Abstract
To study both the pathophysiologic and the prognostic value of ADAMTS13 in thrombotic microangiopathies (TMAs), we enrolled a cohort of 35 adult patients combining a first acute episode of TMA, an undetectable (below 5%) ADAMTS13 activity in plasma, and no clinical background such as sepsis, cancer, HIV, and transplantation. All patients were treated by steroids and plasma exchange, and an 18-month follow-up was scheduled. Remission was obtained in 32 patients (91.4%), and 3 patients died (8.6%) after the first attack. At presentation, ADAMTS13 antigen was decreased in 32 patients (91.4%), an ADAMTS13 inhibitor was detectable in 31 patients (89%), and an anti-ADAMTS13 IgG/IgM/IgA was present in 33 patients (94%). The 3 decedent patients were characterized by the association of several anti-ADAMTS13 Ig isotypes, including very high IgA titers, while mortality was independent of the ADAMTS13 inhibitor titer. In survivors, ADAMTS13 activity in remission increased to levels above 15% in 19 patients (59%) but remained undetectable in 13 patients (41%). Six patients relapsed either once or twice (19%) during the follow-up. High levels of inhibitory anti-ADAMTS13 IgG at presentation were associated with the persistence of an undetectable ADAMTS13 activity in remission, the latter being predictive for relapses within an 18-month delay.
Introduction
Thrombotic microangiopathies (TMAs) are defined by the association of acute mechanical hemolytic anemia, thrombocytopenia, and visceral ischemic manifestations related to the formation of platelet thrombi in the microcirculation.1 Clinically, TMA includes mainly the thrombotic thrombocytopenic purpura (TTP) and the hemolytic uremic syndrome (HUS) characterized by a multivisceral ischemia and a renal ischemia, respectively.2 Although mechanisms for HUS remain very heterogeneous, pathophysiology for most forms of TTP is related to a severe deficiency of a plasma metalloprotease, ADAMTS13 (adisintegrin and metalloprotease with thrombospondin type 1 repeats).3-6 Physiologically, ADAMTS13 is the specific cleaving protease for von Willebrand factor (VWF), a large multimeric glycoprotein crucial for both platelet adhesion and aggregation in the high stress–associated hemodynamic conditions of the microcirculation.7 A severe enzymatic deficiency of ADAMTS13 causes highly adhesive unusually large multimers of VWF to accumulate in plasma, which may spontaneously bind to platelets and thus induce the formation of platelet thrombi in the microcirculation. In rare cases, clinically relevant ADAMTS13 severe deficiency is related to compound heterozygous or homozygous mutations of the ADAMTS13 gene (Upshaw-Schulman syndrome).8-10 In most cases, severe ADAMTS13 deficiency is secondary to the development of anti-ADAMTS13 autoantibodies (auto-Abs).11,12 Anti-ADAMTS13 auto-Abs can be detected in vitro either functionally because of their inhibitory effect on ADAMTS13 enzymatic activity13,14 or, more recently, physically as immunoglobulin G (IgG) or IgM by enzyme-linked immunosorbent assay (ELISA).15,16 In more than 80% of acquired TTP, anti-ADAMTS13 antibodies are inhibitory IgG.11,12 In some cases, the mechanisms for acquired TTP may be different, involving either anti-ADAMTS13 noninhibitory IgG or IgM15,16 or circulating inhibitors not related to an IgG or an IgM.17 However, although most undetectable ADAMTS13-associated TMA overlaps with the clinical entity named TTP, some exceptions remain, consisting of either clinical TTP with detectable ADAMTS13 activity or clinical HUS with undetectable ADAMTS13 activity.18,19 This observation is currently motivating experts in the field to reconsider a new classification of TMA more focused on pathophysiological mechanisms instead of clinical symptoms.3,20
Beyond the elucidation of pathophysiological mechanisms, a second step crucial for TMA characterization is the identification of prognostic factors. Indeed, TMAs are severe diseases with acute events that may be life-threatening in the absence of fast and appropriate treatment (plasmatherapy) and with a global mortality rate that still remains close to 10%.21,22 In addition, in patients who achieved remission after an acute event, the risk of relapse is estimated to be at least 11%, with extreme rates reported at 73% as a function of the clinical background.2,3 However, identifying prognostic factors for both short-term and long-term outcome still remains difficult considering the broad heterogeneity of TMA patients. This heterogeneity including both the clinical background (idiopathic versus disease-associated TMA, sporadic versus recurrent TMA) and the ADAMTS13 plasmatic features (undetectable versus detectable ADAMTS13 activity, presence versus absence of an ADAMTS13 inhibitor) is well emphasized as a limit to determine clear prognostic factors in the recent literature.3,23-27
More specifically, the first acute TMA episode, classically described as a “storm in a blue sky,” remains a crucial challenge for physicians in terms of both pathophysiologic and prognostic issues. The first TMA attack is always a trauma whether it is idiopathic and then a first step within an autoimmune disease or whether it is associated with a preexisting clinical context and then a crucial turn in the course of the latter. More pathophysiologic explanations and prognostic markers are obviously needed. Undetectable ADAMTS13 activity–associated TMA, in addition to being a homogeneous entity, has the advantage of offering an exciting biologic parameter on which to focus, especially thanks to the recent advances in both ADAMTS13 antigen (ADAMTS13Ag) and auto-Ab characterization.16 Thus, in the current study, we chose to focus on both a pathophysiologic and a prognostic analysis (initial outcome and 18-month follow-up) of a homogeneous cohort of adult patients characterized by 3 criteria: (1) a first episode of acute TMA; (2) an undetectable ADAMTS13 activity; and (3) no severe clinical background that could, in itself, influence the prognosis (ie, severe sepsis, cancer, HIV infection, or organ transplantation). Standard clinical and biologic features as well as specific VWF/ADAMTS13-related parameters (VWF antigen [VWFAg], ADAMTS13 activity and antigen, ADAMTS13 inhibitors, and anti-ADAMTS13 IgG/IgM/IgA) were evaluated at presentation in all patients. In patients who achieved remission, ADAMTS13 activity was reevaluated during the follow-up. Thus, we evaluated the correlation of ADAMTS13-related parameters at presentation with the initial clinical and biologic outcome (mortality versus remission, recovery versus nonrecovery of ADAMTS13 activity in remission) as well as with the relapse rate during the follow-up.
Patients, materials, and methods
Patients
All adult patients (more than 18 years old) admitted in the 10 participating French centers with a clinical diagnosis of acute TMA between January 2003 and December 2004 were eligible and tested for ADAMTS13 activity in plasma. Diagnostic criteria for acute TMA were as follows: (1) microangiopathic hemolytic anemia (hemoglobin level below 120 g/L [12 g/dL]), negative direct antiglobulin test, at least 2 schistocytes per high-power field in the peripheral blood smear, lactate dehydrogenase above 450 U/L, and undetectable serum haptoglobin; (2) thrombocytopenia (platelet count below 150 × 109/L); (3) no requirement for fever, neurologic symptoms, or renal failure. For each patient, a questionnaire was completed, allowing initial clinical and laboratory evaluation. Inclusion criteria combined both a first acute event of TMA and an ADAMTS13 activity in plasma below 5%. Exclusion criteria were severe sepsis, cancer, HIV infection, organ or hematopoeitic stem cell transplantation, and disseminated intravascular coagulation. As a first intention treatment, all enrolled patients received both steroids (1 mg prednisone per kilogram of body weight daily) and plasma exchange (exchanging 1.0 to 1.5 times the predicted plasma volume of the patient) at the discretion of the attending physician. Evaluation for the initial outcome (achievement of remission or death) and an 18-month follow-up were performed. Patients were enrolled after appropriate consent was obtained in agreement with the institutional review board of Assistance Publique-Hôpitaux de Paris (Comité Consultatif pour la Protection des Personnes dans la Recherche Biomédicale, Ile-de-France–Paris–Saint Antoine) and the Declaration of Helsinki.
Clinical definition
Idiopathic TMA was defined as TMA occurring in patients with no apparent preexisting disease. Remission was defined by a normal platelet count (above 150 × 109/L) and no plasma exchange treatment for 30 days (day 1 starting on remission) or more.
Sample collection
Before any treatment was initiated, venous blood was collected into 1:10 final volume of 3.8% sodium citrate. Platelet-poor plasma was obtained by centrifugation at 2500g for 20 minutes, and aliquoted samples were stored at −80°C before being tested. Plasma samples were collected both at presentation and in remission (between day 1 and day 7 of remission according to the previous definition).
VWFAg
VWF antigen (VWFAg) levels in plasma were measured using an enzyme-linked immunosorbent assay (ELISA Asserachrom VWF; Diagnostica Stago, Asnières, France).
ADAMTS13 activity ELISA
Measurement of ADAMTS13 activity in plasma was performed as previously described18 with minor modifications. Briefly, the method relies on the hydrolysis of a constant amount of wild-type recombinant VWF used as substrate by serial dilutions of tested plasma used as ADAMTS13 provider. Plasma samples treated with Pefabloc (Roche Applied Science, Indianapolis, IN) (2 mM final) for 10 minutes were serially diluted from 1:10 to 1:640 in 5 mM Tris-HCl, pH 8, 1.5 M urea. Aliquots of 90 μL were preincubated with 10 μL of 100 mM BaCl2 for 5 minutes at room temperature. This mixture (60 μL) was added into wells of microtitration plates to 40 μL wild-type recombinant VWF (0.05 IU) previously dialyzed against 5 mM Tris-HCl, pH8, 1.5 M urea. Incubation was performed for 48 hours at 37°C. The proteolysis was stopped by addition of 5 μL of 200 mM EDTA in water. The residual VWFAg contained in the hydrolysate was thus estimated by a 2-site ELISA using the anti–C-terminal VWF MoAb 453 for coating and a pool of immunoperoxidase-conjugated anti–N-terminal VWF MoAbs for staining.28 Normal pooled plasma (NPP) was arbitrarily defined as containing 100% of ADAMTS13 activity and used as an internal control.
ADAMTS13 inhibitor assay
Patient plasma was heat treated at 56°C for 30 minutes to inactivate any endogeneous ADAMTS13 activity. Circulating inhibitor for ADAMTS13 was assayed by measuring the residual ADAMTS13 activity in mixtures of TMA patient plasma and NPP at 3 distinct volume-volume ratios: 1:1, 2:1, and 3:1 as previously described.18 Titer was semiquantitatively defined as high, medium, or low for a residual ADAMTS13 activity below 10% in 1:1, 2:1, and 3:1 mixtures, respectively.
ADAMTS13 inhibitor assay was systematically performed in addition to the ADAMTS13 activity assay in all patients.
Anti-ADAMTS13 antibody ELISA
Anti-ADAMTS13 IgG, IgM, and IgA were tested in all patients at presentation. Anti-ADAMTS13 IgG and IgM antibodies were detected as published by Rieger et al.16 Briefly, microtiter plates were coated with an anti-His tag antibody (overnight incubation), nonspecific binding sites were blocked for 2 hours at room temperature with PBS-BSA, and recombinant His-tagged ADAMTS13 was added and incubated for 3 hours at room temperature. Serial dilutions of plasma samples were incubated overnight at +4°C. After washing, plates were incubated with an alkaline phosphatase–conjugated goat anti–human IgG, IgM, or IgA antibody (Sigma, Saint Louis, MO) for 2 hours at room temperature. After washing, the enzyme substrate p-nitrophenyl phosphate (PNPP) was added and incubated 45 minutes. Absorbance was read at 405 nm. Anti-ADAMTS13 IgG/IgM/IgA antibodies titers were calculated according to Rieger et al.16 In patients whose anti-ADAMTS13 IgG titer was lower than the detection limit of our developed ELISA (IgG negative), anti-ADAMTS13 IgGs were retested using the TECHNOZYME ADAMTS13-INH commercial kit (Technoclone, Vienna, Austria) to improve sensitivity in the detection (IgG positive if above 15 U/mL). The IgG anti-ADAMTS13 ELISA using the commercial kit was performed according the manufacturer's instructions.
ADAMTS13 Ag ELISA test
ADAMTS13 Ag was measured using a recently developed ADAMTS13 Ag ELISA.29 Briefly, microtiter plates were coated with a polyclonal rabbit IgG against human ADAMTS13. Dilutions of plasma samples were incubated overnight at room temperature. After washing, plates were incubated with a horseradish peroxidase–conjugated polyclonal rabbit IgG against human ADAMTS13 for 2 hours at room temperature. After washing, a chromogenic substrate (Sure Blue TMB Microwell Peroxidase Substrate; KPL, Gaithersburg, MD) was added. The color reaction was stopped by 1N HCl solution, and absorbance was read at 450 nm. The reference interval for the ELISA is 740 to 1420 ng ADAMTS13 per milliliter, and the limit of quantification is 62.5 ng ADAMTS13 per milliliter. Samples with antigen levels under the limit of quantification are expressed as less than 62.5 ng ADAMTS13 per milliliter.
Statistical analysis
Statistics were performed using the computer-assisted program Statview (Abacus Concepts, Berkeley, CA). Means and standard deviations (SD) were calculated for each continuous variable. Comparisons were performed using an independent sample t test for continuous parametric variables and a χ2 test for categoric variables. Relationships between ADAMTS13-related parameters and other variables were studied using univariate and multivariate analysis with both linear and logistic regression analysis.
Results
Patients
Over a 24-month period, 755 adult patients with a clinically suspected acute TMA were enrolled in our national registry and screened for ADAMTS13 activity. A total of 658 patients exhibited a normal or partially decreased ADAMTS13 activity, and 97 patients had an undetectable ADAMTS13 activity. Among the latter, 47 patients had previously been included in our registry because of 1 or several acute TMA episodes, while 50 patients had a first TMA episode. Among the latter, 15 had exclusion criteria such as cancer, HIV, severe sepsis, organ transplantation. Thus, finally, a cohort of 35 consecutive patients with a first acute episode of TMA associated with an undetectable ADAMTS13 activity (below 5%) in plasma was enrolled. Demographic, clinical, and standard biologic features of each patient are presented in Table 1. Mean age was 36 years (SD = 12.7), and the sex ratio was 2.5:1 (25 women and 10 men). Twenty-two patients exhibited an idiopathic TMA, while 13 patients had a TMA associated either with pregnancy (n = 6), the postpartum state (n = 1), oral contraceptives (n = 1), Crohn disease (n = 1), psoriasis (n = 1), chronic hepatitis C (n = 1), diabetes (n = 1), or hypertension (n = 1). At inclusion during the acute TMA, fever was present in 5 patients (14.3%), and neurologic symptoms including either headache, altered mental status, aphasia, or convulsions were observed in 18 patients (51.4%); anemia (mean hemoglobin level ± SD, 71 ± 18 g/L and thrombocytopenia (mean platelet count ± SD, 17 ×109/L ± 13 × 109/L) were present in all patients. The mean creatinine level was 111 ± 52 μM, and only 3 patients exhibited a creatinine level higher than 200 μM. Three patients (8.6%) died of acute multivisceral TMA involvement with acute renal failure within a delay of 10 days of extensive plasma exchanges. Remission was obtained in 32 patients (91.4%) with the classical “steroids plus plasma exchanges” protocol described in “Patients” within a mean delay of 4 ± 1 weeks; no survivor patient exhibited a flare-up episode. The 18-month follow-up showed that 6 patients relapsed.
Demographic, clinical, and standard biologic features of each TMA patient (n = 35)
| Pt no. . | Age, y . | Sex . | Context . | Fever . | CNS symptoms . | Platelet count, × 109/L . | Hb level, g/L . | Creatinine level, μM . | Initial outcome . | No. of relapses* . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | M | Idiopathic | No | None | 10 | 63 | 116 | R | 0 |
| 2 | 19 | F | Idiopathic | No | None | 3 | 44 | 88 | R | 0 |
| 3 | 27 | M | Idiopathic | Yes | None | 10 | 69 | 85 | R | 0 |
| 4 | 35 | F | Idiopathic | Yes | None | 21 | 42 | 90 | R | 0 |
| 5 | 57 | F | Idiopathic | No | Aphasia | 28 | 51 | 92 | R | 0 |
| 6 | 41 | F | Idiopathic | No | Aphasia | 27 | 81 | 83 | R | 1 |
| 7 | 53 | F | Idiopathic | Yes | Aphasia | 10 | 50 | 77 | R | 0 |
| 8 | 35 | F | Idiopathic | No | None | 9 | 62 | 81 | R | 0 |
| 9 | 39 | M | Idiopathic | No | None | 10 | 91 | 85 | R | 0 |
| 10 | 50 | F | Idiopathic | No | None | 15 | 80 | 87 | R | 0 |
| 11 | 29 | F | Idiopathic | No | None | 30 | 70 | 90 | R | 0 |
| 12 | 34 | F | Idiopathic | No | AMS | 15 | 80 | 83 | R | 0 |
| 13 | 40 | M | Idiopathic | Yes | None | 15 | 57 | 100 | R | 1 |
| 14 | 31 | M | Idiopathic | No | Headache | 23 | 110 | 159 | R | 2 |
| 15 | 61 | M | Idiopathic | No | AMS | 4 | 62 | 240 | D | — |
| 16 | 38 | F | Idiopathic | No | None | 5 | 81 | 100 | R | 2 |
| 17 | 45 | M | Idiopathic | No | None | 9 | 60 | 175 | D | — |
| 18 | 28 | M | Idiopathic | Yes | AMS | 15 | 72 | 88 | R | 0 |
| 19 | 39 | F | Idiopathic | No | Aphasia | 17 | 85 | 105 | R | 0 |
| 20 | 16 | F | Idiopathic | No | None | 10 | 70 | 100 | R | 0 |
| 21 | 26 | F | Idiopathic | No | None | 12 | 68 | 81 | R | 1 |
| 22 | 70 | F | Idiopathic | No | Aphasia | 13 | 52 | 195 | D | — |
| 23 | 30 | F | Pregnancy | No | None | 11 | 83 | 82 | R | 0 |
| 24 | 29 | F | Pregnancy | No | Aphasia | 7 | 67 | 139 | R | 0 |
| 25 | 27 | F | Pregnancy | No | None | 20 | 100 | 300 | R | 0 |
| 26 | 24 | F | Pregnancy | No | Headache | 35 | 81 | 79 | R | 0 |
| 27 | 34 | F | Pregnancy | No | None | 10 | 71 | 82 | R | 1 |
| 28 | 33 | F | Pregnancy | No | AMS | 36 | 67 | 85 | R | 0 |
| 29 | 23 | F | Postpartum | No | Headache | 11 | 64 | 81 | R | 0 |
| 30 | 43 | F | Contraceptives | No | AMS | 11 | 72 | 76 | R | 0 |
| 31 | 23 | F | Crohn | No | AMS | 27 | 44 | 78 | R | 0 |
| 32 | 45 | M | Psoriasis | No | None | 17 | 116 | 90 | R | 0 |
| 33 | 59 | F | HCV | No | Convulsions | 14 | 70 | 95 | R | 0 |
| 34 | 29 | F | Diabetes | No | Headache | 20 | 73 | 97 | R | 0 |
| 35 | 31 | M | Hypertension | No | AMS | 80 | 106 | 210 | R | 0 |
| Pt no. . | Age, y . | Sex . | Context . | Fever . | CNS symptoms . | Platelet count, × 109/L . | Hb level, g/L . | Creatinine level, μM . | Initial outcome . | No. of relapses* . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | M | Idiopathic | No | None | 10 | 63 | 116 | R | 0 |
| 2 | 19 | F | Idiopathic | No | None | 3 | 44 | 88 | R | 0 |
| 3 | 27 | M | Idiopathic | Yes | None | 10 | 69 | 85 | R | 0 |
| 4 | 35 | F | Idiopathic | Yes | None | 21 | 42 | 90 | R | 0 |
| 5 | 57 | F | Idiopathic | No | Aphasia | 28 | 51 | 92 | R | 0 |
| 6 | 41 | F | Idiopathic | No | Aphasia | 27 | 81 | 83 | R | 1 |
| 7 | 53 | F | Idiopathic | Yes | Aphasia | 10 | 50 | 77 | R | 0 |
| 8 | 35 | F | Idiopathic | No | None | 9 | 62 | 81 | R | 0 |
| 9 | 39 | M | Idiopathic | No | None | 10 | 91 | 85 | R | 0 |
| 10 | 50 | F | Idiopathic | No | None | 15 | 80 | 87 | R | 0 |
| 11 | 29 | F | Idiopathic | No | None | 30 | 70 | 90 | R | 0 |
| 12 | 34 | F | Idiopathic | No | AMS | 15 | 80 | 83 | R | 0 |
| 13 | 40 | M | Idiopathic | Yes | None | 15 | 57 | 100 | R | 1 |
| 14 | 31 | M | Idiopathic | No | Headache | 23 | 110 | 159 | R | 2 |
| 15 | 61 | M | Idiopathic | No | AMS | 4 | 62 | 240 | D | — |
| 16 | 38 | F | Idiopathic | No | None | 5 | 81 | 100 | R | 2 |
| 17 | 45 | M | Idiopathic | No | None | 9 | 60 | 175 | D | — |
| 18 | 28 | M | Idiopathic | Yes | AMS | 15 | 72 | 88 | R | 0 |
| 19 | 39 | F | Idiopathic | No | Aphasia | 17 | 85 | 105 | R | 0 |
| 20 | 16 | F | Idiopathic | No | None | 10 | 70 | 100 | R | 0 |
| 21 | 26 | F | Idiopathic | No | None | 12 | 68 | 81 | R | 1 |
| 22 | 70 | F | Idiopathic | No | Aphasia | 13 | 52 | 195 | D | — |
| 23 | 30 | F | Pregnancy | No | None | 11 | 83 | 82 | R | 0 |
| 24 | 29 | F | Pregnancy | No | Aphasia | 7 | 67 | 139 | R | 0 |
| 25 | 27 | F | Pregnancy | No | None | 20 | 100 | 300 | R | 0 |
| 26 | 24 | F | Pregnancy | No | Headache | 35 | 81 | 79 | R | 0 |
| 27 | 34 | F | Pregnancy | No | None | 10 | 71 | 82 | R | 1 |
| 28 | 33 | F | Pregnancy | No | AMS | 36 | 67 | 85 | R | 0 |
| 29 | 23 | F | Postpartum | No | Headache | 11 | 64 | 81 | R | 0 |
| 30 | 43 | F | Contraceptives | No | AMS | 11 | 72 | 76 | R | 0 |
| 31 | 23 | F | Crohn | No | AMS | 27 | 44 | 78 | R | 0 |
| 32 | 45 | M | Psoriasis | No | None | 17 | 116 | 90 | R | 0 |
| 33 | 59 | F | HCV | No | Convulsions | 14 | 70 | 95 | R | 0 |
| 34 | 29 | F | Diabetes | No | Headache | 20 | 73 | 97 | R | 0 |
| 35 | 31 | M | Hypertension | No | AMS | 80 | 106 | 210 | R | 0 |
Patients 15, 17, and 22 are dead.
Pt indicates patient; CNS, central nervous system; Hb, hemoglobin; M, male; R, remission; F, female; AMS, altered mental status; D, death; —, not applicable; HCV, hepatitis C virus.
During an 18-month follow-up.
VWF- and ADAMTS13-related parameters in patients at presentation
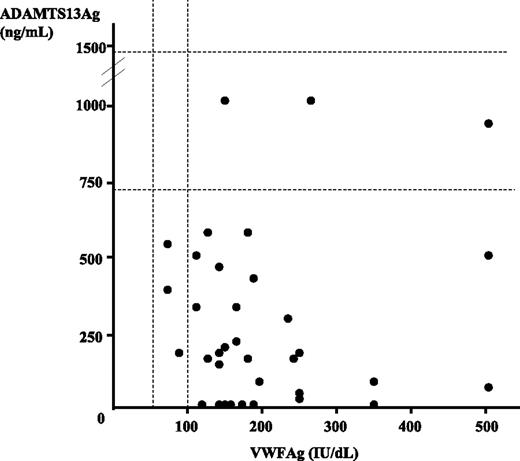
Results of both VWFAg and ADAMTS13Ag are presented in Figure 1. VWFAg levels were higher than 100 IU/dL in 32 patients (91.4%), including 11 patients with levels higher than 200 IU/dL (31.4% of total). ADAMTS13Ag levels were decreased in 32 patients (91.4%) (including 7 patients with undetectable levels) and normal in 3 patients (8.6%). However, statistical analysis showed no correlation between VWFAg and ADAMTS13Ag levels.
von Willebrand factor antigen (VWFAg) and ADAMTS13 antigen (ADAMTS13Ag) levels in plasma in a cohort of 35 patients with acute TMA. Normal ranges are indicated by dashed lines (50 to 100 UI/dL for VWFAg and 740 to 1420 ng/mL for ADAMTS13Ag). Each TMA patient is represented by a circle. All but 3 patients exhibited a VWFAg level higher than 100 IU/dL, while all but 3 patients had a decreased ADAMTS13Ag level. Statistical analysis showed no correlation between VWFAg and ADAMTS13Ag levels.
von Willebrand factor antigen (VWFAg) and ADAMTS13 antigen (ADAMTS13Ag) levels in plasma in a cohort of 35 patients with acute TMA. Normal ranges are indicated by dashed lines (50 to 100 UI/dL for VWFAg and 740 to 1420 ng/mL for ADAMTS13Ag). Each TMA patient is represented by a circle. All but 3 patients exhibited a VWFAg level higher than 100 IU/dL, while all but 3 patients had a decreased ADAMTS13Ag level. Statistical analysis showed no correlation between VWFAg and ADAMTS13Ag levels.
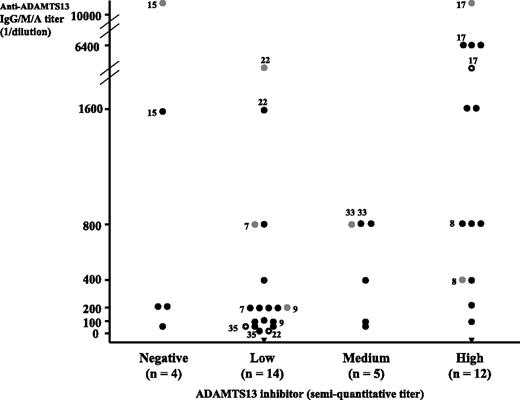
Titrations for both ADAMTS13 inhibitor and anti-ADAMTS13 IgG, IgM, and IgA are presented in Figure 2. An ADAMTS13 inhibitor was detected in 31 patients (89%), while an anti-ADAMTS13 IgG (titer ranging from 25 to 6400) and/or IgM (titer from 20 to 3200) and/or IgA (titer from 200 to more than 10 000 arbitrary units) were detected in 33 patients (94%) (Figure 2). A mild correlation between ADAMTS13 inhibitor titer and anti-ADAMTS13 Ig titer was present with an overlapping between both parameters in 29 of 35 patients (inhibitory anti-ADAMTS13 Ig, 83%). In contrast, in the absence of ADAMTS13 inhibitor, an anti-ADAMTS13 Ig was detected in 4 of 35 patients (noninhibitory anti-ADAMTS13 Ig, 11.5%). Plasma of 2 patients inhibited ADAMTS13 activity despite the absence of anti-ADAMTS13 IgG/IgM/IgA (Figure 2). Statistical analysis showed no correlation between ADAMTS13Ag levels and either ADAMTS13 inhibitor or anti-ADAMTS13 IgG/IgM/IgA titers, respectively.
ADAMTS13 inhibitor and anti-ADAMTS13 IgG/IgM/IgA in a cohort of 35 patients with acute TMA. Each TMA patient is represented by a symbol. Black circles are for patients with IgG, gray circles for patients with IgA, white circles for patients with IgM, and black triangles for patients with no detectable IgG/IgM/IgA. IgG/IgM/IgA titers are expressed as the reciprocal of patient plasma dilution. An isolated IgG was detected in 26 patients, the combination IgG and IgM in 1 patient, the combination IgG and IgA in 4 patients, and the combination of IgG, IgM, and IgA in 2 patients (indicated by numbers referring to patients in Table 1). Two patients had neither an IgG, IgM, nor IgA. ADAMTS13 inhibitors are expressed as semiquantitative titers: 4 patients had no detectable ADAMTS13 inhibitor, 14 patients a low titer, 5 patients a medium titer, and 12 patients a high titer. Twenty-nine patients had both an ADAMTS13 inhibitor and an anti-ADAMTS13 IgG/IgM/IgA (inhibitory anti-ADAMTS13 antibodies), and 4 patients had an anti-ADAMTS13 IgG/IgA with no ADAMTS13 inhibitor (noninhibitory anti-ADAMTS13 antibodies). Two patients had no IgG/IgM/IgA but an ADAMTS13 inhibitor.
ADAMTS13 inhibitor and anti-ADAMTS13 IgG/IgM/IgA in a cohort of 35 patients with acute TMA. Each TMA patient is represented by a symbol. Black circles are for patients with IgG, gray circles for patients with IgA, white circles for patients with IgM, and black triangles for patients with no detectable IgG/IgM/IgA. IgG/IgM/IgA titers are expressed as the reciprocal of patient plasma dilution. An isolated IgG was detected in 26 patients, the combination IgG and IgM in 1 patient, the combination IgG and IgA in 4 patients, and the combination of IgG, IgM, and IgA in 2 patients (indicated by numbers referring to patients in Table 1). Two patients had neither an IgG, IgM, nor IgA. ADAMTS13 inhibitors are expressed as semiquantitative titers: 4 patients had no detectable ADAMTS13 inhibitor, 14 patients a low titer, 5 patients a medium titer, and 12 patients a high titer. Twenty-nine patients had both an ADAMTS13 inhibitor and an anti-ADAMTS13 IgG/IgM/IgA (inhibitory anti-ADAMTS13 antibodies), and 4 patients had an anti-ADAMTS13 IgG/IgA with no ADAMTS13 inhibitor (noninhibitory anti-ADAMTS13 antibodies). Two patients had no IgG/IgM/IgA but an ADAMTS13 inhibitor.
Analysis of decedent patients
Three patients died after the first acute TMA episode (patients 15, 17, and 22; Table 1). Table 1 indicates their specific clinical characteristics, and Table 2 indicates their main VWF/ADAMTS13 features. All patients had an idiopathic TMA, none of them had fever at presentation, while 2 of them exhibited neurologic symptoms (altered mental status and aphasia). In all of them, the platelet count was lower than 15 × 109/L and the hemoglobin level lower than 65 g/L. Interestingly, all of them also had a significant alteration of renal function (creatinine level higher than 175 μM), and 2 of them were older than 60 years. (Table 1). Analysis of the VWF/ADAMTS13 features (Table 2) shows a remarkably increased VWFAg level in 2 patients (350 and 540 IU/dL in patients 15 and 22, respectively) and a remarkably decreased ADAMTS13Ag in 2 patients (less than 62.5 ng/mL in patients 15 and 17). An ADAMTS13 inhibitor was either detectable in 2 patients (low or high titer) or not detectable in 1 patient. Interestingly, all patients were characterized by the association of several anti-ADAMTS13 Ig isotypes (2 patients with IgG, IgM, and IgA and 1 patient with both IgG and IgA) with very high Ig titers, especially for IgA (Table 2).
Main VWF/ADAMTS13 features of the 3 decedent patients
| Patient no. . | VWFAg, IU/dL . | ADAMTS13Ag, ng/mL . | ADAMTS13 inhibitor titer . | Anti-ADAMTS13 IgG titer* . | Anti-ADAMTS13 IgM titer* . | Anti-ADAMTS13 IgA titer* . |
|---|---|---|---|---|---|---|
| 15 | 350 | < 62.5 | − | 1600 | 0 | > 10 000 |
| 17 | 150 | < 62.5 | +++ | 6400 | 3200 | > 10 000 |
| 22 | 540 | 85 | + | 1600 | 20 | 3 200 |
| Patient no. . | VWFAg, IU/dL . | ADAMTS13Ag, ng/mL . | ADAMTS13 inhibitor titer . | Anti-ADAMTS13 IgG titer* . | Anti-ADAMTS13 IgM titer* . | Anti-ADAMTS13 IgA titer* . |
|---|---|---|---|---|---|---|
| 15 | 350 | < 62.5 | − | 1600 | 0 | > 10 000 |
| 17 | 150 | < 62.5 | +++ | 6400 | 3200 | > 10 000 |
| 22 | 540 | 85 | + | 1600 | 20 | 3 200 |
− indicates negative; +++, high; +, low.
1/dilution.
Analysis of ADAMTS13 in survivors at initial clinical remission
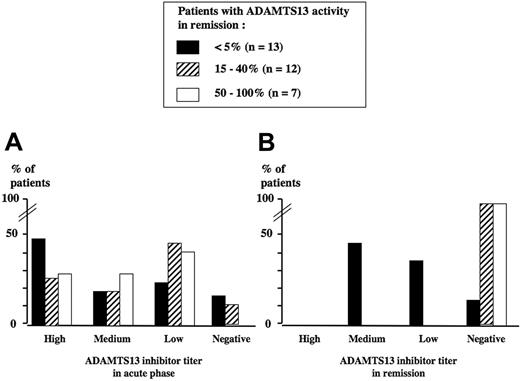
ADAMTS13 activity measured at initial clinical remission showed 3 distinct courses: It either remained undetectable (below 5%) in 13 patients (41%) or became detectable but partially decreased (15% to 40%) in 12 patients (38%) or normal (50% to 100%) in 7 patients (21%). ADAMTS13 inhibitors in remission were still detectable only in patients whose ADAMTS13 activity remained below 5%, with titers either similar or decreased when compared with those of the acute episode (Figure 3).
ADAMTS13 inhibitor titer. Titer is shown in the acute phase (A) and in remission (B) in 32 surviving TMA patients. The level of ADAMTS13 activity in survivors at initial clinical remission defines 3 groups of TMA patients: below 5% (group 1, n = 13, black histograms), 15% to 40% (group 2, n = 12, dashed histograms), and 50% to 100% (group 3, n = 7, white histograms). The percentage of patients with either high, medium, low, or negative ADAMTS13 inhibitor titers within each group is presented both in the acute phase (A) and in remission (B). In group 1, ADAMTS13 inhibitor titers remained identical or decreased in remission when compared with the acute phase. In groups 2 and 3, all ADAMTS13 inhibitors, when present in the acute phase, disappeared in remission.
ADAMTS13 inhibitor titer. Titer is shown in the acute phase (A) and in remission (B) in 32 surviving TMA patients. The level of ADAMTS13 activity in survivors at initial clinical remission defines 3 groups of TMA patients: below 5% (group 1, n = 13, black histograms), 15% to 40% (group 2, n = 12, dashed histograms), and 50% to 100% (group 3, n = 7, white histograms). The percentage of patients with either high, medium, low, or negative ADAMTS13 inhibitor titers within each group is presented both in the acute phase (A) and in remission (B). In group 1, ADAMTS13 inhibitor titers remained identical or decreased in remission when compared with the acute phase. In groups 2 and 3, all ADAMTS13 inhibitors, when present in the acute phase, disappeared in remission.
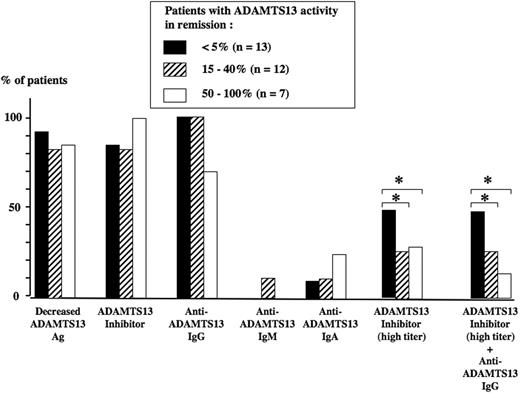
Correlation between ADAMTS13 parameters at presentation and ADAMTS13 activity at initial clinical remission is presented in Figure 4. Neither ADAMTS13 Ag levels nor the presence of an ADAMTS13 inhibitor (independently of the titer) nor the presence of anti-ADAMTS13 IgG, IgM, or IgA at presentation were predictive of the course of ADAMTS13 activity in remission. In contrast, the presence of a high titer of ADAMTS13 inhibitor, either alone or combined with an anti-ADAMTS13 IgG, was associated with the persistence of an undetectable ADAMTS13 activity (below 5%) in remission (Figure 4).
ADAMTS13-related parameters at presentation in 32 surviving TMA patients. The level of ADAMTS13 activity in survivors at initial clinical remission defines 3 groups of TMA patients: below 5% (group 1, n = 13, black histograms), 15% to 40% (group 2, n = 12, dashed histograms), and 50% to 100% (group 3, n = 7, white histograms). At presentation, the percentage of patients with either a decreased ADAMTS13Ag level, an ADAMTS13 inhibitor (independently of the titer), or the presence of an anti-ADAMTS13 IgG, IgM, or IgA was not significantly different between the 3 groups. In contrast, at presentation, the presence of either a high titer of ADAMTS13 inhibitor or a highly inhibitory anti-ADAMTS13 IgG was significantly (*P < .05) more frequent in group 1 when compared with group 2 and group 3, respectively.
ADAMTS13-related parameters at presentation in 32 surviving TMA patients. The level of ADAMTS13 activity in survivors at initial clinical remission defines 3 groups of TMA patients: below 5% (group 1, n = 13, black histograms), 15% to 40% (group 2, n = 12, dashed histograms), and 50% to 100% (group 3, n = 7, white histograms). At presentation, the percentage of patients with either a decreased ADAMTS13Ag level, an ADAMTS13 inhibitor (independently of the titer), or the presence of an anti-ADAMTS13 IgG, IgM, or IgA was not significantly different between the 3 groups. In contrast, at presentation, the presence of either a high titer of ADAMTS13 inhibitor or a highly inhibitory anti-ADAMTS13 IgG was significantly (*P < .05) more frequent in group 1 when compared with group 2 and group 3, respectively.
Follow-up of the survivors
Of the 32 patients who achieved clinical remission after the first acute TMA episode, 6 patients relapsed either once (patients 6, 13, 21, and 27; Table 1) or twice (patients 14 and 16; Table 1) during the 18-month follow-up. Delay for relapses ranged from 5 to 17 months after the first acute TMA episode, and 50% of relapses occurred as soon as the first year. Interestingly, all relapses were associated with an undetectable ADAMTS13 activity and an ADAMTS13 inhibitor titer similar to that at first diagnosis (data not shown).
Both the mean age (36 years old) and the sex ratio (2:1 female-male) of relapsing patients were similar to those of the cohort; all but 1 patient whose acute TMA occurred during pregnancy (patient 27) had an idiopathic TMA, and both their clinical and standard biologic parameters at presentation were similar to the ones of nonrelapsing patients (Table 1). VWF/ADAMTS13-related parameters of the 6 relapsing patients are presented in Table 3. At presentation of the initial acute TMA episode, VWFAg levels were either normal or moderately increased (70 to 250 IU/dL), ADAMTS13Ag levels were either decreased or normal (75 to 1010 ng/mL), an ADAMTS13 inhibitor was detected in 5 of 6 patients, while an anti-ADAMTS13 IgG was present in all patients. Interestingly, at initial remission, ADAMTS13 activity was still undetectable (below 5%) in 5 patients together with ADAMTS13 inhibitor titers either identical or decreased when compared with inhibitor titers of the initial acute phase (Table 3).
Main VWF/ADAMTS13 features at presentation and at initial remission of the first TMA attack of the 6 relapsing patients during the 18-month follow-up
| Patient no. . | VWFAg, IU/dL . | ADAMTS13 Ag, ng/mL . | ADAMTS13 inhibitor titer . | Anti-ADAMTS13 IgG titer, 1/dilution . | ADAMTS13 activity, %; inhibitor in remission . |
|---|---|---|---|---|---|
| 6 | 140 | 450 | +++ | 200 | < 5; ++ |
| 13 | 180 | 145 | +++ | 800 | < 5; ++ |
| 14 | 245 | 75 | + | 400 | < 5; + |
| 16 | 140 | 1010 | +++ | 800 | < 5; ++ |
| 21 | 70 | 300 | +++ | 1600 | 15; − |
| 27 | 140 | 160 | − | 200 | < 5; − |
| Patient no. . | VWFAg, IU/dL . | ADAMTS13 Ag, ng/mL . | ADAMTS13 inhibitor titer . | Anti-ADAMTS13 IgG titer, 1/dilution . | ADAMTS13 activity, %; inhibitor in remission . |
|---|---|---|---|---|---|
| 6 | 140 | 450 | +++ | 200 | < 5; ++ |
| 13 | 180 | 145 | +++ | 800 | < 5; ++ |
| 14 | 245 | 75 | + | 400 | < 5; + |
| 16 | 140 | 1010 | +++ | 800 | < 5; ++ |
| 21 | 70 | 300 | +++ | 1600 | 15; − |
| 27 | 140 | 160 | − | 200 | < 5; − |
Anti-ADAMTS13 IgM titer and anti-ADAMTS13 IgA titer equal 0 for all patients listed in this table.
+++ indicates high; ++, medium; +, low; −, negative.
The relapse rate was significantly higher in patients whose ADAMTS13 activity at initial remission was still undetectable (below 5%) (5 of 13; 38.5%) when compared with patients whose ADAMTS13 activity at initial remission was detectable (above 15%) (1 of 19; 5%).
Discussion
In adult patients, an autoimmunization against ADAMTS13 inducing a severe ADAMTS13 functional deficiency in plasma has been described as a key mechanism for most undetectable ADAMTS13-associated TMA.3
The first goal of the current study was to explore the pathophysiology for ADAMTS13 severe deficiency in a cohort of adult patients who underwent a first acute episode of TMA. Because none of these patients had ever received plasma infusions before, any potential alloimmunization against ADAMTS13 and variations in anti-ADAMTS13 auto-Abs titers after plasmatherapy30 could be excluded. Interestingly, this study is the first one to show anti-ADAMTS13 IgA in TMA patients. In all cases, however, these IgAs were associated with IgG. In our cohort, a circulating inhibitor against ADAMTS13 was detected with a lower frequency (89%) than an anti-ADAMTS13 IgG/IgM/IgA (94%) as previously reported by Rieger at al,16 who recently demonstrated a higher sensitivity of IgG/IgM detection (97%) versus inhibitor detection (83%) in 59 patients with an undetectable ADAMTS13-associated acute TMA. This discrepancy between inhibitors and Ig rates is, however, not surprising considering the broad range (50% to almost 90%) of circulating ADAMTS13 inhibitors reported in TMA patients3,11-13,18,31,32 and the established variability of sensitivity of the miscellaneous methods used for the detection of these inhibitors.14
In our cohort, the overlapping rate between ADAMTS13 inhibitors and anti-ADAMTS13 IgG/IgM/IgA was 83%, corresponding to inhibitory IgG/IgM/IgA, while the rate for noninhibitory IgG/IgM/IgA was 11.5%. These proportions are in agreement with the literature15,16,33 and confirm that most anti-ADAMTS13 auto-Abs are functional (inhibitory) in vitro and potentially in vivo. However, the decrease of ADAMTS13Ag plasma levels found in 91.4% of patients in our cohort suggests that the formation of immune complexes that may be rapidly cleared in vivo also strongly participates in ADAMTS13 deficiency whatever the inhibitory or noninhibitory capacity of ADAMTS13 auto-Abs, as previously reported.34,35 Also, in addition to the inhibitory effect on ADAMTS13 function, the residual levels of both ADAMTS13 activity and ADAMTS13Ag in plasma may be influenced by several other parameters—either Ig related (ie, the affinity for ADAMTS13, the epitope(s) on ADAMTS13,36-38 a proteolytic degradation of ADAMTS1339 ) or non-Ig related (ie, a decreased liver synthesis,40 an inactivation by thrombin or plasmin,41 or an in vitro inhibition by hemoglobin,17 the latter being excluded in our patients). Finally and interestingly, we found that anti-ADAMTS13 IgG associated with a high inhibitor titer at presentation was predictive of a nonrecovery of ADAMTS13 activity in clinical remission (Figure 4). The pathophysiology for this discrepancy between clinical remission and ADAMTS13-related biologic remission in some patients remains, however, unclear.
The second goal of our study was to search for prognostic factors among ADAMTS13-related parameters at presentation. The originality and strength of the current study was to specifically include patients with a first acute TMA episode associated with an undetectable ADAMTS13 activity and to exclude patients whose clinical background in itself could have influenced the prognosis (cancer, HIV infection, organ transplantation, severe sepsis).
These exclusion criteria may therefore explain the mortality rate of 8.6%, which is lower than most rates reported in the literature.3,21,22 Interestingly, a detailed analysis of the 3 decedent patients reveals a specific association between mortality and the combination of several Ig isotypes (IgG, IgM, and IgA) with the presence of very high IgA titers in all patients (Table 2). The association of both an IgG and an IgM has already been described in a 70-year-old patient with a fatal TTP.15 The presence of anti-ADAMTS13 IgA has never been described in any patient to date, and both their etiology and their potential toxicity remain unknown. However, IgAs are known to be associated with a bad prognosis with several pathological conditions such as nephropathies,42 myeloma,43 celiac disease,44 and heparin-induced thrombocytopenia.45 In our patients, the role of anti-ADAMTS13 IgA toxicity on renal tissue may be questionable, because all of them had a severe alteration of renal function as soon as at presentation. Also, in our 3 decedent patients, the inhibitory function of the anti-ADAMTS13 IgG/IgM/IgA does not appear closely related to mortality although ADAMTS13Ag levels were severely decreased. This last point underlines that an immune-depletion mechanism leading to a severe ADAMTS13 quantitative deficiency is the most important mechanism for the severe ADAMTS13 enzymatic deficiency identified in the decedent patients. The high titers of the anti-ADAMTS13 IgG/IgM/IgAs are likely to be strongly involved in the formation of immune complexes, but a particularly high affinity of these IgG/IgM/IgAs to ADAMTS13 may also be hypothesized. Hence, both the high titer and the potential high affinity of the anti-ADAMTS13 IgG/IgM may also explain the lack of response to the first intention treatment (steroids and plasma exchanges) that led to a fatal outcome with a dramatic multivisceral involvement in our 3 patients. Retrospectively, in these patients, the indication of a first-line immunosuppressive treatment associated with plasmatherapy is debatable.
In our cohort, however, 32 patients of 35 (91.4%) achieved remission within 3 to 5 weeks with a treatment associating steroids and plasma exchanges. No patient required an additive treatment (ie, splenectomy or immunosuppressive agents) to induce remission. This good response to plasmatherapy alone may be explained by both the exclusion of TMA associated with sepsis, cancer, organ transplantation, HIV, and enrollment during a first acute TMA episode. Indeed, TMA refractory to plasmatherapy has been described mainly either in the previous clinical contexts or, independently of these specific clinical backgrounds, preferentially in patients with a recurrent disease.3
Using an 18-month follow-up of the 32 patients who achieved remission, we found a global relapse rate of 19%. Although relapse rates previously reported are very heterogeneous and look very dependent on the clinical background,3,23 the relapse rate of our cohort is, however, in agreement with data from the literature: In adult idiopathic TMA, data from the Oklahoma TTP-HUS registry report a relapse rate of about 20%2 ; in adult TMA occurring concomitantly with another clinical condition (especially pregnancy and autoimmune diseases), the risk of relapse is reported to be higher than 25%.46,47 Also in agreement with these data, of our 6 relapsing patients, 5 had an idiopathic TMA while 1 had a pregnancy-associated TMA, and 50% of relapses occurred within the first year.
Interestingly, the relapse rate was significantly higher in patients whose ADAMTS13 activity in remission was still undetectable (38.5%) when compared with patients who recovered a detectable ADAMTS13 activity in remission (5%). This observation is in agreement with a previous report from our group showing that most patients with multirelapsing TTP becoming progressively refractory to plasmatherapy were characterized by a persistent undetectable ADAMTS13 activity during remission.48 However, the predictive value for relapse of an undetectable ADAMTS13 activity in remission remains debatable: Indeed, in our cohort, 1 patient (patient 21) who recovered a detectable ADAMTS13 activity in remission (15%) relapsed 16 months after the initial episode, while 8 patients with a still undetectable ADAMTS13 activity in remission did not relapse during the 18-month follow-up. On one hand, in our study, the positive predictive value for relapsing in a delay of 18 months of an undetectable ADAMTS13 activity in remission (number of relapsing patients with an undetectable ADAMTS13 activity in remission/total number of patients with an undetectable ADAMTS13 activity in remission) is only 38.5%. These data are not surprising considering that even patients with an inherited, and thus persistent, severe ADAMTS13 deficiency (Upshaw-Schulman syndrome) exhibit greatly heterogeneous clinical phenotypes in terms of both severity and frequency of relapses.49,50 Indeed, similar to the incomplete penetrance and variable expressivity related to gene modifiers observed in most thrombotic diseases,51 both specific environmental and genetic backgrounds are likely to associate synergistically with persistent acquired ADAMTS13 severe deficiency in the trigger of TMA relapses. On the other hand, the negative predictive value for relapse of a detectable ADAMTS13 activity in remission (number of nonrelapsing patients with a detectable ADAMTS13 activity in remission/total number of patients with a detectable ADAMTS13 activity in remission) is 94.7%, which means that recovering a detectable ADAMTS13 in remission may be considered as a good prognostic factor (lower risk of relapse) in patients after a first acute TMA episode.
In conclusion, this study emphasizes that the pathophysiology for a first TMA attack related to an undetectable ADAMTS13 activity mainly involves anti-ADAMTS13 IgG combining both an inhibitory and an immune complex–depletion mechanism. Among ADAMTS13-related parameters at presentation, the combination of several anti-ADAMTS13 Ig isotypes (IgG, IgM, and IgA), including very high IgA titers, is linked to a higher risk of mortality. In survivors, highly inhibitory anti-ADAMTS13 IgGs at presentation are associated with the persistence of an undetectable ADAMTS13 activity in remission, the latter being predictive for relapses within 18 months. Similarly to other autoimmune diseases,52 the role of anti-ADAMTS13 auto-Abs in acquired TMA as prognostic markers needs to be further addressed using longitudinal studies involving large numbers of patients, ideally as soon as the first TMA attack.
Authorship
Contribution: A.V., F.S., and S.F. designed the study, analyzed the results, and wrote the paper; S.F. and G.M. did all the antibody assay work; the study protocol was discussed with P.C., E.A., C.B.-B., F.F., J.-P.M., E.O., E.R., B.S., and J.-P.V., who were also in charge of patients; P.P., N.S., J.-L.T., and P.V. were in charge of patients; and A.V., D.M., J.-P.G., M.W., F.S., M.R., G.M., and S.F. were responsible for the VWF and ADAMTS13 assays.
Conflict-of-interest disclosure: S.F., M.R., G.M., and F.S. are employees of Baxter Healthcare Inc. They declare that they have no commercial interest in the assay systems developed to detect anti-ADAMTS13 antibodies.
A complete list of the members of the French Clinical and Biological Network on Adult Thrombotic Microangiopathies appears as a data supplement to the online version of this article (Document S1, available on the Blood website; see the Supplemental Material link at the top of the online article).
Correspondence: Agnès Veyradier, Service d'Hématologie Biologique, Hôpital Antoine Béclère, 157, rue de la Porte-de-Trivaux, 92140 Clamart cedex, France; e-mail: agnes.veyradier@abc.aphp.fr.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The authors are grateful to Sylvaine Savigny, Véronique Dael, and Monique Lepka (Service d'Hématologie Biologique, Hôpital Antoine Béclère) for expert technical assistance. The authors also thank the biologists from the hemostasis and plasmapheresis departments of the participating centers for their help in the collection and the shipment of the plasma samples (Dr Marie-Lorraine Scrobohacci, Hôpital Saint-Louis; Dr Valérie Eschwege and Dr Annie Robert, Hôpital Saint-Antoine; Dr Dominique Lasne, Hôpital Necker; Dr Farhad Heshmati and Dr Annette Bussel, Hôpital Cochin; Dr Katia Pouimayou and Prof Irène Juhan-Vague, Hôpital de la Timone Marseille; Dr Valérie Proulle and Dr Marie Dreyfus, Hôpital de Bicêtre; Dr Isabelle Martin and Dr Annick Ankri, Hôpital de la Pitié-Salpêtrière; Dr Michèle Gouault-Heilmann, Hôpital Henri Mondor Créteil; and Dr Nathalie Parquet, Hôpital Saint Louis).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal