Abstract
Arterial calcification (AC) is generally regarded as an independent risk factor for cardiovascular morbidity and mortality. Matrix Gla protein (MGP) is a potent inhibitor of AC, and its activity depends on vitamin K (VK). In rats, inactivation of MGP by treatment with the vitamin K antagonist warfarin leads to rapid calcification of the arteries. Here, we investigated whether preformed AC can be regressed by a VK-rich diet. Rats received a calcification-inducing diet containing both VK and warfarin (W&K). During a second 6-week period, animals were randomly assigned to receive either W&K (3.0 mg/g and 1.5 mg/g, subsequently), a diet containing a normal (5 μg/g) or high (100 μg/g) amount of VK (either K1 or K2). Increased aortic calcium concentration was observed in the group that continued to receive W&K and also in the group changed to the normal dose of VK and AC progressed. Both the VK-rich diets decreased the arterial calcium content by some 50%. In addition, arterial distensibility was restored by the VK-rich diet. Using MGP antibodies, local VK deficiency was demonstrated at sites of calcification. This is the first study in rats demonstrating that AC and the resulting decreased arterial distensibility are reversible by high-VK intake.
Introduction
Arterial calcification is an important independent risk factor for the development of atherosclerosis, myocardial infarction, stroke, and renal disease.1,2 Patients with manifest arterial calcification have an unfavorable prognosis compared with patients with no or mild calcification.3,4 Therefore, the prevention or reversal of arterial calcification may lead to improved patient outcomes.
For a long time it has been thought that calcification was a passive process and the end stage of cardiovascular disease. During the past 10 years, however, it has become clear that several osteoregulatory proteins, both stimulatory and inhibitory, are involved in the calcification of vascular tissue.5-8 One of the strongest in vivo inhibitors of arterial calcification is matrix Gla protein (MGP). MGP was first discovered in bone,9 but it is mainly produced by vascular smooth muscle cells and chondrocytes. Its function became clear in MGP-deficient mice,10 which died within 6 to 8 weeks after birth as a result of rupture of the large arteries. Histochemical evaluation demonstrated complete calcification of the elastic fibers in the arterial vessels and a phenotypic change of smooth muscle cells into chondrocytes. MGP acts by direct inhibition of calcium crystal formation and regulates bone morphogenetic protein-2, a growth factor responsible for osteogenic differentiation.11-13 Murshed et al14 demonstrated that restoration of MGP exclusively in the vascular smooth muscle cells of the MGP-null mice completely rescued the vascular calcification phenotype. For this effect the MGP needed to be γ-carboxylated because mutating the Gla residues into aspartic acid residues led to the synthesis of nonfunctional MGP and to the death of all animals.
Vitamin K is an essential cofactor in the γ-carboxylation of glutamate residues in a small group of proteins, including MGP.15 The activity of these vitamin K–dependent or Gla proteins is strictly dependent on the presence of γ-carboxyglutamate (Gla) residues at a number of well-defined positions. The oxidation of vitamin K-hydroquinone (KH2) into vitamin K-epoxide (KO) provides the energy required for Gla formation, and the KO formed can be reused after subsequent reduction by the enzyme vitamin K-epoxide reductase (VKOR).16,17 Coumarin derivatives such as warfarin specifically block VKOR, leading to exhaustion of the available vitamin K stores and to the synthesis of noncarboxylated, inactive Gla proteins. Mainly in the liver, a second pathway for KH2 formation is present. The key enzyme in this pathway is the NAD(P)H-dependent DT-diaphorase. In extrahepatic tissues, such as the arterial vessel wall, DT-diaphorase activity is low,18 which explains why high–vitamin K intake effectively counteracts the effect of warfarin in the liver but not in bone19 and the arterial vessel wall.20,21 Thus, by subjecting experimental animals to a regimen of warfarin + vitamin K, the synthesis of Gla proteins can be blocked in the extrahepatic tissues, without affecting coagulation factor synthesis in the liver. Using this regimen of warfarin and vitamin K, Price et al20 showed that this induced medial calcifications of the elastic lamellae in arteries and heart valves of rats within 3 to 5 weeks through inhibition of the γ-glutamyl carboxylation of matrix Gla protein.
Vitamin K consists of 2 forms, namely phylloquinone (vitamin K1; K1) and the menaquinones (vitamin K2; K2). It has been reported that K1 can be converted into vitamin K2 (specifically MK4, menaquinone-4); the accumulation appears to be specific for extrahepatic tissues.22,23 A recent study by Thijssen et al24 revealed that menadione (2-methyl-1,4-naphthoquionone) is a product of vitamin K catabolism and the likely intermediate in the synthesis of MK4 that is found in the arterial vessel wall. We have used this arterial calcification model20 (the calcification-inducing regimen of warfarin and vitamin K) and found that high–vitamin K2 supplementation was able to inhibit warfarin-induced arterial calcification in rats.25 Moreover, we and others have reported that the use of coumarin-type anticoagulants is associated with increased cardiac valve calcification.26,27 Further studies using immunohistochemical analysis with conformation-specific antibodies demonstrated that in calcifying carotid arteries MGP predominantly occurs in the noncarboxylated form, suggesting that the local vitamin K status is suboptimal.13,28
The rat arterial calcification model, as developed by Price et al20 and used by others,21,25,29 has thus far only looked at the development of arterial calcification. The aim of the present study was to use the rat arterial calcification model to investigate whether maximal MGP activity, ascertained by high–vitamin K intake, may stop the progression or even induce a reversal of warfarin-induced arterial calcification and the associated decrease in arterial distensibility.
Materials and methods
Animals and diet
Male Wistar Kyoto rats were purchased from the Maastricht University. Rats were 10 weeks old when entering the study, and all animals were housed in normal cages with free access to water and the indicated foods. Irradiated (900 Gy [0.9 Mrad]) vitamin K–deficient food was from Hope Farms, Woerden, The Netherlands. Vitamins K1 and K2 were dissolved in corn oil prior to adding to the vitamin K–deficient food in the required amounts. Warfarin was added directly to the food. All experimental protocols were approved by the Experimental Animal Ethics Committee of the Maastricht University.
To induce vascular calcification, rats (n = 30) received a diet containing warfarin (3 mg/g food) and vitamin K1 (1.5 mg/g food; the minimal dose required for rats is 0.5 μg/g food), according to the method described earlier by our group.25 These animals are designated as the W&K group. Control rats (n = 18) received no warfarin and a normal dose of vitamin K1 (5 μg/g food; this is equivalent to the vitamin K amount in normal standard rat food). From the control group, 6 rats where killed at the start of the experiment to measure the baseline calcium content of the abdominal aorta and left carotid artery. After 6 weeks of treatment, 6 control rats and 6 W&K rats were killed to monitor the effect of treatment. The remaining rats in the W&K group (n = 24) were subdivided into 4 groups of 6 rats for another 6-week treatment. One group continued the W&K diet, whereas warfarin was discontinued in the remaining 3 groups: one group received normal vitamin K1 (5 μg/g food), one group received high vitamin K1 (100 μg/g food; the dietary vitamin K requirements for rats are 0.5 μg/g food to maintain normal blood clotting), and the last group received high vitamin K2 (menaquinone-4, 100 μg/g food). In addition, the remaining 6 control rats continued their diet for another 6 weeks (see Figure 1 for schematic overview).
Antibodies and chemicals
Monoclonal and polyclonal antibodies against various epitopes of MGP were raised according to standard procedures. The following MGP-derived peptides were used for immunization: amino acid residues 61 to 79 (tMGP), residues 35 to 53 (4 Gla residues; cMGP), and residues 35 to 53 (4 Glu-residues; ucMGP). The corresponding antibodies are designated as poAb–anti-tMGP (recognizing all forms of MGP), moAb–anti-cMGP (recognizing carboxylated MGP; cMGP), and poAb–anti-ucMGP (recognizing no-carboxylated MGP; ucMGP), respectively. Vitamin K1 and warfarin were purchased from Sigma (St Louis, MO); vitamin K2 (menaquinone-4) was a kind gift from Eisai (Tokyo, Japan). All chemicals were of analytical grade or better.
Experimental procedures
Rats were anesthetized with sodium pentobarbital. Blood was collected in 105 mM trisodium citrate either by tail vein puncture or from the portal vein (at the end of the experiment), and plasma aliquots were frozen at −80°C. Before collecting all required tissues, the vasculature was perfused with a sterile vasodilating saline solution (150 mM saline, 100 pM sodium nitroprusside) via the portal vein. The aortic arch, thoracic and abdominal aortas, and right and left carotid arteries were dissected and transferred to a physiologic salt solution in a silicon-coated Petri dish, and adipose and connective tissues were carefully removed. The abdominal aorta and left carotid artery were frozen in liquid nitrogen for assessment of the calcium content. The aortic arch and thoracic aorta were fixed in 1% (vol/vol) HEPES-buffered formaldehyde overnight at 4°C for immunohistochemistry. The right carotid artery was used for monitoring the distensibility and compliance.
Biochemical and immunohistochemical measurements
Tissue calcium was determined after lyophilization and expressed per gram of dry weight, the freeze-dried tissues were extracted with a 10-fold excess (wt/vol) of 10% formic acid (overnight at 4°C), and calcium concentrations were measured using atomic absorption spectrometry (Department of Clinical Chemistry, University Hospital Maastricht, The Netherlands). Immunohistochemistry was performed after embedding the tissues in paraffin and subsequent sectioning (4 μm thick). Each seventh section was used for calcium detection by Von Kossa staining. Each subsequent section was stained for hematoxylin/eosin, macrophages (mouse anti-rat CD68; Serotec, Oxford, United Kingdom), apoptosis using ApopTag apoptosis detection kit (Chemicon, Temecula, CA), poAb-tMGP (5 μg), moAb-cMGP (1 μg), and poAb-ucMGP (1 μg), respectively. Immunostaining was performed using either biotinylated sheep anti–mouse IgG (Amersham Biosciences, Little Chalfont, United Kingdom) or biotinylated swine anti–rabbit IgG (Dako, Golstrup, Denmark) as a second antibody (60 minutes at RT), followed by incubation with avidin-linked alkaline phosphatase complex (30 minutes at RT; Dako); staining was performed by the alkaline phosphatase kit I (staining 5 minutes at RT; Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin and mounted with coverslips. Each antibody staining was performed in one batch.
The relative extent of MGP staining and apoptosis was measured using a microscope coupled to a computerized morphometry system (quantimed 57; Leica, Wageningen, The Netherlands). Three sections (28 μm apart) were used for morphometric analysis, and quantification was expressed as percentage staining of the total arterial medial area. To reliably compare the different antibodies (anti-tMGP, anti-cMGP, and anti-ucMGP) both microscope and camera adjustments were kept constant.26,30
Vitamin K and KO determination
Concentrations of vitamin K1, K1-O, MK4, and MK4-O were analyzed by high-performance liquid chromatography (HPLC) as described previously.25 Briefly, part of the aorta was weighed and homogenized in ethanol/water (vol/vol) at a ratio of 1:2 using a blender (Ultra Turrax; Janke and Kunkel, Staufen, Germany). Samples were supplemented with 100 ng vitamin K125 (GLSynthesis, Worcester, MA) as an internal standard, extracted with 4 volumes of n-hexane, and prepurified on silica columns as described previously.32 Quantification of vitamin K was performed by HPLC with fluorescence detection (excitation at 244 nm, emission at 430 nm) after postcolumn reduction on a 10 × 0.2-cm column filled with zinc powder (Riedel-DeHaën, Seelze, Germany) at 40°C. The mobile phase consisted of ethanol/acetonitrile/reduction buffer/water at a ratio (vol/vol) of 360:90:4:3 and was degassed continuously with helium. Reduction buffer contained 1 M ZnCl2, 1 M NaOAc, and 1 M AcOH in MeOH.
Arterial distensibility
The right carotid artery from all animals (t = 12-week point) was used to determine arterial distensibility as described.33 Artery segments (3-4 mm) were mounted in an arteriograph (Living System Instrumentation, Burlington, VT) in which the arterial diameter could be continuously monitored. Both ends of the vessels were cannulated on 120- to 150-μm wide glass micropipettes and tied with two 17-μm thin nylon threads. Arterial segments were bathed in a 10-mL organ chamber filled with calcium-free physiologic salt solution (composition in mmol/L: NaCl 144, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, HEPES 14.9, and glucose 5.5, pH 7.4) which was maintained at 37°C and gassed with 95% O2 and 5% CO2. After administration of 10 μM Na-nitroprusside to assure maximal vasodilatation, intra-arterial pressure was gradually increased from 10 to 200 mm Hg. Arterial distensibility or the relative change in arterial lumen volume induced by a given increase in pressure, was estimated by: DC = ΔA/An-1 × ΔP (DC indicates distensibility; A, area; P, pressure).34
Statistical analysis
Values are expressed as mean ± SD. The difference between 2 groups was determined by Wilcoxson ranked nonpaired test. Differences for multiple comparisons were determined by ANOVA with Bonferroni correction. Differences were considered to be significant at P less than .05.
Results
Arterial calcification
The plasma calcium concentration was not affected by the different regimens and ranged between 2.25 and 2.33 mM (mean ± SD: 2.28 ± 0.03) (see Table 1). During the first 6 weeks of W&K treatment, all 6 rats analyzed in this group displayed a significantly increased aortic calcium content (mean ± SD in μg/mg dry tissue: 0.24 ± 0.02 versus 1.62 ± 0.36; P < .001). As is shown in Table 1, aortic calcification further increased when the W&K treatment was continued from week 7 to 12. Remarkably, calcification continued at a comparable rate in animals receiving a normal dose of K1 during this period. In contrast, high–vitamin K intake (both K1 and K2) not only blocked the progress of further calcium accumulation but also lead to a greater than 37% reduction of previously accumulated arterial calcium precipitates within 6 weeks (53% as compared with the 12-week W&K time point). A similar observation was made in the left carotid artery (reduction of 44% compared with the 6-week time point W&K), and there was a good correlation between the calcium content of the abdominal aorta and that of the left carotid artery in the various groups (r2 = 0.85, P < .001). In the thoracic aorta, calcification was visualized by Von Kossa staining (Figures 2, 3, and 4). In the control rats (at 0, 6, and 12 weeks) no calcification was detected; however, extensive calcifications were found in all animals receiving the W&K diet (both at 6 and at 12 weeks). Also all rats treated with normal K1 during weeks 7 to 12 displayed extensive calcifications. In rats treated with high K1 1 rat did not have visible calcium precipitates, whereas 5 animals had decreased but still visible calcifications. In the K2 group, calcium precipitates were absent in 2 rats, whereas in 4 rats remaining calcifications were observed.
Arterial calcium contents after various treatments
| Diet . | Calcium . | Total MGP . | ||
|---|---|---|---|---|
| Aorta (μg/g) . | Carotid artery (μg/g) . | Plasma (mmol/L) . | Plasma (nmol/L) . | |
| Week 0 | ||||
| Standard chow | 0.24 ± 0.02 | 0.36 ± 0.11 | 2.29 ± 0.04 | 6.91 ± 1.21 |
| Week 6 | ||||
| Standard chow | 0.25 ± 0.02 | 0.34 ± 0.09 | 2.29 ± 0.05 | 6.40 ± 1.44 |
| W&K | 1.62 ± 0.36* | 10.58 ± 6.00* | 2.28 ± 0.05 | 1.83 ± 0.33* |
| Week 12 | ||||
| Standard chow | 0.28 ± 0.03 | 0.39 ± 0.08 | 2.29 ± 0.03 | 6.79 ± 1.51 |
| W&K | 2.40 ± 0.46* | 13.01 ± 3.08* | 2.30 ± 0.04 | 1.65 ± 0.79* |
| Normal K1 | 2.31 ± 0.57* | 13.39 ± 2.89* | 2.27 ± 0.02 | 1.93 ± 0.55* |
| High K1 | 1.02 ± 0.33*† | 5.91 ± 4.42*† | 2.28 ± 0.03 | 2.99 ± 0.48*† |
| High K2 | 1.12 ± 0.44*† | 7.58 ± 3.27*† | 2.27 ± 0.03 | 2.63 ± 0.42*† |
| Diet . | Calcium . | Total MGP . | ||
|---|---|---|---|---|
| Aorta (μg/g) . | Carotid artery (μg/g) . | Plasma (mmol/L) . | Plasma (nmol/L) . | |
| Week 0 | ||||
| Standard chow | 0.24 ± 0.02 | 0.36 ± 0.11 | 2.29 ± 0.04 | 6.91 ± 1.21 |
| Week 6 | ||||
| Standard chow | 0.25 ± 0.02 | 0.34 ± 0.09 | 2.29 ± 0.05 | 6.40 ± 1.44 |
| W&K | 1.62 ± 0.36* | 10.58 ± 6.00* | 2.28 ± 0.05 | 1.83 ± 0.33* |
| Week 12 | ||||
| Standard chow | 0.28 ± 0.03 | 0.39 ± 0.08 | 2.29 ± 0.03 | 6.79 ± 1.51 |
| W&K | 2.40 ± 0.46* | 13.01 ± 3.08* | 2.30 ± 0.04 | 1.65 ± 0.79* |
| Normal K1 | 2.31 ± 0.57* | 13.39 ± 2.89* | 2.27 ± 0.02 | 1.93 ± 0.55* |
| High K1 | 1.02 ± 0.33*† | 5.91 ± 4.42*† | 2.28 ± 0.03 | 2.99 ± 0.48*† |
| High K2 | 1.12 ± 0.44*† | 7.58 ± 3.27*† | 2.27 ± 0.03 | 2.63 ± 0.42*† |
Calcium was measured in the abdominal aorta, in the left carotid artery, and in plasma. MGP was measured in plasma using the Biomedica assay. All values are expressed as mean ± SD per group of 6 animals.
Significant differences (P < .01) compared with controls (compared with the same time point).
Significant differences (P < .01) compared with the W&K diet.
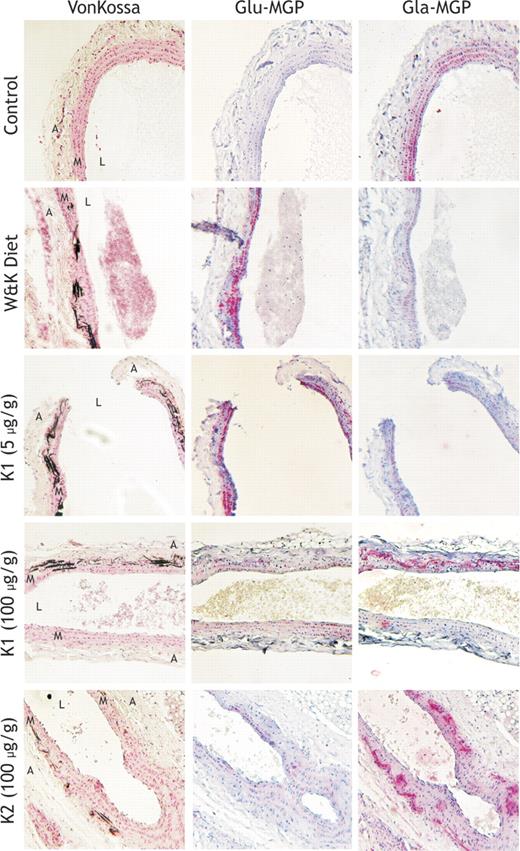
Effect of different dietary treatments on aortic calcification at the 12-week time point in male Wistar Kyoto rats. Rats (n = 6 per group) were treated with the following dietary regimens; row 1 represents 12 weeks of normal vitamin K diet (5 μg/g K1), row 2 represents 12 weeks of the W&K diet (3 mg/g warfarin and 1.5 mg/g vitamin K1), row 3 represents 6 weeks of normal vitamin K (5 μg/g K1) after 6 weeks of W&K, row 4 represents 6 weeks of high–vitamin K1 (100 μg/g) diet after 6 weeks of W&K, and row 5 represents 6 weeks of the high–vitamin K2 (100 μg/g) diet after 6 weeks of W&K. The thoracic aorta segment (between the aortic arch and the renal branch) was removed immediately after killing the animals in each diet group and fixed in 1% buffered formalin. Subsequently, longitudinal sections of each aorta were stained for mineral by von Kossa stain (column 1), ucMGP (column 2), and cMGP (column 3). Red stain indicates MGP, black stain indicates calcium, and blue indicates cell nuclei. Magnification, × 100. Acquisition was performed using an Axioskop 40 microscope (Carl Zeiss, Sliedrecht, The Netherlands) equipped with Achroplan 10×/0.25 objective lens and using an E-PI 10×/20 aperture. Pictures were taken using a Canon Powershot G5 (Canon, Hoofddorp, The Netherlands) and corrected for contrast and brightness using Adobe Photoshop CS2 for Windows (Adobe Systems, San Jose, CA).
Effect of different dietary treatments on aortic calcification at the 12-week time point in male Wistar Kyoto rats. Rats (n = 6 per group) were treated with the following dietary regimens; row 1 represents 12 weeks of normal vitamin K diet (5 μg/g K1), row 2 represents 12 weeks of the W&K diet (3 mg/g warfarin and 1.5 mg/g vitamin K1), row 3 represents 6 weeks of normal vitamin K (5 μg/g K1) after 6 weeks of W&K, row 4 represents 6 weeks of high–vitamin K1 (100 μg/g) diet after 6 weeks of W&K, and row 5 represents 6 weeks of the high–vitamin K2 (100 μg/g) diet after 6 weeks of W&K. The thoracic aorta segment (between the aortic arch and the renal branch) was removed immediately after killing the animals in each diet group and fixed in 1% buffered formalin. Subsequently, longitudinal sections of each aorta were stained for mineral by von Kossa stain (column 1), ucMGP (column 2), and cMGP (column 3). Red stain indicates MGP, black stain indicates calcium, and blue indicates cell nuclei. Magnification, × 100. Acquisition was performed using an Axioskop 40 microscope (Carl Zeiss, Sliedrecht, The Netherlands) equipped with Achroplan 10×/0.25 objective lens and using an E-PI 10×/20 aperture. Pictures were taken using a Canon Powershot G5 (Canon, Hoofddorp, The Netherlands) and corrected for contrast and brightness using Adobe Photoshop CS2 for Windows (Adobe Systems, San Jose, CA).
Effect of W&K treatment (3 mg/g warfarin and 1.5 mg/g vitamin K1) on the presence of calcification and MGP at the 12-week time point. Sections were prepared as described in the legend of Figure 2. Longitudinal sections of each thoracic aorta were stained with von Kossa (A) and immunohistochemically with anti–t-MGP (B), anti-ucMGP (C), and anti-cMGP (D) (see “Materials and methods” for details). It is clearly demonstrated that because of the W&K diet arterial calcification was significantly present. In panel B total MGP is up-regulated in the calcified area. From panel D it can be seen that cMGP is almost absent, whereas significant amounts of the inactive ucMGP are present around the calcified area (C). Arrow indicates same area. Red stain indicates MGP, black stain indicates calcium, and blue indicates cell nuclei. Magnification, × 400. A indicates adventitia; M, media; and L, lumen. Details of image acquisition are provided in the legend of Figure 2.
Effect of W&K treatment (3 mg/g warfarin and 1.5 mg/g vitamin K1) on the presence of calcification and MGP at the 12-week time point. Sections were prepared as described in the legend of Figure 2. Longitudinal sections of each thoracic aorta were stained with von Kossa (A) and immunohistochemically with anti–t-MGP (B), anti-ucMGP (C), and anti-cMGP (D) (see “Materials and methods” for details). It is clearly demonstrated that because of the W&K diet arterial calcification was significantly present. In panel B total MGP is up-regulated in the calcified area. From panel D it can be seen that cMGP is almost absent, whereas significant amounts of the inactive ucMGP are present around the calcified area (C). Arrow indicates same area. Red stain indicates MGP, black stain indicates calcium, and blue indicates cell nuclei. Magnification, × 400. A indicates adventitia; M, media; and L, lumen. Details of image acquisition are provided in the legend of Figure 2.
Effect of the high-K2 treatment (100 μg/g vitamin K2) on the presence of calcification and MGP at the 12-week time point. Sections were prepared as described in the legend of Figure 2. Longitudinal sections of each thoracic aorta were stained with von Kossa (A) and immunohistochemically with anti–t-MGP (B), anti-ucMGP (C), and anti-cMGP (D) (see “Materials and methods” for details). It is shown that because of the high-K2 diet cMGP (D) is up-regulated in the calcified area (A; along the elastic fibers). From panel C it can be seen that ucMGP is almost absent. Arrow indicates same area. Red stain indicates MGP, black stain indicates calcium, and blue indicates cell nuclei. Magnification, × 400. A indicates adventitia; M, media; and L, lumen. Details of image acquisition are provided in the legend of Figure 2.
Effect of the high-K2 treatment (100 μg/g vitamin K2) on the presence of calcification and MGP at the 12-week time point. Sections were prepared as described in the legend of Figure 2. Longitudinal sections of each thoracic aorta were stained with von Kossa (A) and immunohistochemically with anti–t-MGP (B), anti-ucMGP (C), and anti-cMGP (D) (see “Materials and methods” for details). It is shown that because of the high-K2 diet cMGP (D) is up-regulated in the calcified area (A; along the elastic fibers). From panel C it can be seen that ucMGP is almost absent. Arrow indicates same area. Red stain indicates MGP, black stain indicates calcium, and blue indicates cell nuclei. Magnification, × 400. A indicates adventitia; M, media; and L, lumen. Details of image acquisition are provided in the legend of Figure 2.
MGP measurements
To investigate matrix Gla protein in relation to calcification of vascular tissue we used conformation-specific antibodies against MGP. With poAb anti-tMGP, we identified MGP in the arteries from control animals, where it was primarily associated with the elastin fibers (data not shown). Subsequent staining with conformation-specific antibodies revealed that virtually all of this MGP consisted of carboxylated (ie, active) MGP (Figure 2). Much higher total MGP concentrations were found, however, in arteries from the W&K group, where it colocalized with the calcium deposits (Figures 2–3). Also in the arteries from animals treated with low and high vitamin K the total MGP was elevated compared with the controls (Figure 2). Staining with conformation-specific antibodies revealed that in animals from both the W&K group (Figures 2–3) and the normal-dose vitamin K group (Figure 2) most of the MGP occurred in the noncarboxylated (ie, inactive) form, whereas in rats subjected to high–vitamin K treatment (both K1 and K2) the MGP deposits were mostly present in the carboxylated (ie, active) form (Figures 2, 4). Moreover, mainly the noncarboxylated form of MGP colocalized with vascular calcifications. The above-described immunohistochemical staining of MGP was quantified (percentage staining of MGP/total arterial medial layer) and plotted in Figure 5.
Quantification of MGP at the 12-week time point. Three sections, each 28 μm apart, were measured using a microscope coupled to a computerized morphometry system (for details see “Materials and methods”). Quantification was expressed as the percentage staining of the total arterial medial area. Diets represent 12-week control, W&K (3 mg/g warfarin and 1.5 mg/g vitamin K1), normal vitamin K diet (5 μg/g K1), high vitamin K1 (100 μg/g K1), and high vitamin K2 (100 μg/g K2) after 6 weeks of W&K. ▪ represent total MGP, ⊡ represent cMGP, and □ represent ucMGP. Quantification was performed by 2 independent persons. Bars represent mean values ± SEM (n = 6).
Quantification of MGP at the 12-week time point. Three sections, each 28 μm apart, were measured using a microscope coupled to a computerized morphometry system (for details see “Materials and methods”). Quantification was expressed as the percentage staining of the total arterial medial area. Diets represent 12-week control, W&K (3 mg/g warfarin and 1.5 mg/g vitamin K1), normal vitamin K diet (5 μg/g K1), high vitamin K1 (100 μg/g K1), and high vitamin K2 (100 μg/g K2) after 6 weeks of W&K. ▪ represent total MGP, ⊡ represent cMGP, and □ represent ucMGP. Quantification was performed by 2 independent persons. Bars represent mean values ± SEM (n = 6).
Measurement of total MGP in plasma revealed a 4-fold decrease after 6 weeks of warfarin treatment (from 6.9 ± 1.2 to 1.8 ± 0.3 nmol/L) as compared with the control rats. After another 6 weeks of the warfarin diet or normal vitamin K1 diet, MGP levels had not changed from the values at 6 weeks in the W&K and normal K1 groups. However, a significant rise in plasma MGP was noticed after the subsequent high–vitamin K treatment (Table 1). Staining for macrophages revealed that these were absent in the vascular media layer (Figure 6E-F). However, measuring the rate of apoptosis using TUNEL staining (Figure 6A-D) demonstrated that in the W&K-treated animals significantly more (11%) apoptotic vascular smooth muscle cells were present than in those receiving the control diet (< 1%). During high–vitamin K treatment the rate of apoptosis was significantly lower (4% lower in the K1 group and 3% in the K2 group) than during W&K (P < .02) and normal vitamin K1 treatment (7%; P < .05).
Effect of different dietary treatments on apoptotic activity at the 12-week time point. Sections were prepared as described in the legend of Figure 2. Longitudinal sections of each thoracic aorta were stained by immunohistochemical techniques with an antimacrophage antibody and with a terminal dUTP nick-end labeling (TUNEL) staining for apoptosis (see “Materials and methods” for details). Panel A represents 12 weeks of normal vitamin K diet (5 μg/g K1), panels B and E represent 12 weeks of W&K diet (3 mg/g warfarin and 1.5 mg/g vitamin K1), panel C represents normal K (5 μg/g) after 6 weeks of W&K, and panels D and F represent 6 weeks of high vitamin K (100 μg/g K1 or K2) after 6 weeks of W&K. Macrophages are absent in the medial layer of aortic tissue, both in rats treated with W&K (E) and high vitamin K (F). Also, in control animals macrophages were absent (data not shown). In panel E a positive control is shown as an inset (arrow indicates macrophage). The W&K-treated animals (B), however, have significantly increased apoptosis of vascular smooth muscle cells (VSMCs) compared with control animals (A), in which apoptosis is hardly measurable. Also in the normal K-treated animals, after 6 weeks of W&K, (C) apoptosis is clearly visible. The rats treated with high vitamin K (D) showed significantly reduced apoptosis as compared with the normal-treated animals (C). See “Results” for details. Magnification, × 400. Details of image acquisition are provided in the legend of Figure 2.
Effect of different dietary treatments on apoptotic activity at the 12-week time point. Sections were prepared as described in the legend of Figure 2. Longitudinal sections of each thoracic aorta were stained by immunohistochemical techniques with an antimacrophage antibody and with a terminal dUTP nick-end labeling (TUNEL) staining for apoptosis (see “Materials and methods” for details). Panel A represents 12 weeks of normal vitamin K diet (5 μg/g K1), panels B and E represent 12 weeks of W&K diet (3 mg/g warfarin and 1.5 mg/g vitamin K1), panel C represents normal K (5 μg/g) after 6 weeks of W&K, and panels D and F represent 6 weeks of high vitamin K (100 μg/g K1 or K2) after 6 weeks of W&K. Macrophages are absent in the medial layer of aortic tissue, both in rats treated with W&K (E) and high vitamin K (F). Also, in control animals macrophages were absent (data not shown). In panel E a positive control is shown as an inset (arrow indicates macrophage). The W&K-treated animals (B), however, have significantly increased apoptosis of vascular smooth muscle cells (VSMCs) compared with control animals (A), in which apoptosis is hardly measurable. Also in the normal K-treated animals, after 6 weeks of W&K, (C) apoptosis is clearly visible. The rats treated with high vitamin K (D) showed significantly reduced apoptosis as compared with the normal-treated animals (C). See “Results” for details. Magnification, × 400. Details of image acquisition are provided in the legend of Figure 2.
Vitamin K measurements
To investigate whether both forms of vitamin K are transported equally well to the arteries, we have measured the arterial vitamin K content following the different food regimens. Control rats had accumulated both K1 and K2 in their arteries (Table 2); although these animals had received only K1, the tissue concentrations of K2 were 2 times higher than those of K1. During the W&K diet, the animals received high doses of K1, and consequently high levels of both K1 and K1O had accumulated in the arteries (Table 2). In this case no K2 species were found because warfarin blocks the conversion from K1 to K2.35-37 It is remarkable that, although K1 was used (as measured by K1O) substantial tissue calcification was observed in this group (see Figure 2). After stopping the warfarin treatment, animals receiving the normal dose of K1 showed vascular K1 and K2 levels that were in the range of control rats. No vitamin K epoxides were found in this group. In animals receiving a high dose of K1 the arterial concentrations of both K1 and K2 were approximately 8-fold higher (Table 2) than after normal-dose K1 treatment, and trace amounts of the respective epoxides were found. Rats receiving the high-K2 diet had accumulated exclusively K2 and trace amounts of K2O (Table 2).
Vitamin K concentrations in the rat aortas at time point of 12 weeks
| Diet . | Vitamin K . | |||
|---|---|---|---|---|
| K1 (ng/g) . | K1O (ng/g) . | K2 (MK4) (ng/g) . | K2O (MK4) (ng/g) . | |
| Control | 8.5 ± 1.9 | ND | 16.2 ± 4.1 | ND |
| W&K | 531 ± 196 | 967 ± 419 | ND | ND |
| K1 (5 μg/g) | 13.2 ± 21.1 | ND | 24.4 ± 29.3 | ND |
| K1 (100 μg/g) | 69 ± 84 | 2.3 ± 1.9 | 183 ± 79 | 6.5 ± 2.6 |
| K2 (100 μg/g) | ND | ND | 248 ± 112 | 5.2 ± 4.8 |
| Diet . | Vitamin K . | |||
|---|---|---|---|---|
| K1 (ng/g) . | K1O (ng/g) . | K2 (MK4) (ng/g) . | K2O (MK4) (ng/g) . | |
| Control | 8.5 ± 1.9 | ND | 16.2 ± 4.1 | ND |
| W&K | 531 ± 196 | 967 ± 419 | ND | ND |
| K1 (5 μg/g) | 13.2 ± 21.1 | ND | 24.4 ± 29.3 | ND |
| K1 (100 μg/g) | 69 ± 84 | 2.3 ± 1.9 | 183 ± 79 | 6.5 ± 2.6 |
| K2 (100 μg/g) | ND | ND | 248 ± 112 | 5.2 ± 4.8 |
All values are expressed as mean ± SD per group of 6 animals. Detection limit for K1 and K2 (MK4) is 0.05 ng/g.
ND means not detectable.
Mechanical properties of isolated carotid arteries
Figure 7 summarizes the pressure-diameter relations of isolated carotid arteries during maximal vasodilatation (4 groups from the 7- to 12-week experiment). The minimal arterial diameter at low distending pressure (10 mm Hg) was significantly larger in the W&K group when compared with control rats and especially in comparison to the rats treated with either the high-K1 diet or the high-K2 diet. Furthermore, within a physiologic pressure range (100-140 mm Hg) the arterial distensibility was significantly smaller in the W&K group than in the control and high–vitamin K groups. At 100 mm Hg, the distensibility averaged 8.5 ± 0.5, 3.6 ± 0.6, 7.5 ± 0.6, and 10.0 ± 0.7 × 10−3 mm Hg−1 for control, W&K, high K1, and high K2, respectively (significance P < .05 compared with W&K).The maximal diameter at high distending pressure (200 mm Hg) did not differ significantly between the experimental groups (1391 ± 21, 1381 ± 19, 1377 ± 14, and 1351 ±19 μm for control, W&K, high K1, and high K2, respectively). Collectively these findings indicate that the W&K treatment increased the arterial stiffness of the arteries and that this was reversed by both the high-K1 and -K2 intake.
Effects of vitamin K status on the mechanical properties of isolated rat carotid arteries. The pressure-diameter relation was monitored for animals receiving the control diet (n = 6; ▵), those after 12 weeks of the W&K diet (n = 6; ○), and animals after 6 weeks of W&K and subsequently 6 weeks of high vitamin K (both K1 (□) and K2 (▪; n = 6 per group). The arterial diameter is shown as a function of increasing pressure. Data are shown as mean ± SD.
Effects of vitamin K status on the mechanical properties of isolated rat carotid arteries. The pressure-diameter relation was monitored for animals receiving the control diet (n = 6; ▵), those after 12 weeks of the W&K diet (n = 6; ○), and animals after 6 weeks of W&K and subsequently 6 weeks of high vitamin K (both K1 (□) and K2 (▪; n = 6 per group). The arterial diameter is shown as a function of increasing pressure. Data are shown as mean ± SD.
Discussion
In this study we provide evidence that warfarin-induced medial vascular calcification in rats is preventable or even reversible by high–vitamin K intake, with a putative role for the vitamin K–dependent protein MGP. Although it is well known that MGP is important in the prevention of calcification,14,28 its contribution to regression of arterial calcification is a novel finding.
Traditionally, vascular calcification has been thought to be a passive end process and, once it was present, an irreversible feature. Now it is known that both intimal and medial calcification is an active process with inhibitors and stimulators of calcification. Thus far, research has mainly focused on the prevention or retardation of arterial calcification using lipid-lowering drugs such as statins or bisphosphonates,38-42 but all failed in regressing existing arterial calcification. Recently, however, it was shown that medial elastocalcinosis can be reversed,43,44 suggesting that calcium resorption, like its deposition, is an actively regulated process.
It has been shown previously that arterial calcification can be induced with the warfarin-containing diet.20,25 Here, we addressed the question of whether progression of further calcification could be stopped and whether existing mineral deposits could be diminished by a high–vitamin K diet in rats. Our experiments indicate that in healthy, nontreated animals a relatively low vitamin K intake is sufficient for complete MGP carboxylation and for preventing arterial calcification (Figure 2), whereas warfarin-initiated calcium accumulation in the arteries increased accumulation of MGP in these areas. This can be explained by assuming a feedback mechanism by which increased local calcium stimulates MGP expression in an attempt to prevent calcification. It has been shown in cell cultures45 and vascular tissue20 that warfarin up-regulates the mRNA expression of MGP. However, under the conditions used in our experiment, this increase in MGP did not prevent calcium deposition in the vascular tissue. Using conformation-specific antibodies, we demonstrated that during warfarin treatment most of the MGP had been synthesized as undercarboxylated, inactive species. The fact that the serum MGP concentration declined to approximately 20% of normal is in agreement with observations by Price et al20,46 and suggests a different balance between tissue MGP and circulating MGP levels.
Continuation of the warfarin-containing diet between weeks and 12 led to an almost linear increase of the arterial calcium content. An unexpected finding was that calcium accumulation also continued in the normal vitamin K group even after warfarin treatment had been stopped. We speculate that the calcium salt precipitates (as identified by Von Kossa staining) induce a high MGP expression level, causing a high local vitamin K requirement which is not met by the normal vitamin K diet. This can be seen in Figures 2 and 3 in which the majority of the newly synthesized MGP is produced in the undercarboxylated, inactive form. This is in agreement with work from Sweatt et al13 who showed that in aging rats ucMGP was associated with arterial calcification as demonstrated with polyclonal conformation-specific MGP antibodies. The researchers concluded that inactive MGP because of vitamin K deficiency could lead to arterial calcifications.
Arterial calcification could, however, be reversed by high–vitamin K intake. After a 6-week period (weeks 7-12) some 40% of the preformed calcium salts had been removed. In an attempt to find the mechanism underlying this observation, we monitored the presence of macrophages. It is known that bone is resorbed by osteoclastic activity and that in the vessel wall also macrophages can clear hydroxyapatite by phagocytosis.44,47 Staining for macrophages revealed that the arterial media areas of rats in all groups were free from macrophage infiltration (Figure 6E-F), which can thus be excluded as a possible mechanism of calcium removal in this experiment. Staining for apoptosis, however, demonstrated that during the warfarin treatment apoptosis was up-regulated (Figure 6A-D). It has been shown that apoptosis precedes calcification,48 and this seems to be the likely mechanism for calcium salt deposition in the W&K animals. VSMC–derived apoptotic vesicles are loaded with calcification inhibitors, including MGP, and these vesicles have promineralizing properties when MGP function is impaired.49 Here, we demonstrate that high–vitamin K intake is associated with significantly less VSMC apoptosis and with significant regression of arterial calcification. It has been shown that another vitamin K–dependent protein synthesized by VSMCs, growth arrest specific gene-6 protein (gas6), is involved in the survival of VSMCs50 and in the clearance of apoptotic bodies from the vasculature.51 Also gas6 requires Gla residues and hence vitamin K for its activity.52 On the basis of our data we conclude that vitamin K and the vitamin K–dependent protein MGP are involved in the observed regression of arterial calcification. However, we cannot conclude whether and to which extent other vitamin K–dependent proteins such as gas6 are involved in the observed regression of arterial calcification. Because macrophages were absent in the vascular media during high–vitamin K treatment, we postulate that the calcium deposits were removed by phagocytosis carried out by the surrounding VSMCs under conditions of maximal calcification inhibition provided by the high–vitamin K diet. This is consistent with a paper by Proudfoot et al53 which reported that phagocytosis is a normal property of VSMCs.
In addition, the regression of arterial calcification was accompanied by restoration of arterial distensibility to a similar level as in the control rats. The fact that K1 and K2 (MK4) had similar effects in this model seems to be in contradiction to previous data in which it was demonstrated that K2 (MK4) is more effective than K1 in preventing calcification during warfarin treatment.25 An explanation for this apparent discrepancy may be found in the fact that certain tissues (including the vessel wall) specifically accumulate K2, even when the diet contains exclusively K1.54 The conversion of K1 into MK4 is blocked, however, during warfarin treatment.35-37 In the experiments performed by Spronk et al25 as well as in our experiments, K2 (MK4) or K2-O (MK4-O) were nearly absent in arteries from rats treated with W&K. Also in rats fed the normal vitamin K1 diet, only small amounts of K2 (MK4) could be identified. In the high-K1 group, however, K1 had been converted to K2 to such an extent that in the high-K1 group arterial K2 had comparable tissue concentrations as in the K2 (MK4)–treated group. We conclude that at very high intakes of K1, (200-fold the daily requirement of the liver) both vitamers may help decrease arterial calcification.
The decreased MGP levels in the plasma of animals with substantial arterial calcifications are consistent with the outcomes of previous studies in rats.20 Also in humans it was reported that calcification is associated with decreased circulating MGP levels.55 Both carboxylated and noncarboxylated MGP have a high affinity for hydroxyapatite; hence, the most plausible explanation for this observation is that in the case of arterial mineralization most of the MGP produced is directly bound to the calcium salts and not set free in the circulation. After feeding rats either a high-K1 or a high-K2 (MK4) diet for 6 weeks, their plasma MGP levels had increased significantly. This may be related to the decreased vascular calcium content providing fewer matrixes for MGP binding but also to an increased transport of MGP that had bound to the dissolving matrix. Moreover, it has been reported that the transport of calcium from calcified tissue may occur via a fetuin-MGP-calcium phosphate complex, which has a much longer plasma half-life than free MGP.56 If complexed MGP is detected in our assay, the slow elimination of such complexes might contribute to the relatively high-MGP concentration in the serum of rats on a high–vitamin K diet.
We measured the arterial distensibility as a clinical parameter of vascular elasticity. The experiment shown in Figure 7 demonstrates that warfarin induced stiffening of the arterial vessel wall. This is consistent with work from Essalihi et al21 which showed that warfarin treatment resulted in increases of aortic pulse pressure, pulse pressure, and systolic blood pressure. In our model, normal vitamin K1 in the diet was not capable of affecting arterial distensibility, whereas during the high–vitamin K diet (both K1 and K2) the vascular properties that were lost by warfarin-induced calcification were restored.
The animal model we used mimics arterial media sclerosis (also known as Mönckeberg sclerosis). Media sclerosis is particularly common in diabetes mellitus, end-stage renal disease, and aging. Notably, patients with chronic kidney disease (CKD) are at high risk of cardiovascular disease.57 These patients often receive a high-calcium diet (to complex phosphate), vitamin D, and warfarin (to prevent thrombotic events). It was demonstrated, however, that each of these treatments is associated with an increased risk of arterial calcification.26,46,49,58 Given that arterial calcifications are predictive of cardiovascular events, regression of arterial calcification may help to reduce the risk of death in patients with CKD and coronary artery disease. Whether increased vitamin K intake could have such an effect in humans has to be investigated. Obviously, this is only possible in patients not receiving oral anticoagulant treatment.
Authorship
Contribution: L.J.S. designed the study, compiled and analyzed the data, and wrote the first manuscript draft; H.M.H.S. and B.A.M.S. performed research and analyzed data; P.M.S. performed research; J.G.R.D.M. analyzed data and contributed to the manuscript writing; C.V. designed the study and contributed to the manuscript writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leon J. Schurgers, VitaK—University of Maastricht, PO Box 616, 6200 MD Maastricht, The Netherlands; e-mail: l.schurgers@bioch.unimaas.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal