Abstract
In chronic HIV infection, most untreated patients lose naive CD4+ and CD8+ T cells, whereas a minority preserve them despite persistent high viremia. Although antiretroviral therapy (ART)–mediated viral suppression generally results in a rise of naive and total CD4+ T cells, certain patients experience very little or no T-cell reconstitution. High peripheral T-cell activation has been linked to poor clinical outcomes, interfering with previous evaluations of thymic function in disease progression and therapy-mediated T-cell recovery. To circumvent this, we used the sj/βTREC ratio, a robust index of thymopoiesis that is independent of peripheral T-cell proliferation, to evaluate the thymic contribution to the preservation and restoration of naive CD4+ T cells. We show that the loss of naive and total CD4+ T cells is the result of or is exacerbated by a sustained thymic defect, whereas efficient thymopoiesis supports naive and total CD4+ T-cell maintenance in slow progressor patients. In ART-treated patients, CD4+ T-cell recovery was associated with the normalization of thymopoiesis, whereas the thymic defect persisted in aviremic patients who failed to recover CD4+ T-cell counts. Overall, we demonstrate that efficient thymopoiesis is key in the natural maintenance and in therapy-mediated recovery of naive and total CD4+ T cells.
Introduction
A hallmark of human immunodeficiency virus 1 (HIV-1) infection is the progressive loss of CD4+ T cells, which ultimately leads to severe immunodeficiency. The contraction of the CD4+ T-cell population reflects the sum of shifts between de novo production, proliferation, differentiation, and death of thymic emigrants, naive, effector, and memory T cells.1 Indeed, although a vast majority of the gut-associated short-lived CCR5+CD4+-activated T cells are depleted during acute HIV infection,2-4 a commonly observed immune perturbation is the reduction of the naive T-cell compartment within blood as disease progresses.5 The exact mechanisms underlying this change is likely the result of a combination of direct and indirect cytopathogenicity, disorganization of lymphoid organs, heightened immune activation, and/or loss of thymic function.6 Nevertheless, HIV-1–induced CD4+ T-cell lymphopenia evokes the need for the replenishment of lymphocyte numbers through an input of naive CD4+ T cells. This can be accomplished through proliferation and expansion of the preexisting pool of naive CD4+ T cells7 or through de novo thymic production, with the latter leading to the establishment of a CD4+ T-cell pool with various T-cell receptors. The relative contribution of proliferation and thymic output to the size of the CD4+ T-cell compartment in treated and untreated chronic HIV infection remains uncertain.
T-cell receptor excision circle (TREC) frequencies at HIV-1 seroconversion have been closely associated with the hazard of death.8 However, it is nevertheless important to note that these observed low TREC values may not only be a result of suppression of thymic activity, but could also reflect heightened immune activation and proliferation, which also have been related to poor prognosis.9-12 Thus, in resolving this issue, the sjTREC frequency is not a suitable marker of thymic function, particularly in HIV-infected patients. We recently developed a new tool to estimate thymic activity, based on the measurement of the extent of intrathymic precursor T-cell proliferation occurring between late TN and early DP thymocyte differentiation stages that is proportional to thymic output. Because DJβTRECs (byproducts of the TCRB rearrangement) are generated before this proliferation, DJβTREC frequency in DP cells is inversely proportional to the number of division cycles these cells have undergone during their differentiation process. Moreover, because the sjTREC molecule is produced after this extensive proliferation, the sjTRECs are not diluted by this intrathymic cell division. Accordingly, the sj/βTREC ratio (sjTREC frequency/DJβTREC frequency), measured in peripheral blood mononuclear cells, is a direct measure of the proliferative history of recent thymic emigrants and represents the extent of thymic output over the preceding weeks. Using this tool, we have demonstrated that HIV-1 infection generally suppresses thymic activity as early as 3 months after infection in untreated subjects.13
Following the administration of highly active antiretrovial therapy (HAART), the majority of patients experience viral suppression and a concomitant increase in CD4+ naive and memory T-cell numbers,14 whereas some patients (8%-17%) fail to reconstitute their CD4+ T-cell numbers despite viral control.15,16 During this immune reconstitution, radiographic evidence of thymic tissue, greater TREC levels, and reduced peripheral T-cell activation are closely associated with the extent of naive T-cell restoration.17-20 This suggests, but does not prove, that the thymus plays an important role in the repopulation of the CD4+ T-cell pool. If thymic output determines the magnitude of cellular restoration, it would be expected that the lack of such thymic activity would result in a poor immunologic response to HAART despite control of viremia.
In the present study, the sj/βTREC ratio (a robust index of thymic activity) and Ki-67 staining to measure naive T-cell entry into cell cycle were used to decipher the relative contributions of thymic output and peripheral proliferation to CD4+ and CD8+ naive T-cell homeostasis during the natural history of HIV infection as well as during HAART-induced immune reconstitution.
Patients, materials, and methods
Patients and control persons
Blood samples were obtained from 38 healthy volunteers (control group) and 49 HIV-1–infected patients in the chronic phase of infection. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll Hypaque density sedimentation, frozen, and stored for further use. A summary of the virologic and immunologic status of these patients is shown in Table 1. Twenty-two highly viremic HIV-infected patients did not receive therapy by preference or following medical recommendation. They were grouped according to CD4+ T-cell counts into progressor (P, n = 10) and slow progressor (SLP, n = 12) patients. The other 27 patients receiving HAART had undetectable plasma viral load at the time of sampling and were subcategorized into 2 groups according to their rate of CD4+ T-cell reconstitution: 14 immunologic responders (IRs) and 13 poor immunologic responders (PIRs). In all HAART-treated patients, sampling was done during the well-described secondary phase of CD4+ T recovery, a median of 22 months after HAART initiation. Although 6 patients in the PIR group exhibited minor blips of viremia during the follow-up period, most patients had an undetectable viral load throughout this same period. Because of sample availability, only subgroups of patients from the SLP and PIR groups were analyzed by fluorescence-activated cell sorting (FACS). However, these subgroups were similar to their respective patient populations as well as to the P and IR groups, respectively, with respect to age and viral load. Patients from the P and IR groups were collected from the McGill University Health Center Montreal Chest Hospital and its collaborators. Patients from the SLP and PIR groups were collected from the John Carey Special Immunology Unit at Case Western Reserve University, University Hospitals of Cleveland. Clinical protocols conformed to ethical guidelines of the authors' institutions and the US Department of Health and Human Services' human experimentation guidelines. Samples were obtained with the subjects' informed consent, in accordance with the Declaration of Helsinki.
Flow cytometry
Frozen PBMCs were thawed and resuspended in PBS 2% FCS. Approximately 2 to 3 × 106 cells were stained for surface expression of CD3, CD4, CD8, CD45RA, and CD27 (Becton Dickinson, San Jose, CA). This was followed by fixation/permeabilization by using the BD FACSLysing and FACSPermeabilizing buffer as directed by the manufacturer (Becton Dickinson, Pharmingen). For each sample, intracellular staining was carried out though the incubation of the permeabilized cells with mAb directed against Ki-67 (Becton Dickinson, Pharmingen) or its respective isotype for 30 minutes on ice. The cells were then washed, fixed in 2% PFA for 30 minutes, rewashed, and resuspended in PBS 2% FCS overnight. Data were acquired on a Becton Dickinson LSR II system and analyzed using DiVa software (Becton Dickinson Systems). A minimum of 3 × 105 events in the live cell gate, as defined by forward and side scatter, was accumulated for each sample.
TREC quantification
As previously described,13,21,22 parallel quantification of each TREC together with the CD3γ chain (used as a housekeeping gene) was performed for each sample using the LightCycler technology (Roche Diagnostics, Mannheim, Germany). PBMCs were lysed in Tris (10 mM, pH 8.2) Tween-20 (0.05%), NP-40 (0.05%), and Proteinase K (100 μg/mL) for 30 minutes at 56°C and then 15 minutes at 98°C. Multiplex polymerase chain reaction (PCR) was performed for sjTREC or each of the 6 Dβ1Jβ TRECs, together with the CD3γ chain (10 minutes initial denaturation at 95°C, 30 seconds at 95°C, 30 seconds at 60°C, 2 minutes at 72°C for 22 cycles) using outer 3′/5′ primer pairs. PCR conditions in the LightCycler experiments, performed on 1/100th of the initial PCR, were as follows: 1 minute initial denaturation at 95°C, 1 second at 95°C, 10 seconds at 60°C, 15 seconds at 72°C for 40 cycles. Fluorescence measurements were performed at the end of the elongation steps. TRECs and CD3γ LightCycler quantifications were performed in independent experiments, but on the same first-round, serially diluted standard curve. This highly sensitive, nested quantitative PCR assay allows the detection of one copy of 105 cells for each TREC. The sjTRECs were quantified in triplicate, and duplicate experiments were performed for each individual DβJβ TREC for all studied samples. The sum of DJβTREC frequencies is calculated as 2.16-fold the sum of the 6 measured DJβTREC frequencies (to extrapolate to the 13 principal DβJβ TRECs). The sj/βTREC ratio is the sjTREC frequency divided by the sum of DJβTREC frequencies (sj/β TREC = sjTREC/sum DJβ TRECs).
Statistics
Statistical analysis (Mann-Whitney test and Spearman correlation test, r, and P values) was performed using the Vassar college website (http://faculty.vassar.edu/lowry/VassarStats.html) and StatView F-4.5 software (Abacus Concepts, Berkeley, CA). An r value of at least 0.3 or no more than −0.3 and a P value no more than .05 were considered nominally significant.
Results
Naive T-cell counts are maintained in slow progressor patients despite sustained viremia
A subgroup of HIV chronically infected patients maintain high CD4+ T-cell counts (ie, comparable to those in uninfected persons) for prolonged periods of time despite sustained high-level viremia.23 These slow-progressing patients contrast with the majority of chronically infected patients who show a dramatic decline in CD4+ T-cell counts when viremia is left uncontrolled over time. We analyzed 2 groups of patients: slow progressors (SLPs, n = 12) who had high CD4+ T-cell counts at the time of sampling (median, 535 CD4+ T cells/μL; range, 308-970 CD4+ T cells/μL; median time from first HIV positivity, 41 months; range, 14-178 months) and maintained high CD4+ T-cell counts thereafter (median follow-up, 51 months; range, 19-221 months; median CD4+ counts, 482 CD4+ T cells/μL; range, 380-975 CD4+ T cells/μL) and patients who progressed more rapidly to disease (progressors [Ps], n = 10; 202 CD4+ T cells/μL; range, 50-254 CD4+ T cells/μL; 21 months after HIV-positivity; range, 14-36 months) (Table 1). Comparing these 2 groups of patients allowed us to assess the contribution of peripheral proliferation (ie, antigen- or cytokine-driven or homeostatic proliferation) and thymic output to the maintenance of both naive (CD45RA+CD27+) and total CD4+ T-cell counts.
Patient characteristics
| . | HIV+ . | HIV+ treated . | ||
|---|---|---|---|---|
| P . | SLP . | IR . | PIR . | |
| No. | 11 | 12 | 14 | 12 |
| Age, y | 38 (23-50) | 36 (19-49) | 41.5 (20-51) | 43.5 (32-57) |
| VL, log copies/mL | 4.66 (4.04-6.27) | 4.9 (4.48-5.45) | 1.70 (1.70-2.70) | 1.70 (1.70-2.64) |
| CD4, cells/μL | 210 (50-254) | 535 (380-970) | 630 (210-1130) | 121 (46-277) |
| CD8, cells/μL | 654 (390-870) | 991 (560-2098) | 918 (380-2347) | 842 (253-1540) |
| Time after first HIV+, mo | 21 (14-36) | 41 (14-178) | — | — |
| Time after Tx, mo | — | — | 22 (7-66) | 24 (5-80) |
| Time follow-up | NA | 55 (19-221) | 41 (12-70) | 65 (33-111) |
| CD4 at follow-up | NA | 482 (380-975) | 672 (371-1055) | 260 (75-470) |
| CD4 change,* cells/y | −151 (−433 to −117) | 19 (−57 to 78) | 230 (132 to 923) | 32 (−7 to 90) |
| . | HIV+ . | HIV+ treated . | ||
|---|---|---|---|---|
| P . | SLP . | IR . | PIR . | |
| No. | 11 | 12 | 14 | 12 |
| Age, y | 38 (23-50) | 36 (19-49) | 41.5 (20-51) | 43.5 (32-57) |
| VL, log copies/mL | 4.66 (4.04-6.27) | 4.9 (4.48-5.45) | 1.70 (1.70-2.70) | 1.70 (1.70-2.64) |
| CD4, cells/μL | 210 (50-254) | 535 (380-970) | 630 (210-1130) | 121 (46-277) |
| CD8, cells/μL | 654 (390-870) | 991 (560-2098) | 918 (380-2347) | 842 (253-1540) |
| Time after first HIV+, mo | 21 (14-36) | 41 (14-178) | — | — |
| Time after Tx, mo | — | — | 22 (7-66) | 24 (5-80) |
| Time follow-up | NA | 55 (19-221) | 41 (12-70) | 65 (33-111) |
| CD4 at follow-up | NA | 482 (380-975) | 672 (371-1055) | 260 (75-470) |
| CD4 change,* cells/y | −151 (−433 to −117) | 19 (−57 to 78) | 230 (132 to 923) | 32 (−7 to 90) |
VL indicates viral load; Tx, treatment; —, not applicable because not treated at sampling time; NA, not applicable because of beginning of treatment.
CD4 changes are calculated differently for the 4 groups of patients: P was calculated from first seropositivity to sampling time (14-36 months); SLP was calculated from first seropositivity to follow-up time (19-221 months); IR was calculated from the initiation of treatment to the first time point of the plateau phase of CD4 gain (12-70 months); PIR was calculated during the follow-up period (33-111 months).
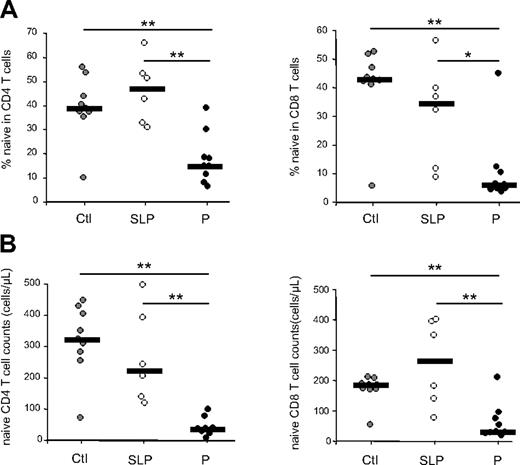
Despite sustained viral replication (see Table 1) and an inverted CD4/CD8 T-cell ratio, SLP patients maintained normal or near-normal proportions and absolute numbers of circulating CD4+ and CD8+ naive T cells (Figure 1). This contrasts to what was observed in patients who progress more rapidly to disease (group P), whereby naive T-cell proportions in the CD4+ compartment as well as the absolute number of naive CD4+ T cells were reduced when compared with these numbers in uninfected, healthy, age-matched persons (P = .003 and P = .001, respectively; Figure 1). This was also the case for the CD8+ T-cell compartment (P = .007 and P = .007). Although a greater naive T-cell depletion cannot be excluded in these patients, the fact that we observed a significant decrease in both CD4+ and CD8+ naive T-cell counts suggests the possibility of a thymic defect in P patients.
Evolution of naive T-cell subsets during chronic HIV-1 infection. Naive T-cell (CD45RA+, CD27+) frequencies (A) and numbers (B) were quantified in CD4+ and CD8+ peripheral T cells from HIV-1–infected patients. Slow progressor (SLP, n = 6) as well as progressor (P, n = 9) patients are compared with healthy control persons (Ctl, n = 9). Statistical differences between groups are shown on top (*P < .05; **P < .01). The subgroups of patients analyzed are representative of their respective patient population described in Table 1 with respect to age, CD4 counts, and viral load.
Evolution of naive T-cell subsets during chronic HIV-1 infection. Naive T-cell (CD45RA+, CD27+) frequencies (A) and numbers (B) were quantified in CD4+ and CD8+ peripheral T cells from HIV-1–infected patients. Slow progressor (SLP, n = 6) as well as progressor (P, n = 9) patients are compared with healthy control persons (Ctl, n = 9). Statistical differences between groups are shown on top (*P < .05; **P < .01). The subgroups of patients analyzed are representative of their respective patient population described in Table 1 with respect to age, CD4 counts, and viral load.
Maintenance of the naive T-cell compartment in untreated viremic patients: thymic output or peripheral proliferation?
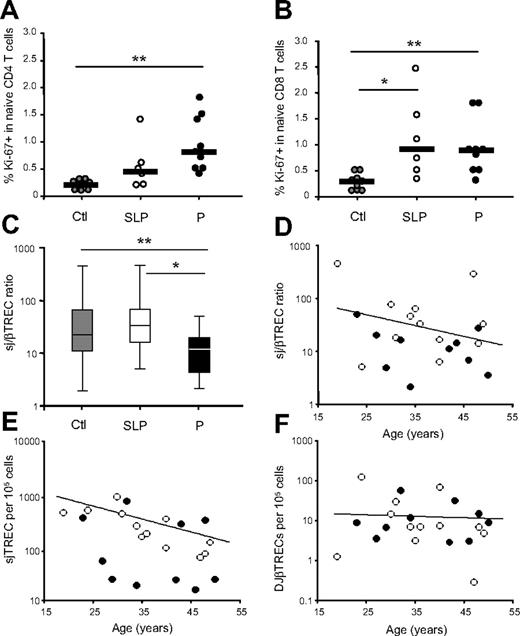
The maintenance of peripheral naive T-cell numbers rests on the thymus's ability to produce new T cells, on their survival, and on the magnitude of their peripheral proliferation. We thus sought to determine and compare the level of proliferation of naive T cells in both groups through flow cytometric analysis of the expression of the nuclear antigen Ki-67, expressed in cells that have recently entered into cycle.24 When comparing both P and SLP patients to the uninfected control group, a greater proportion of naive CD4+ T cells from patients with low CD4+ T-cell counts (group P) expressed Ki-67 (median, 0.8% versus 0.2% in healthy control subjects; mean, 0.9% versus 0.2%; P = .002). However, Ki-67 expression among naive CD4+ T cells in the SLP patients, although slightly higher, remained comparable to that observed in healthy persons (median, 0.5% versus 0.2%; mean, 0.6% versus 0.2%; P = NS) (Figure 2A). The proportion of proliferating CD8+ naive T cells in these HIV-infected patients showed greater variability but was higher in both groups of patients than in healthy control subjects (median, 0.92% and 1.15% for group SLP and P, versus 0.28% in healthy controls; means, 1.12% and 0.93% versus 0.27%; P = .006 and P = .05 respectively; Figure 2B). These results indicate that in rapid progressors, high levels of cell-cycle entry and activation, shown by a greater proportion of Ki-67–expressing cells, are insufficient to maintain naive and total CD4+ T-cell numbers.
Naive T-cell cycling and thymic function during chronic HIV-1 infection. Naive T-cell cycling was quantified through Ki-67 expression on CD45RA+, CD27+ cells in the CD4+ (A) and CD8+ (B) T-cell compartments. Slow progressor (SLP, n = 6) as well as progressor (P, n = 9) patients are compared with healthy control persons (Ctl, n = 9). The subgroups of patients analyzed are representative of their respective patient population described in Table 1 with respect to age, CD4 counts, and viral load. For panels A and B, horizontal lines represent median values. Statistical differences between groups are shown on top (*P < .05; **P < .01). (C) Thymic function was estimated through the calculation of the sj/βTREC ratio in the 3 groups of individuals (group P, n = 10; group SLP, n = 12; controls, n = 38). Horizontal lines represent maximal values, third quartiles, medians, first quartiles, and minimal values, from top to bottom. sj/βTREC ratio (D), sjTREC (E), and DJβTREC (F) frequencies are presented as a function of age for group P (close symbols) and group SLP (open symbols). The lines represent the linear regressions for the control group.
Naive T-cell cycling and thymic function during chronic HIV-1 infection. Naive T-cell cycling was quantified through Ki-67 expression on CD45RA+, CD27+ cells in the CD4+ (A) and CD8+ (B) T-cell compartments. Slow progressor (SLP, n = 6) as well as progressor (P, n = 9) patients are compared with healthy control persons (Ctl, n = 9). The subgroups of patients analyzed are representative of their respective patient population described in Table 1 with respect to age, CD4 counts, and viral load. For panels A and B, horizontal lines represent median values. Statistical differences between groups are shown on top (*P < .05; **P < .01). (C) Thymic function was estimated through the calculation of the sj/βTREC ratio in the 3 groups of individuals (group P, n = 10; group SLP, n = 12; controls, n = 38). Horizontal lines represent maximal values, third quartiles, medians, first quartiles, and minimal values, from top to bottom. sj/βTREC ratio (D), sjTREC (E), and DJβTREC (F) frequencies are presented as a function of age for group P (close symbols) and group SLP (open symbols). The lines represent the linear regressions for the control group.
The depletion of both CD4+ and CD8+ naive T cells in P patients argues for a defect in thymic output. We therefore assessed thymic function through determination of the intrathymic precursor T-cell proliferation by quantification of the sj/βTREC ratio. Intrathymic precursor T-cell proliferation was significantly reduced in group P as compared with healthy persons (median sj/βTREC ratio = 12.6 versus 22; means, 15.7 versus 50.3; P = .05) (Figure 2C). In SLP patients, however, the sj/βTREC ratio was similar to that of healthy control subjects (median sj/βTREC ratio = 33.4; mean = 88.8; P = NS). Because thymic function is age dependant,18,25 we analyzed the sj/βTREC ratio in both viremic patient groups (P and SLP) as a function of age. This analysis revealed that a majority of P patients (8 of 10) consistently showed a sj/βTREC ratio below the average level of age-matched healthy persons, whereas the sj/βTREC ratio values obtained in SLP patients bracketed the normal range for all age groups (Figure 2D). Hence, thymic function is significantly greater in viremic patients who maintain CD4+ T-cell counts than in patients who have progressed more rapidly to disease (P = .02) (Figure 2C). These data demonstrate that in the absence of efficient thymopoiesis (the enhanced cell cycling observed in progressor patients) is not able to maintain naive T-cell numbers, whereas, in slow progressors, efficient thymopoiesis seems sufficient to maintain CD4+ T-cell counts.
Modification of naive T-cell homeostasis during chronic HIV infection
In a previous study13 we demonstrated that, despite a reduced thymic function and an increased peripheral naive T-cell proliferation, patients' sjTREC levels during primary HIV infection remained close to those of healthy persons. Moreover, the DJβTREC frequencies were significantly higher in these patients than in uninfected control subjects. These data suggested that a peripheral mechanism was initiated during primary HIV infection to compensate for the impairment of thymic function, allowing the maintenance of peripheral naive CD4+ T-cell counts.13 When analyzing these 2 types of TRECs in chronically infected patients, we observed a strong reduction of sjTREC frequencies in group P as compared with frequencies in both SLP patients and healthy control subjects, with the latter 2 being comparable (median sjTREC = 46/105 cells in P versus 243 and 310 in SLP and control subjects, respectively; means = 205, 329, and 599; P = .051 and .021, respectively) (Figure 2E). Similar results were obtained for the absolute numbers of sjTREC per milliliter of blood (median sjTREC = 336/mL in P versus 6750 and 6191 in SLP and control subjects, respectively; mean = 1870 versus 6323 and 11 983; P < .001 and .012, respectively). In contrast, DJβTREC frequencies in SLP patients were identical to those of aged-matched healthy control subjects, whereas only a trend to loss is observed in P patients as compared with healthy persons (median DJβTREC = 6/105 cells in SLP versus 7.7 and 14.9 in P and controls, respectively; means = 19.8, 12.4, and 19.2; P = NS; Figure 2F). These data suggest that the compensatory mechanism that maintains stable sjTREC frequencies and increased DJβTREC frequencies observed in primary infection is lost in chronic progressor patients. However, this peripheral compensatory mechanism is not needed in SLP patients, given that the naive T-cell pool is maintained and supported by efficient thymopoiesis.
All these indices were affected regardless of age in the untreated P patients, illustrating that chronic HIV infection disrupts thymic productivity and naive T-cell homeostasis independently of age. Importantly, these experiments show that such disruptions are not present in SLP patients, thereby stressing the role of thymic activity in maintaining CD4+ T-cell counts despite high viremia.
Antiretroviral treatment leads to increased counts but not to increased proportions of naive T cells in immunologic responders, whereas poor immunologic responders do not restore either.
HIV-infected persons differ in their capacity to reconstitute CD4+ T-cell numbers following HAART-mediated viral suppression. Although the majority of patients do exhibit a significant rise in CD4+ T-cell numbers, some patients maintain low CD4+ T-cell counts despite adequate viral control (8%-17%).15,16 To test whether differences in the kinetic and magnitude of immune reconstitution in these groups of patients could be attributed to perturbations in thymic output and/or peripheral T-cell proliferation, we analyzed 2 groups of HAART-treated, virologically suppressed patients (Table 1). Immunologic responders (IR, n = 14) were defined as gaining more than 100 CD4+ T cells/μL per year during the major phase of T-cell recovery (median CD4+ increase = 230 CD4+ T cells/year; range, 132-923 CD4+ T cells/year). They were sampled at 22 months after onset of treatment (range, 7-66 months) and demonstrated high CD4+ T-cell counts (median = 672 CD4/μL; range, 371-1055 CD4/μL) at follow-up (median = 41 months after treatment; range, 12-70 months). Poor immunologic responders (PIR, n = 12) demonstrated a slow increase in their CD4+ T-cell counts (median = 32 CD4+ T cells/year; range,−7 to 90 T cells/year) over the follow-up period (median follow-up = 65 months; range, 33-111 months; Table 1).
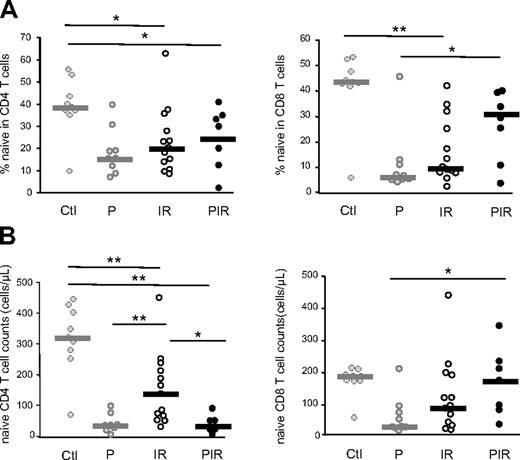
In immunologic responders, antiretroviral treatment leads to greater CD4+ naive T-cell counts (Figure 3B) than those found in P patients (P = .004) after a median of 22 months of treatment. Of note, although clearly significant, this restoration was only partial inasmuch as the circulating naive CD4+ T-cell counts did not reach the level of those in healthy control subjects (P = .004). However, the naive T-cell frequencies within the CD4+ and CD8+ compartments remained similar to those of untreated patients (P = 0.8), demonstrating that the naive T-cell niche disrupted in progressor patients is not completely restored following viral suppression (Figure 3A-B).
Evolution of naive T-cell subsets under HAART. Naive T-cell (CD45RA+, CD27+) frequencies (A) and numbers (B) were quantified in CD4+ and CD8+ peripheral T cells from HIV-–infected patients. Immunologic responder (IR; n = 13) as well as poor immunologic responder (PIR; n = 7) patients are compared with untreated infected persons (P). Statistical differences between groups are shown on top (*P < .05; **P < .01). The subgroups of patients analyzed are representative of their respective patient population described in Table 1 with respect to age, CD4 counts, and viral load.
Evolution of naive T-cell subsets under HAART. Naive T-cell (CD45RA+, CD27+) frequencies (A) and numbers (B) were quantified in CD4+ and CD8+ peripheral T cells from HIV-–infected patients. Immunologic responder (IR; n = 13) as well as poor immunologic responder (PIR; n = 7) patients are compared with untreated infected persons (P). Statistical differences between groups are shown on top (*P < .05; **P < .01). The subgroups of patients analyzed are representative of their respective patient population described in Table 1 with respect to age, CD4 counts, and viral load.
In contrast, in the PIR group, the influence of viral suppression differed on naive T-cell restoration. Although both the frequency of naive T cells within the CD4+ compartment and the absolute number of naive CD4+ T cells remained similar to those of untreated patients (Figure 3), a significant increase was observed in both the frequency (P = .06) and absolute counts of CD8+ naive T cells (P = .013; Figure 3).
Recovery of the naive T-cell compartment in patients under HAART: thymic output or peripheral proliferation?
To assess the relative contribution of naive T-cell cycling and thymic output to the efficiency of immune reconstitution, we measured Ki-67 along with the sj/βTREC ratio as a measure of intrathymic precursor T-cell proliferation in the 2 groups of virologically suppressed patients (IR and PIR). These numbers were compared with those observed for untreated progressors (group P).
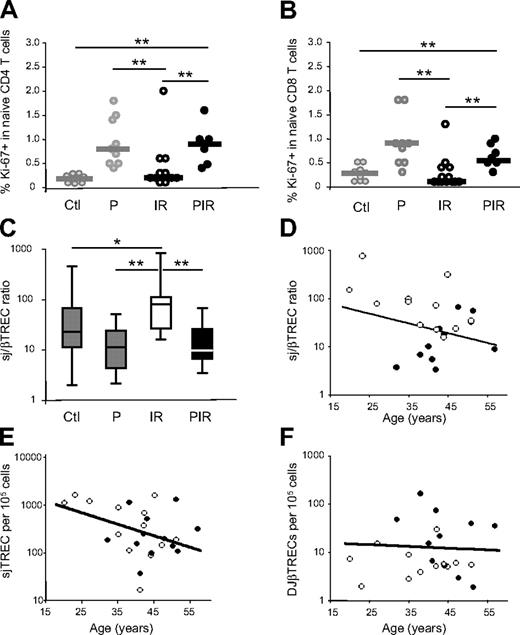
The frequency of CD4+ and CD8+ naive T cells expressing Ki-67 was strongly reduced in group IR as compared with group P (4-fold in CD4+ and 6-fold in CD8+ naive T cells; P = .004 and P = .002, respectively), returning to levels similar to those observed in healthy control subjects (Figure 4A-B). In contrast, naive T-cell Ki-67 expression in poor immunologic responders remained high following viral suppression. These data suggest that the observed cell-cycle entry of peripheral naive T cells in these patients is not dependent on plasma viremia but may reflect the expansion of the remaining cells needed to fill the void left by peripheral T-cell lymphopenia, as has been reported in other lymphopenic settings.26-28
Naive T-cell cycling and thymic function under HAART. Naive T-cell cycling was quantified through Ki-67 expression on CD45RA+, CD27+ cells in the CD4+ (A) and CD8+ (B) compartments. Immunologic responders (IRs; n = 13) as well as poor responders (PIRs, n = 7) are compared with healthy control subjects (Ctl) and untreated HIV-infected persons (P). The subgroups of patients analyzed are representative of their respective patient population described in Table 1 with respect to age, CD4 counts, and viral load. Statistical differences between groups are shown on top (*P < .05; **P < .01). (C) Thymic function was estimated through the calculation of the sj/βTREC ratio in the 4 groups of individuals (PIR, n = 11; IR, n = 12). Horizontal lines represent maximal values, third quartiles, medians, first quartiles, and minimal values, from top to bottom. The IR patient with an sjTREC frequency of 17 sjTREC/105 cells did not show any detectable DJβTREC; thus, his sj/βTREC ratio was impossible to calculate. The sj/βTREC ratio (D), sjTREC (E), and DJβTREC (F) frequencies are presented as a function of age for group PIR (closed symbols) and IR (open symbols). For one IR and one PIR patient, DJβTRECs were undetectable in triplicate experiments, the sj/βTREC ratio was thus impossible to calculate for these patients. The lines represent the linear regressions for the control group.
Naive T-cell cycling and thymic function under HAART. Naive T-cell cycling was quantified through Ki-67 expression on CD45RA+, CD27+ cells in the CD4+ (A) and CD8+ (B) compartments. Immunologic responders (IRs; n = 13) as well as poor responders (PIRs, n = 7) are compared with healthy control subjects (Ctl) and untreated HIV-infected persons (P). The subgroups of patients analyzed are representative of their respective patient population described in Table 1 with respect to age, CD4 counts, and viral load. Statistical differences between groups are shown on top (*P < .05; **P < .01). (C) Thymic function was estimated through the calculation of the sj/βTREC ratio in the 4 groups of individuals (PIR, n = 11; IR, n = 12). Horizontal lines represent maximal values, third quartiles, medians, first quartiles, and minimal values, from top to bottom. The IR patient with an sjTREC frequency of 17 sjTREC/105 cells did not show any detectable DJβTREC; thus, his sj/βTREC ratio was impossible to calculate. The sj/βTREC ratio (D), sjTREC (E), and DJβTREC (F) frequencies are presented as a function of age for group PIR (closed symbols) and IR (open symbols). For one IR and one PIR patient, DJβTRECs were undetectable in triplicate experiments, the sj/βTREC ratio was thus impossible to calculate for these patients. The lines represent the linear regressions for the control group.
Thymic production, as measured by the quantification of the sj/βTREC ratio, was greatest in IR patients (median sj/βTREC ratio = 76.6 as compared with 12.6 in group P; mean = 147.4 versus 15.8; P = .002) (Figure 4C), at levels even greater than those of healthy control subjects (median sj/βTREC ratio = 22; mean = 50.3; P = .01). In contrast, the thymic function of PIR patients remained similar to that of group P (median sj/βTREC ratio = 9.8; mean = 20.6), clearly demonstrating that these patients do not restore thymic function despite viral suppression. Figure 4D further clarifies this point and illustrates how the sj/βTREC ratio is particularly low in the younger PIR persons.
With IR patients exhibiting greater thymic function, one would expect greater levels of sjTRECs than among healthy control subjects. Individual TREC quantification showed, however, that sjTREC levels were similar in both treated patient groups and control subjects (Figure 4E), with DJβTRECs frequencies significantly lower in the IR group patients (median DJβTREC = 5.6 per 105 cells as compared with 14.97 in control subjects; mean = 8.11 versus 19; P = .005; Figure 4F). This lack of sjTREC increase suggests that the peripheral homeostatic mechanisms involved in the regulation of RTE and naive T-cell counts remain perturbed in the IR group (see “Discussion”).
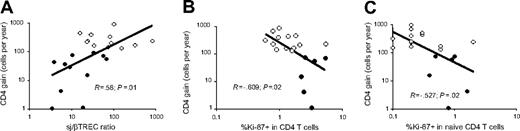
Interestingly enough, the overall yearly gain of CD4+ T cells observed in all treated patients (IR and PIR) correlated with thymic function as measured by the sj/βTREC ratio (R = 0.58, P = .01) (Figure 5A). In contrast, a greater proportion of cycling (homeostatic or antigen-driven) CD4+ T cells or CD4+ naive T cells did not lead to an efficient restoration of CD4+ T cells, inasmuch as a negative correlation was observed between the gain of CD4+ T cells and both indices (R = −0.609, P = .02; R = −0.572, P = .02, respectively; Figure 5B-C).
CD4+ T-cell counts correlates with intrathymic precursor T–cell cycling. Relationship between CD4+ gain in PIR (closed symbols) or IR (open symbols) and intrathymic precursor T-cell proliferation (A), CD4+ T–cell cycling (B), or peripheral naive CD4+ T–cell cycling (C).
CD4+ T-cell counts correlates with intrathymic precursor T–cell cycling. Relationship between CD4+ gain in PIR (closed symbols) or IR (open symbols) and intrathymic precursor T-cell proliferation (A), CD4+ T–cell cycling (B), or peripheral naive CD4+ T–cell cycling (C).
Impact of thymic function and peripheral proliferation on naive CD4+ T-cell counts
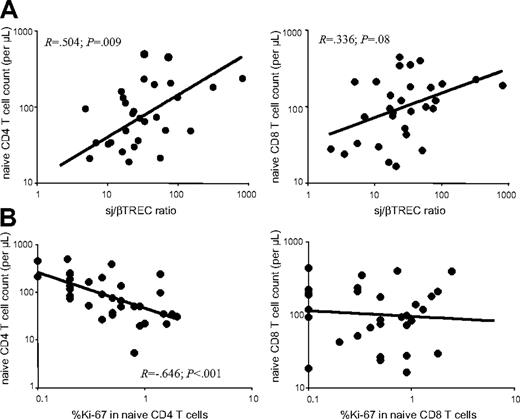
We then sought to evaluate the relative impact of de novo production from the thymus and peripheral proliferation on the number of both CD4+ and CD8+ naive T cells in the 4 groups of patients. By analyzing all samples from HIV-infected patients, for which both thymic function and FACS analysis were performed (n = 29), a strong positive correlation was observed between thymic production, as measured by the sj/βTREC ratio, and CD4+ naive T-cell counts (R = 0.504, P = .009) (Figure 6A left). Although the correlation was less pronounced in the CD8+ compartment, a trend was also observed between naive T-cell counts and intrathymic proliferation (R = 0.336, P = .08; Figure 6A right). In contrast, peripheral naive CD4+ T-cell cycling negatively correlates with naive CD4+ T-cell counts (R = −0.646, P < .001; Figure 6B left). However, peripheral proliferation of naive CD8+ T cells was not linked to naive CD8+ T-cell counts (Figure 6B right).
Thymic function is associated with peripheral naive CD4+ T-cell counts. Naive T-cell (CD45RA+, CD27+) counts were quantified in CD4+ (left) and CD8+ (right) peripheral T cells from HIV-1 infected patients (n = 29). (A) Correlation between naive T-cell counts and thymic function as defined by the sj/βTREC ratio. (B) Correlation between naive T-cell counts and the percentage of cycling cells in CD4+ or CD8+ naive T-cell compartments.
Thymic function is associated with peripheral naive CD4+ T-cell counts. Naive T-cell (CD45RA+, CD27+) counts were quantified in CD4+ (left) and CD8+ (right) peripheral T cells from HIV-1 infected patients (n = 29). (A) Correlation between naive T-cell counts and thymic function as defined by the sj/βTREC ratio. (B) Correlation between naive T-cell counts and the percentage of cycling cells in CD4+ or CD8+ naive T-cell compartments.
These analyses suggest that thymopoiesis is the main source of T-cell production in treated or untreated HIV-1–infected persons. The efficiency of thymic function is crucial to both maintaining naive T-cell counts in slow progressor HIV-infected patients and restoring CD4+ T-cell counts in treated patients. In contrast, naive T-cell peripheral cycling, either induced in response to CD4+ lymphopenia as previously observed in other lymphopenic settings26-28 or as a consequence of immune activation, does not help in restoring peripheral naive T-cell counts.
Discussion
In this report, we have evaluated thymic function as well as cell cycling in homogeneous groups of patients with divergent responses to persistent viremia or who differentially reconstitute CD4+ T-cell numbers following viral suppression under HAART. We show that during chronic HIV-1 infection, a defect in intrathymic precursor T-cell proliferation is a rate-limiting step that governs the maintenance of naive CD4+ and CD8+ T-cell counts. Indeed, by comparing 2 similarly viremic groups with distinct CD4+ T-cell outcomes, we have found that the inability to generate new T cells in the thymus is a key element in disease progression. In addition, we have demonstrated that the speed of CD4+ T-cell reconstitution in patients with HAART-mediated viral suppression is linked to their capacity to efficiently restore de novo naive T-cell production through efficient thymopoiesis. In contrast, the negative correlation observed between Ki-67 expression and naive T-cell numbers illustrates the inability of naive T-cell cycling to rescue low CD4+ T-cell numbers.
Variation of the sjTREC frequency alone does not accurately reflect the perturbations of thymic function in HIV-infected patients. Indeed, changes in sjTREC frequencies can be the consequence of either or both reduced thymic production and increased peripheral proliferation as a consequence of generalized immune activation.12,18,29,30 However, the sj/βTREC ratio exclusively depends on intrathymic precursor T-cell proliferation and is not affected by changes in the frequency of peripheral T cells in cycle, or their survival or death rate.13 As the extent of thymic export relies mainly on the number of cells reaching positive and negative selection steps, variations in precursor T-cell proliferation are certainly reflected by modifications in thymic production.31 The sj/βTREC ratio is thus an accurate marker for thymic output, even during chronic HIV infection when generalized immune activation strongly enhances peripheral T-cell proliferation.
Slow disease progression has been shown to be related to both viral and host factors such as viral deficiency (nef-, Vpr-, Vif-, …),32,33 tropism (lack of drift to CXCR4 tropic viruses),34 and particular genotypes (mutations in CCR5, CCR2, SDF1)35 or HLA type (HLA-A3,-B14,-B17,-B27,-DR6, and DR7).35 Moreover, it has been argued that recurrent or persistent immune activation also leads to disease progression.4,36 However, the viremic, slow-progressing patients studied here harbor higher proportions of Ki-67+ than healthy persons in both naive (Figure 2) and effector/memory T cells (data not shown). With this in mind, our results demonstrate for the first time that a specific characteristic of viremic, slow progressor patients is a good thymic function (Figure 2), most likely enabling the maintenance of CD4+ T-cell counts despite active viral replication. Finally, the fact that the slowly progressing patients exhibit a slow CD4+ T-cell loss in the presence of high-level viremia excludes an inherent reduced replicative capacity of the virus as a cause of slow disease progression.
On initiation of HAART, T-cell reconstitution in HIV-infected patients occurs in 2 major phases. The first takes place within the first months of treatment and is characterized mainly by an upsurge of circulating memory CD4+ T cells largely redistributed from lymphoid tissue and the normalization of T-cell activation.14,37,38 Although an increase in sjTRECs has been observed in this first phase of immune reconstitution, the differential sjTREC frequency changes in lymph nodes and peripheral blood suggest that redistribution of TREC-rich cells also occurs in this early phase of HAART.39 This is followed by a second phase of effective T-cell reconstitution under HAART that takes place during the subsequent year, when naive T cells steadily rise.37 This naive T-cell increase has been shown to correlate with a positive change in thymic size, accompanied by an increase in sjTREC frequency and absolute sjTREC content.40-42 The question of whether the rise in sjTRECs was a direct consequence of thymic output remained controversial because a reduction in immune T-cell activation, halting the suspected heightened TREC dilution before HAART, was shown to precede these events.14,43 The analysis of the sj/βTREC ratio within this second reconstitution phase allowed us to definitively prove that immunologic responders demonstrate a significant supranormal thymic activity (Figure 4C-D) during this reconstitution phase.
Interestingly, despite reduced thymic activity, peripheral naive CD4+ T-cell counts never completely recovered in immunologic responders during the course of this study (Figure 3B). Moreover even with strong intrathymic proliferation (Figure 4C-D) and very little naive T-cell cycling (Figure 4A-B), the sjTREC levels remained nearly identical to those observed in uninfected healthy control subjects (Figure 4E). Thus, the TREC-bearing cells must either be recruited to the effector and memory T-cell pool (followed by proliferation) or selectively die.14 Both of these scenarios ultimately prevent the increase in sjTREC levels, as might be expected from the greater sj/βTREC ratio, and limit naive T-cell count restoration (Figure 3A-B). The lack of naive T-cell survival potential has been also observed in other immune reconstitution settings. For example, the poor reconstitution of naive T-cell numbers following allogeneic hematopoietic stem cell transplantation has been attributed to the low CD127 and bcl-2 expression levels.21 Nevertheless, HIV-infected immunologic responders under antiretroviral therapy do experience a rise in CD4+ T cells, demonstrating the positive impact of a functional thymus in the face of important changes in T-cell turnover.
However, unsuccessful naive CD4+ T-cell recovery despite HAART-mediated viral control in PIR is a result of a profound lack of thymic activity. This finding is consistent with previous work showing that treated HIV-infected patients with a weak immunologic response to HAART have a smaller thymus than good responders.17,19 Also, poor immunologic responders exhibit enhanced proportions of Ki-67–expressing cells in both naive (Figure 4A-B) and memory T cells (Ki-67+ in effector memory T cells, 3.75% versus 1.2% in healthy control subjects; P = .013), linking high levels of T-cell proliferation to a lack of CD4+ T-cell recovery in such cases. Whether Ki-67 expression illustrates the progeny of a successful cell division or the first steps of immune activation rendering a cell more prone to die,44,45 the outcome remains the same, namely, naive T-cell maintenance or reconstitution is not supported by the attempts of naive T cells to expand in the periphery (Figure 5B-C). These data corroborate with previous findings of shorter telomeres in CD4+ T cells from poor immunologic responders to HAART,17 thereby illustrating a greater proliferative history of CD4+ T cells in these patients, again linking peripheral proliferation to low CD4+ T-cell reconstitution. Of note, we also observed high CD8+ naive T-cell cycling in the PIR group, the members of which have near-normal CD8+ naive T-cell counts. This finding illustrates that, unlike what is observed in CD4+ T cells, self-renewal through peripheral proliferation may participate in naive T-cell recovery for the CD8+ compartment. However, these CD3+CD8+CD45RA+CD27+ cells may include a subset of effector T cells that share this phenotype and may be especially enriched for cells in cycle (B. Yassin-Diab, personal oral communication, June 2006), possibly misrepresenting the actual state of CD8+ naive T cells. Nevertheless, the high frequency of cycling CD4+ naive T cells observed in the PIR group is probably indicative of a failed homeostatic attempt to replenish the naive CD4+ T-cell compartment.
Mounting evidence supports the fact that HIV infection induces important perturbations in the “thymus-naive T-cell” axis. We have previously shown that acute HIV infection is characterized by a severely impaired thymopoiesis, as illustrated by an early drop of the sj/βTREC ratio.13 However, the interpretation of the individual dynamics of sjTREC and DJβTRECs have demonstrated the existence of compensatory mechanisms (possibly working through increased thymic input and/or enhanced peripheral naive T-cell survival) that lead to maintained naive T-cell counts despite impaired thymic function. Such a compensation was suggested by the limited decrease in sjTREC-containing cells in primary HIV infection.29 The data presented here demonstrate that these compensatory mechanisms are lost in chronic infection. Indeed, because thymic activity remained low in patients progressing to disease (low sj/βTREC ratio; Figure 2C-D), the RTE frequency eventually dropped, as shown by the observed low sjTREC levels (Figure 2E; Douek et al,18 Rodes etal,23 and Lewin et al46 ), and ultimately contributed to the decrease in size of the naive T-cell compartment (Figure 1; Roederer et al47 ). It is plausible to suggest that this state of affairs could be a result of the previously elucidated HIV-induced accumulation of collagen within the lymph node architecture, ultimately limiting the size of the residing T-cell pool, including naive T cells.48
In this study, we have clearly demonstrated that efficient thymopoiesis is a key element in the maintenance of CD4+ T-cell counts in slow progressor patients and in CD4+ T-cell recovery in virally suppressed, HAART-treated, chronically HIV-infected patients. However, enhanced peripheral naive T-cell cycling is associated with CD4+ lymphopenia and does not seem to efficiently compensate for this deficiency. Indeed, our results provide strong evidence for the existence of a close positive relationship between thymic activity and delayed disease progression as well as with the CD4+ T-cell gain subsequent to viral suppression under HAART.
Authorship
Contribution: M.-L.D. performed research, collected and analyzed data, participated in the research design, and wrote the paper; R.B. performed research, collected data, and participated in data analysis; J.Z. performed research; R.A., M.-R.B., and J.-P.R. provided specific clinical samples and collected data; M.M.L. provided specific clinical samples, collected data, and participated in writing the paper; R.-P.S. designed research and wrote the paper; and R.C. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rafick-Pierre Sekaly, Laboratoire d'Immunologie, Centre de Recherches du Centre Hospitalier de l'Université de Montréal (CHUM), Hôpital Saint Luc, 264 René-Lévesque est, Bureau 1307, Montréal, Québec, Canada H2X 1P1; e-mail: rafick-pierre.sekaly@umontreal.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Zvi Grossman, Dr Antonio Freitas, Dr Nicolas Chomont, and Dr Stephanie Beq for constructive discussions and their critical reading of this manuscript. We also thank Carmen Estrela and Mario Legault for administrative assistance.
This work was supported by grants from the National Institutes of Health (NIH) (R.-P.S.), the Canadian Institute of Health Research (CIHR) (R.-P.S.), les Fonds de la Recherche en Santé du Québec (FRSQ) (R.-P.S.), the Canadian Network for Vaccines and Immunotherapeutics (CANVAC) (R.-P.S.), and the Center for Aids Research (CFAR) at Case Western Reserve University (grant AI 36219) (M.M.). M.-L.D. received a doctoral research award from the CIHR. J.-P.R. is a scientific scholar receiving support from the Réseau Sida et Maladies Infectieuses du FRSQ. R.-P.S. is the Canada Research Chair (CRC) in Human Immunology.
This work was carried out in partial fulfillment of a doctoral thesis at McGill University, Montreal, QC, Canada, for M.-L.D.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal