7 The thymi of WT and LckCrePtenflox/flox mice contained equal numbers of αGalCer-CD1d+TCRβ+NKT cells (Vα14iNKT cells) (Figure 2C–D, left panels), indicating that Pten deficiency has no effect on total Vα14iNKT cell numbers. We then determined CD44/NK1.1 profiles10,11 to follow the development of Vα14iNKT cells in thymus. While about 80% of thymic Vα14iNKT cells in Ptenflox/flox mice were mature and had reached stage 3 (CD44highNK1.1+), only 9% of Vα14iNKT cells in LckCrePtenflox/floxmice were at stage 3 and most had arrested at stage 2 (immature CD44highNK1.1− cells) (Figure 2C, right panel, and E). No differences were observed between the WT and mutant thymus in the percentage of stage 1 NKT precursors (CD44lowNK1.1−) present. When proliferative capacity was examined, there was no difference between the WT and the mutant in the percentage of BrdU-positive cells in the stage 2 population (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Thus, a block in differentiation is the most likely explanation for the observed increase in stage 2 cells in the mutant thymus. In the periphery, Vα14iNKT cells in LckCrePtenflox/flox mice were decreased in the spleen and liver to 51% and 48% of WT values, respectively (Figure 2C–D). These decreases had to be due to reductions in mature Vα14iNKT cells because no increases were observed in the percentage of NK1.1− Vα14iNKT cells in mutant spleen and liver (Figure S1B).
Vα14iNKT cells are positively selected in the thymus via the interaction of their TCRs with CD1d expressed by DP cortical thymocytes and negatively selected by CD1d expressed by antigen-presenting cells such as DCs.8,12 Thus, normal CD1d expression on DP thymocytes and thymic/peripheral DCs is required for proper NKT cell development.8,9 Flow cytometric analysis revealed no differences in CD1d levels expressed by DP thymocytes or thymic or splenic DCs from Ptenflox/flox and LckCrePtenflox/flox mice (Figure S1C). These data indicate that the impaired development of Pten-deficient Vα14iNKT cells is not due to altered CD1d expression by antigen-presenting cells.
The developmental failure of Vα14iNKT cells in LckCrePtenflox/flox mice is cell autonomous
The Pten gene is deleted in both NKT cells and T cells in LckCrePtenflox/flox mice, and DP T cells are an important stromal component for normal NKT cell development in the thymus.8,9,12 To rule out the possibility that it was the Pten mutation in DP T cells that caused the decrease in NKT cells in LckCrePtenflox/flox mice, purified CD44highNK1.1−αGalCer-CD1d+TCRβ+ immature Vα14iNKT cells (stage 2) from Ptenflox/flox and LckCrePtenflox/flox mice were transferred into Jα18−/− fetal thymus (which lacks all Vα14iNKT cells) and cultured for 9 days using the FTOC method.11 As shown in Figure 3, more than 60% of immature Ptenflox/flox Vα14iNKT cells developed into mature Vα14iNKT cells (CD44highNK1.1+αGalCer-CD1d+TCRβ+; stage 3) in this environment. In contrast, almost all transferred immature LckCrePtenflox/flox Vα14iNKT cells remained at stage 2. This result clearly indicates that the reduced number of Vα14iNKT cells in LckCrePtenflox/flox mice was due to a cell-autonomous defect.
Failure of Pten-deficient immature Vα14iNKT cells to mature in vitro. CD44highNK1.1− (stage 2, immature) Vα14iNKT cells were purified from the thymi of Ptenflox/flox or LckCrePtenflox/flox mice and injected into FTOCs derived from a Jα18−/− embryo. (Left) Flow cytometric analysis of cell populations injected. (Right) At 9 days after injection, Vα14iNKT cells in the FTOC were isolated and analyzed by flow cytometry to detect stage 3 (mature) cells. Mature Vα14iNKT cells were very rare in FTOCs receiving Pten-deficient stage 2 NKT cells. One result representative of 3 experiments is shown.
Failure of Pten-deficient immature Vα14iNKT cells to mature in vitro. CD44highNK1.1− (stage 2, immature) Vα14iNKT cells were purified from the thymi of Ptenflox/flox or LckCrePtenflox/flox mice and injected into FTOCs derived from a Jα18−/− embryo. (Left) Flow cytometric analysis of cell populations injected. (Right) At 9 days after injection, Vα14iNKT cells in the FTOC were isolated and analyzed by flow cytometry to detect stage 3 (mature) cells. Mature Vα14iNKT cells were very rare in FTOCs receiving Pten-deficient stage 2 NKT cells. One result representative of 3 experiments is shown.
Impaired activation of Pten-deficient NKT cells in vitro and in vivo
We next investigated the functional status of Pten-deficient Vα14iNKT cells by determining their proliferation and cytokine production. When total cells from WT thymus were stained with CD1d dimer–Ig and anti-TCRβ antibody, the Vα14iNKT fraction was found to be slightly activated prior to any exogenous stimulation. We therefore purified NK1.1+TCRβ+ cells from Ptenflox/flox and LckCrePtenflox/flox thymi and activated these cells using DCs that had been loaded in vitro with αGalCer. In response to this activation, NK1.1+TCRβ+ mature NKT cells from Ptenflox/flox thymus showed high levels of proliferation (Figure 4A) as well as vigorous production of IL-4 (Figure 4B) and IFNγ (Figure 4C). In contrast, NK1.1+TCRβ+ mature NKT cells from LckCrePtenflox/flox thymus showed severely impaired proliferation and cytokine production (Figure 4A–C). These results suggest that Pten is required not only for the normal development of NKT cells but also for their functions.
Impaired activation of Pten-mutant NKT cells in vitro and in vivo. (A) Impaired proliferation. NK1.1+TCRβ+ cells were purified from the thymi of Ptenflox/flox or LckCrePtenflox/flox mice and cocultured with DCs loaded with vehicle (DC) or αGalCer (DC-αGC). After 48 hours, cells were pulsed with [3H]-thymidine for 12 hours and thymidine uptake was measured. (B-C) Impaired cytokine production. IL-4 and IFNγ in the supernatants of the cultures in panel A were measured by ELISA. (D-E) Impaired response of Vα14iNKT cells to αGalCer administration in vivo. αGalCer was intraperitoneally injected into Ptenflox/flox, Pten+/−, or LckCrePtenflox/flox mice, and serum concentrations of IL-4 (D) and IFNγ (E) were evaluated at 2 hours and 12 hours after αGalCer injection, respectively. For all panels, data are expressed as the mean value ± SEM for 3 mice per group. **P < .01.
Impaired activation of Pten-mutant NKT cells in vitro and in vivo. (A) Impaired proliferation. NK1.1+TCRβ+ cells were purified from the thymi of Ptenflox/flox or LckCrePtenflox/flox mice and cocultured with DCs loaded with vehicle (DC) or αGalCer (DC-αGC). After 48 hours, cells were pulsed with [3H]-thymidine for 12 hours and thymidine uptake was measured. (B-C) Impaired cytokine production. IL-4 and IFNγ in the supernatants of the cultures in panel A were measured by ELISA. (D-E) Impaired response of Vα14iNKT cells to αGalCer administration in vivo. αGalCer was intraperitoneally injected into Ptenflox/flox, Pten+/−, or LckCrePtenflox/flox mice, and serum concentrations of IL-4 (D) and IFNγ (E) were evaluated at 2 hours and 12 hours after αGalCer injection, respectively. For all panels, data are expressed as the mean value ± SEM for 3 mice per group. **P < .01.
To examine cytokine production by Vα14iNKT cells in response to αGalCer in vivo, we intraperitoneally injected αGalCer into Ptenflox/flox, Pten+/−, and LckCrePtenflox/flox mice. Administration of αGalCer to WT mice led to a rapid increase in serum IL-4 and IFNγ levels within 2 hours and 12 hours, respectively (Figure 4D–E). It is known that Vα14iNKT cells are solely responsible for the production of these cytokines at these time points.22 Accordingly, in both Pten+/− and LckCrePtenflox/flox mice that received intraperitoneal injection of αGalCer, serum levels of IFNγ were severely decreased. αGalCer-treated LckCrePtenflox/flox mice also showed a significant decrease in serum IL-4 whereas Pten+/− mice showed only a slight (insignificant) reduction. These data indicate that, as well as reducing their numbers, mutation of Pten functionally impairs mature Vα14iNKT cells both in vitro and in vivo.
Pten-deficient Vα14iNKT cells show defective antitumor immunity
Because Vα14iNKT cells were abnormal in number and function in LckCrePtenflox/flox mice, we investigated whether these animals had a defect in Vα14iNKT-mediated antitumor immunity in vivo. First, we purified αGalCer-CD1d+TCRβ+ (Vα14iNKT) cells from Ptenflox/flox mice and adoptively transferred them into αGalCer-treated Jα18−/− (Vα14iNKT cell–deficient) recipients that had been inoculated with B16F10 melanoma cells (Figure 5A). The presence of WT Vα14iNKT cells markedly inhibited the metastasis of B16F10 cells to the lungs (about 60% reduction; Figure 5B–C), consistent with previous observations.36 In contrast, only a minor inhibition of B16F10 metastasis was achieved using Vα14iNKT cells from αGalCer-treated Pten+/− mice (16% reduction; Figure 5C) or αGalCer-treated LckCrePtenflox/flox mice (8% reduction; Figure 5B). These findings show that mutation of Pten in Vα14iNKT cells impairs antitumor immunity.
Defective antitumor surveillance by Pten-deficient Vα14iNKT cells. (A) Scheme describing the experimental protocol used to evaluate antitumor surveillance by NKT cells in vivo. Vα14iNKT cells were purified from mice of the indicated genotypes and adoptively transferred into Jα18−/− mice that had been inoculated with B16F10 melanoma cells. The mice were then injected with αGalCer at 0, 4, and 8 days after NKT cell transfer. After 14 days, numbers of focal lung metastases were counted. (B-C, left panels) Increased lung metastasis. Quadrant scheme in the upper corners shows the labeling of the cell images. “None” indicates controls in which no cells were transferred. Representative images of lung metastases 2 weeks after the transfer of Ptenflox/flox or LckCrePtenflox/flox Vα14iNKT cells (B) or after the transfer of Pten+/+ or Pten+/− Vα14iNKT cells (C) are shown. (B-C, right panels) Relative numbers of the lung metastases identified in the left panels. Results shown are mean ± SEM of 18 mice that received no cells and 6 mice each that received Vα14iNKT cells. **P < .01.
Defective antitumor surveillance by Pten-deficient Vα14iNKT cells. (A) Scheme describing the experimental protocol used to evaluate antitumor surveillance by NKT cells in vivo. Vα14iNKT cells were purified from mice of the indicated genotypes and adoptively transferred into Jα18−/− mice that had been inoculated with B16F10 melanoma cells. The mice were then injected with αGalCer at 0, 4, and 8 days after NKT cell transfer. After 14 days, numbers of focal lung metastases were counted. (B-C, left panels) Increased lung metastasis. Quadrant scheme in the upper corners shows the labeling of the cell images. “None” indicates controls in which no cells were transferred. Representative images of lung metastases 2 weeks after the transfer of Ptenflox/flox or LckCrePtenflox/flox Vα14iNKT cells (B) or after the transfer of Pten+/+ or Pten+/− Vα14iNKT cells (C) are shown. (B-C, right panels) Relative numbers of the lung metastases identified in the left panels. Results shown are mean ± SEM of 18 mice that received no cells and 6 mice each that received Vα14iNKT cells. **P < .01.
PI3K signaling is responsible for the phenotypes of Pten-deficient Vα14iNKT cells
To determine whether PI3K was activated in Vα14iNKT cells that interacted with αGalCer-loaded DCs, we analyzed levels of the PI3K product PIP3. Purified Vα14iNKT cells expressing the fusion bioprobe AktPH-GFP32 were cultured with αGalCer-loaded DCs in fibronectin-coated glass-base dishes. As shown in Figure 6A, the AktPH-GFP bioprobe was recruited to the plasma membrane of WT Vα14iNKT cells that interacted with αGalCer-loaded DCs (solid arrow) but remained in the cytoplasm of WT Vα14iNKT cells that did not make contact with the DCs (dotted arrows). In addition, the bioprobe was not recruited to the plasma membrane of WT Vα14iNKT cells that interacted with αGalCer-loaded DCs in the presence of wortmannin (a pan-PI3K inhibitor) or in Vα14iNKT cells that contacted DCs lacking αGalCer (Figure 6B). These findings suggest that PI3K is activated in Vα14iNKT cells when these cells are stimulated by interaction with αGalCer-loaded DCs.
PI3K is activated in Vα14iNKT cells that interact with αGalCer-loaded DCs. Localization of PIP3 to the NKT cell membrane. TCRβ+NK1.1+HSAlow NKT cells were purified from thymocytes of 8-week-old AktPH-GFPtg/tg mice (WT for Pten) expressing the PIP3-binding AktPH-GFP bioprobe I (left). These Vα14iNKT cells were incubated with αGalCer-loaded DCs, and PIP3 localization was determined by confocal fluorescence (left) and phase contrast (right) microscopy. (A) Vα14iNKT cells that made contact with αGalCer-loaded DCs showed membrane localization of PIP3 (solid arrow), while PIP3 remained in the cytoplasm of Vα14iNKT cells that did not make contact with DCs (dotted arrows). (B) AktPH-GFP bioprobe localization to the plasma membrane of Vα14iNKT cells was inhibited by wortmannin or by contact with DCs lacking αGalCer.
PI3K is activated in Vα14iNKT cells that interact with αGalCer-loaded DCs. Localization of PIP3 to the NKT cell membrane. TCRβ+NK1.1+HSAlow NKT cells were purified from thymocytes of 8-week-old AktPH-GFPtg/tg mice (WT for Pten) expressing the PIP3-binding AktPH-GFP bioprobe I (left). These Vα14iNKT cells were incubated with αGalCer-loaded DCs, and PIP3 localization was determined by confocal fluorescence (left) and phase contrast (right) microscopy. (A) Vα14iNKT cells that made contact with αGalCer-loaded DCs showed membrane localization of PIP3 (solid arrow), while PIP3 remained in the cytoplasm of Vα14iNKT cells that did not make contact with DCs (dotted arrows). (B) AktPH-GFP bioprobe localization to the plasma membrane of Vα14iNKT cells was inhibited by wortmannin or by contact with DCs lacking αGalCer.
The PI3K signaling pathway leads to PKB/Akt activation, an event that is negatively regulated by Pten. Accordingly, Pten deficiency usually leads to enhanced PI3K signaling and cellular hyperactivation and hyperproliferation.26,28 Curiously, Pten deficiency in Vα14iNKT cells led to impaired cell numbers and functions. We therefore examined the role of PI3K in generating the phenotypes of Pten-deficient NKT cells. The PI3K subunit p110δ is highly expressed in hematopoietic cells and is the major catalytic subunit of class IA PI3Ks in T-lineage cells.30 The PI3K subunit p110γ, which is a unique catalytic subunit responsible for class IB PI3K activation downstream of G protein–coupled receptors (GPCRs), is also highly expressed in lymphocytes and important for the normal development of T-lineage cells.29 To determine whether PI3K signaling was responsible for the defects of Pten-deficient Vα14iNKT cells, we examined double-mutant mice lacking Pten and PI3K p110γ29 (LckCrePtenflox/flox/p110γ−/−) or Pten and PI3K p110δ30 (LckCrePtenflox/flox/p110δD910A/D910A). The mutation of p110γ partially rescued Vα14iNKT cell numbers in LckCrePtenflox/flox mice (Figure 7A, upper panel) and completely rescued the proliferation defect of Pten-deficient Vα14iNKT cells (Figure 7B). A slightly weaker but still significant effect was seen upon mutation of p110δ. Moreover, numbers of peripheral Pten-deficient Vα14iNKT cells were restored to near-normal levels in both strains of double-mutant mice (data not shown). These results indicate that excessive PI3K signaling contributes to the phenotypes of Pten-deficient Vα14iNKT cells.
The phenotypes observed in Pten-deficient NKT cells are PI3K dependent. (A, top) Decreased maturation. Thymic Vα14iNKT cells from mice of the indicated genotypes were analyzed by flow cytometry for percentages of stage 3 cells. (A, bottom) Increased expression of Ly49C/I receptors. The αGalCer-CD1d+TCRβ+NK1.1+ cells in the top panel were analyzed by flow cytometry to determine relative expression levels of Ly49C/I. (B) Impaired proliferation. The NK1.1+TCRβ+ cells from panel A were stimulated by incubation with αGalCer-loaded DCs, and [3H]-thymidine incorporation was measured. Data shown are the mean ± SEM for 3 mice per group. **P < .01. For both panels, the defects of Pten-deficient Vα14iNKT cells were partially rescued by mutation of a PI3K subunit (p110γ or p110δ).
The phenotypes observed in Pten-deficient NKT cells are PI3K dependent. (A, top) Decreased maturation. Thymic Vα14iNKT cells from mice of the indicated genotypes were analyzed by flow cytometry for percentages of stage 3 cells. (A, bottom) Increased expression of Ly49C/I receptors. The αGalCer-CD1d+TCRβ+NK1.1+ cells in the top panel were analyzed by flow cytometry to determine relative expression levels of Ly49C/I. (B) Impaired proliferation. The NK1.1+TCRβ+ cells from panel A were stimulated by incubation with αGalCer-loaded DCs, and [3H]-thymidine incorporation was measured. Data shown are the mean ± SEM for 3 mice per group. **P < .01. For both panels, the defects of Pten-deficient Vα14iNKT cells were partially rescued by mutation of a PI3K subunit (p110γ or p110δ).
Enhanced expression of inhibitory Ly49 receptors by Pten-deficient Vα14iNKT cells
Interaction between MHC class I and inhibitory Ly49 receptors (such as Ly49A and LY49C/I) can negatively regulate the responses of T cells to alloantigens,37 suggesting that signaling via the TCR can be regulated by a Ly49-dependent mechanism. With respect to NKT cells, NKT functions are impaired upon inhibitory Ly49–MHC class I interaction,38 and mice transgenic for Ly49A show impaired NKT cell maturation and selection.39 Thus, Ly49-mediated regulation is important for the development and functions of NKT cells, and this regulation may involve modulation of the strength of TCR signaling. These considerations led us to examine the expression of inhibitory Ly49 receptors by our Pten-deficient Vα14iNKT cells. Specifically, we determined the expression of Ly49C/I (which recognizes MHC class I, H-2Kb, H-2Dd) and Ly49A (which recognizes MHC class I, H-2Dd) by thymic Vα14iNKT cells of LckCrePtenflox/flox mice and the double mutants. As expected, the proportion of αGalCer-CD1d+TCRβ+NK1.1+ thymocytes showing strong expression of Ly49C/I (Figure 7A, bottom) and Ly49A (Figure S2) was increased in LckCrePtenflox/flox mice compared with Ptenflox/flox mice. At least for Ly49C/I, this increased expression was mitigated by the presence of the p110δD910A/D910 or p110γ−/− mutation. There were no differences between LckCrePtenflox/flox mice and Ptenflox/flox mice in the H2-Kb levels expressed by thymic or splenic DCs (Figure S1C). Thus, PI3K signaling is responsible for the altered inhibitory Ly49 receptor repertoire of Pten-deficient thymic Vα14iNKT cells. Taken together, our results suggest that the reduced number and functions of Pten-deficient Vα14iNKT cells may be linked to their increased expression of inhibitory Ly49 receptors.
Discussion
Many studies in mouse models have demonstrated that Vα14iNKT cells are activated in a broad range of disorders, including autoimmune diseases, microbial infections, graft rejection, and cancer progression/metastasis.5,19-21 However, little is known about intracellular signal transduction in NKT cells, particularly the role of the Pten/PI3K signaling pathway that is crucial in so many other cell types. Our results are the first to demonstrate that mutation of Pten in murine Vα14iNKT cells impairs the development and functions of these cells and that these defects depend on PI3K activity. An in vivo consequence of the defective Vα14iNKT compartment is enhanced tumor metastasis.
The study of genetically modified mice has begun to yield important insights into the mechanisms controlling the maturation of Vα14iNKT cells. To date, investigations of diverse gene-deficient mice have revealed 3 general categories of Vα14iNKT cell abnormalities.40 In the first category (group 1), the mutant mice show a total absence or a severe reduction in numbers of both immature (stage 2) and mature (stage 3) Vα14iNKT cells. In the second category (group 2), the mutant animals exhibit a block in Vα14iNKT cell development between stage 2 and stage 3 such that stage 2 cells are increased while stage 3 cells are decreased. In the third category (group 3), numbers of stage 3 Vα14iNKT cells are decreased but there is no increase in stage 2 cell numbers. Knockout mice lacking CD1d, Fyn, SAP, IL-7, IKK2, NIK, or RelB all have the group 1 phenotype, indicating that these molecules are essential for the initial development of Vα14iNKT cells from DP precursors.40,41 Mice lacking T-bet42 or Itk43 are members of group 2. These molecules do not affect the earliest stages of NKT differentiation from DP precursors but are critical for the continued maturation of stage 2 Vα14iNKT cells. Mice deficient for IL-15 or transgenic for mIkBα fall into group 3 because these molecules are important for the survival of mature NKT cells.40,44 While the phenotypes observed in the Vα14iNKT cells of T-bet– or Itk-deficient mice are strikingly similar to those we observed for Vα14iNKT cells of LckCrePtenflox/flox mice, there are some fundamental differences. First, although the phenotypes observed in T-bet–deficient mice can be explained by a failure to express the IL-2R/IL-15R common β subunit (CD122),42 the proliferative response to IL-15 by mature NKT cells of LckCrePtenflox/flox mice was normal (Figure S3). Second, Itk-deficient Vα14iNKT cells show reduced expression of the inhibitory NK receptors Ly49C/I and intact IFNγ secretion,43 but Pten-deficient Vα14iNKT cells exhibit enhanced Ly49C/I and Ly49A expression and reduced IFNγ secretion. In addition, because Itk is activated by Pten deficiency,45 reduction of Itk signaling is unlikely to have occurred in our LckCrePtenflox/flox mice. For these reasons, we believe that the phenotypes observed in Vα14iNKT cells of LckCrePtenflox/flox mice cannot be explained by effects attributable to altered T-bet or Itk function. Finally, in all the previously reported mutant mice, the molecules deleted by the gene targeting are known to activate cells. No other mutant mouse to date features the targeting of an intracellular signaling molecule that negatively regulates cellular activation in Vα14iNKT cells.
Ly49 is a multigene receptor family expressed mainly by NK cells and stage 3 mature Vα14iNKT cells.44 Ly49A, Ly49C/I, and Ly49G2 are inhibitory NK receptors, while Ly49D and Ly49H are activatory NK receptors. Both groups of receptors interact with proteins encoded by specific MHC class I alleles.46 It has been clearly shown that the interaction between Ly49A and H-2Dd and between Ly49C and H-2Kb or H-2Dd negatively regulates NK effector functions such as cytotoxicity and bone marrow graft rejection.47,48 Similarly, the appropriate inhibitory Ly49–MHC class I interactions can negatively regulate the maturation, proliferation, and functions of NKT cells.37-39 In our study, we found that most αGalCer-CD1d+TCRβ+NK1.1+ thymocytes in LckCrePtenflox/flox mice showed an enhancement of Ly49C/I expression that was PIP3 dependent. The continuous accumulation of PIP3 that resulted from the Pten deficiency in our mutants may have up-regulated the expression of inhibitory Ly49 receptors. Thus, the phenotypes observed in Pten-deficient Vα14iNKT cells can plausibly be explained by their enhanced expression of inhibitory Ly49 receptors.
Several lines of evidence suggest that developing Vα14iNKT cells undergo negative selection if they encounter high-avidity antigen or abundant self-antigen. First, the addition of αGalCer to early-stage FTOC prevents the development of Vα14iNKT cells in a dose-dependent manner.14,16 Second, overexpression of CD1d on DCs or thymocytes in transgenic mice results in a reduced number of Vα14iNKT cells that exhibit both altered Vβ repertoire usage and reduced sensitivity to antigen.12,14 Third, in mice doubly transgenic for the activatory Ly49D receptor and its ligand H2-Dd, Vα14iNKT cells are absent from the thymus and periphery due to developmental arrest between stage 2 and stage 3. The addition of the inhibitory Ly49A transgene to Ly49D/H2-Dd transgenic mice resulted in a partial rescue of NKT cell development.15 A possible interpretation of these findings is that Vα14iNKT cells with self-recognizing Ly49 inhibitory receptors preferentially survive as a result of decreased negative selection mediated by activatory Ly49. It may be that, as well as TCR avidity, an appropriate balance of signals delivered by activatory and inhibitory Ly49 receptors is required to set the affinity threshold and regulate proper Vα14iNKT cell development. Pten-deficient Vα14iNKT cells exposed to endogenous glycolipid antigens may transmit supraphysiological levels of signaling which, in turn, may drive enhanced negative selection of Vα14iNKT cells. The deficit of Vα14iNKT cells in the peripheral organs and thymus may then be partially restored by repopulation of the mature Vα14iNKT compartment with cells showing increased expression of inhibitory Ly49 receptors, altered Vβ usage, and reduced sensitivity to antigen.14
Pre-TCR signaling in T cells of PI3K p110δ−/−/p110γ−/− double-mutant mice is greatly decreased compared with signaling in T cells of PI3Kδ−/− or γ−/− single-mutant animals.49 In addition, T-cell responses induced by TCR stimulation are much weaker in p110δ−/−50 and p110γ−/−29 mice than in WT controls. In our study, we showed that the phenotypes observed in Pten-deficient NKT cells could be partially rescued by mutation of p110γ. These results suggest that TCR signaling may directly activate both PI3Kδ and PI3Kγ in Vα14iNKT cells. Alternatively, inhibitory Ly49 receptors may directly affect both PI3Kδ and PI3Kγ, which may eventually result in modulated TCR signaling. In support of the latter possibility, there is evidence that SHIP1 (another PIP3 phosphatase) as well as the p85 subunit of PI3K can both be recruited to Ly49A or Ly49C.51
The molecular mechanisms underlying the impaired function of Vα14iNKT cells in patients with autoimmune disease or cancer remain to be determined. Our work suggests that the continuous activation of the PI3K pathway in murine LckCrePtenflox/flox Vα14iNKT cells (due to decreased Pten function) may alter the negative selection, development, function, and tolerance of these cells. In humans, a parallel scenario could lead to the onset of these diseases. It is well known that αGalCer has antitumor effects against cancer cells of various origins and that this protection depends on an initial burst of IFNγ production by NKT cells and subsequent ongoing IFNγ production by NK cells.36 IFNγ itself is not directly toxic to tumor cells, but the IFNγ produced by NKT cells is critical for the recruitment and proliferation of NK cells (and other leukocytes) that are cytolytic to tumor cells. In addition, IFNγ has potent antiangiogenic activity52 and induces other leukocytes to produce IFNγ-inducible factors such as IFN-inducible protein 10 (IP-10) and monokine induced by IFNγ.53 These cytokines may indirectly inhibit tumor neovascularization and thus induce tumor hypoxia. We have demonstrated that Pten-deficient Vα14iNKT cells fail to produce IFNγ both in vitro and in vivo, most likely accounting for the impaired antitumor surveillance observed in LckCrePtenflox/flox mice.
Our study is the first to show that engagement of the TCRs of murine Vα14iNKT cells by αGalCer-loaded DCs leads to the activation of PI3K in the NKT cells. We have also demonstrated that the loss of Pten or activation of PI3K signaling in Vα14iNKT cells leads to a failure in their development. The few mature Vα14iNKT cells that survive are tolerant to αGalCer, resulting in defective antitumor surveillance and accelerated metastasis. Mice heterozygous for the Pten mutation (Pten+/−) showed a significant reduction in serum IFNγ production after administration of αGalCer in vivo. In addition, adoptive transfer of Pten+/− Vα14iNKT cells into αGalCer-treated, B16F10 melanoma cell–inoculated Jα18−/− recipients impaired the inhibition of B16F10 melanoma metastasis to the lungs. We speculate that an identical mechanism may be operating in humans with Cowden disease, a hereditary syndrome of cancer susceptibility caused by heterozygous mutations of PTEN: Namely, an individual who inherits a mutated PTEN allele is not only at risk for additional tumorigenic mutations due to loss of heterozygosity (LOH) of the PTEN gene but may also experience accelerated growth of any incipient tumors due to impaired NKT-mediated antitumor surveillance.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
H.K. and T.O. contributed equally to this work.
Correspondence: Akira Suzuki, Hondo 1-1-1, Akita, 0108543 Japan; e-mail: suzuki@med.akita-u.ac.jp.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants from the Ministry of Education, Science, Sports and Culture, Japan; the Kowa Life Science Foundation; the Daiwa Securities Health Foundation; the Suzuken Memorial Foundation; and the Mochida Memorial Foundation for Medical and Pharmaceutical Research. We thank Dr Tetsuo Noda (Tohoku University), Dr Kiminori Hasagawa, Dr Masato Sasaki, Dr Junko Sasaki, Dr Shunsuke Takasuga, Ms Miki Nishio, Mr Seiichi Kuwajima, and Mr Tomoharu Kanie (all of Akita University) for helpful discussions and technical support.




![Figure 4. Impaired activation of Pten-mutant NKT cells in vitro and in vivo. (A) Impaired proliferation. NK1.1+TCRβ+ cells were purified from the thymi of Ptenflox/flox or LckCrePtenflox/flox mice and cocultured with DCs loaded with vehicle (DC) or αGalCer (DC-αGC). After 48 hours, cells were pulsed with [3H]-thymidine for 12 hours and thymidine uptake was measured. (B-C) Impaired cytokine production. IL-4 and IFNγ in the supernatants of the cultures in panel A were measured by ELISA. (D-E) Impaired response of Vα14iNKT cells to αGalCer administration in vivo. αGalCer was intraperitoneally injected into Ptenflox/flox, Pten+/−, or LckCrePtenflox/flox mice, and serum concentrations of IL-4 (D) and IFNγ (E) were evaluated at 2 hours and 12 hours after αGalCer injection, respectively. For all panels, data are expressed as the mean value ± SEM for 3 mice per group. **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/8/10.1182_blood-2006-07-038059/4/m_zh80080711300004.jpeg?Expires=1765913263&Signature=EiyrFL~JrfGwue-QvGo8pkCFV3tl1xpJHcm4qycfcC~Lhp26EdxxxzMwlCeGOZgFYN46zL1FIuersyApaCWhQF~gau3e6ZbJ-v2mTT8HmLVpD-b7NlqtMGEIaGTGBJjnGcPMJFYpe1t3ggZTJ0FcRhDZhY6UUsK82kWPo8MYtS5UxVLji2njEjkzkTiWsVOtZSHayZCoR9BX3~-HFXvBrMbowxrpYBrOCtMfZ-g5JZFT-TzFoiYmv8-iBSwDXAyUH3rXf4IlrLzvPIksXw0okiAd822rs3FFmUc7bEGmpntBlDC9Tv18jM8dBrfoei1wPdf3DmRlQwshAesp0SJW2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 7. The phenotypes observed in Pten-deficient NKT cells are PI3K dependent. (A, top) Decreased maturation. Thymic Vα14iNKT cells from mice of the indicated genotypes were analyzed by flow cytometry for percentages of stage 3 cells. (A, bottom) Increased expression of Ly49C/I receptors. The αGalCer-CD1d+TCRβ+NK1.1+ cells in the top panel were analyzed by flow cytometry to determine relative expression levels of Ly49C/I. (B) Impaired proliferation. The NK1.1+TCRβ+ cells from panel A were stimulated by incubation with αGalCer-loaded DCs, and [3H]-thymidine incorporation was measured. Data shown are the mean ± SEM for 3 mice per group. **P < .01. For both panels, the defects of Pten-deficient Vα14iNKT cells were partially rescued by mutation of a PI3K subunit (p110γ or p110δ).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/8/10.1182_blood-2006-07-038059/4/m_zh80080711300007.jpeg?Expires=1765913263&Signature=ZEIlXtVoKg9bHUuVb~lFS8O6g-AvtALRfCMDFSXyxjz85erMSzrCcFzRvQaMQpOzxYi6UQ~BZrexN-jLjkOypuzFUyxT5h669DL8gQJOQpJrrIX3fYMGnqU2xngEGyU68oPp4~NfRvPdfzhvc2fdpfzdf1gg0I6lEr-MRhoy-L2WSeL8qbm56FE4wvM78WaVIzSwvljOlQetZVOUyv3gn4c-HAHlQ8MuWbhmQ2j-HKD~h3UCSC~xW8IdE5A92YdLs~kAZsptRCMDRwOkGYPXMMle3IeukBrQXNyE5I5Qd~Y598mDnn~AMg6-joHX-3xFVuHUeorO2TfEUzQH2MKoHg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal