Abstract
Human herpesvirus 8 (HHV-8) is considered the causative agent of Kaposi sarcoma, a highly vascularized neoplasm characterized by spindle-shaped cells of endothelial origin and inflammatory cell infiltration. The cell transforming ability of HHV-8 has been associated with the activation of NF-κB, a nuclear factor playing a pivotal role in promoting inflammation and cell proliferation; however, little is known about NF-κB activation during acute HHV-8 infection. In the present study, we used a recently established in vitro model of HHV-8 acute productive infection in endothelial cells to investigate the effect of HHV-8 on NF-κB activity and function. HHV-8 rapidly and potently induced NF-κB activity in endothelial cells via stimulation of the IκB kinase (IKK). Following IKK activation, HHV-8 selectively triggered the production of high levels of monocyte chemoattractant protein 1 (MCP-1), whereas it did not affect the expression of other NF-κB–dependent proinflammatory proteins, including TNF-α, IL-8, and RANTES. Deletion of NF-κB–binding sites in the MCP-1 enhancer resulted in significant inhibition of HHV-8–induced transcription. Furthermore, MCP-1 production was accompanied by virus-induced capillary-like structure formation at early stages of infection. The results suggest that HHV-8–induced MCP-1 may play an important role in promoting inflammation and pathogenic angiogenesis typical of HHV-8–associated lesions.
Introduction
Human herpesvirus 8 (HHV-8), also known as Kaposi sarcoma-associated herpesvirus (KSHV), is a member of the Rhadinovirus genus in the γ-herpesvirus subfamily first detected in Kaposi sarcoma (KS),1 a neoplasm of endothelial origin. Since then, HHV-8 has been associated with a rare form of B-cell lymphoma known as primary effusion lymphoma (PEL)2 and with the multicentric Castleman disease, a B-cell lymphoproliferative disease,3 and has been shown to be the causative agent of KS.4
The lesions of KS are characterized by inflammation, neoangiogenesis, and the presence of characteristic spindle cells of endothelial origin. In vitro HHV-8 infection of endothelial cells causes dramatic changes in the cellular phenotype resembling the spindle shape of KS lesion cells. Following this observation, a number of studies have characterized the transforming ability of HHV-8, focusing either on the reactivation of latent virus or on the effect of isolated viral genes.4-6 On the other hand, the effects of acute HHV-8 infection on endothelial cell functions, induction of angiogenesis, and triggering of inflammatory processes are still largely unknown.
The cellular transcription factor, nuclear factor-κB (NF-κB), is a critical regulator of the immediate early pathogen response, playing an important role in promoting inflammation, in the control of cell proliferation and survival, and in the regulation of virus replication.7,8 NF-κB normally exists as an inactive cytoplasmic complex, whose predominant form is a heterodimer composed of p50 and p65 (Rel A) subunits, bound to inhibitory proteins of the IκB family (IκBs), and is induced in response to a variety of pathogenic stimuli, including UV radiation, exposure to proinflammatory cytokines or mitogens, and to bacterial or viral infection.9
Different stimuli for NF-κB activation initiate different signal transduction pathways most of which converge on the IκB kinase (IKK) signalosome that plays a major role in NF-κB activation.10 IKK is a multisubunit complex, containing 2 catalytic subunits (IKK-α and IKK-β), which are able to form homodimers or heterodimers, and the IKK-γ or NEMO regulatory subunit, which is not a kinase per se, but acts as a docking protein for IKK kinases or other signaling proteins.10 Following stimulation, the NF-κB/IκB complex is activated via the phosphorylation of the inhibitory protein. Phosphorylation targets IκBs for ubiquitination, which leads to degradation of the inhibitory subunit by the 26S proteasome. Following the degradation of the inhibitory protein and exposure of the nuclear localization signal, freed NF-κB dimers translocate to the nucleus and bind to DNA consensus sequences 5′-GGGACTTTCC-3′ (κB elements), activating the transcription of several target genes encoding, among others, cell adhesion molecules, inflammatory and chemotactic cytokines, cytokine receptors, and enzymes that produce inflammatory mediators.7 More recently, NF-κB activation has been connected with multiple aspects of oncogenesis, including the control of cell proliferation and differentiation, apoptosis, and cell migration.11 NF-κB can, in fact, promote cell proliferation either directly by up-regulating transcription of cyclin D and c-Myc, or via induction of different growth factors and stimulatory cytokines.8 NF-κB is also known to suppress cell death pathways by switching on genes that dampen proapoptotic signals.11 In addition, NF-κB has been shown to induce the expression of chemokines that promote cell migration and angiogenesis, such as IL-8 and vascular endothelial growth factor (VEGF), as well as of several matrix metalloproteinases (MMPs) that promote tumor invasion of surrounding tissue.11,12 Recently, it has been shown that NF-κB is constitutively activated in several types of neoplastic cells, and its activation has been associated with various hematologic malignancies, including PEL.12-14 Finally, functionally important NF-κB–binding sites have been located also in the genome of several viruses.9
It is well known that infection with different RNA and DNA viruses, including herpesviruses, can trigger NF-κB activation via different signaling mechanisms (for a review, see Santoro et al9 ). In the case of HHV-8 it has been shown that activation of NF-κB is required for HHV-8–associated cell transformation15,16 and is necessary for the production of replication-competent infectious progeny from reactivated virus.17 However, even though it has been shown that transfection with several isolated viral genes may induce NF-κB activation,18-23 the role of NF-κB during de novo acute HHV-8 infection in endothelial cells is still largely unknown.
We have recently described an in vitro model of HHV-8 acute productive infection that represents a useful tool to investigate the molecular mechanisms responsible for virus-induced alterations of endothelial cell functions.24 In the present report, this model was used to investigate the role of HHV-8 exogenous infection on NF-κB activity and function in human endothelial cells. We show that acute HHV-8 infection results in a rapid activation of NF-κB in endothelial cells. Activation is mediated by HHV-8–induced stimulation of IKK activity in infected cells. We then investigated the ability of HHV-8 to induce inflammatory processes during acute infection by analyzing the expression of a panel of NF-κB–dependent inflammatory and chemotactic cytokines at different times after infection. We surprisingly found that, among the different proinflammatory genes analyzed, HHV-8 selectively triggered the expression and secretion of high levels of monocyte chemoattractant protein 1 (MCP-1). We also found that this event is accompanied by virus-induced capillary-like structure formation at a very early stage of acute infection.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical vein, characterized as previously described25 and cultured in EGM-2 complete medium (BioWhittaker, Walkersville, MD), using collagen-coated flasks (Biocoat Collagen, BD Biosciences, Bedford, MA). All the experiments were performed on third to fifth passage HUVECs. The PEL-derived BC-3 cell line26 used for HHV-8 production was maintained in RPMI medium (Invitrogen-Gibco, Carlsbad, CA) supplemented with 15% inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen-Gibco).
HHV-8 infection
A cell-free HHV-8 inoculum was obtained by stimulation of BC-3 cells with 20 ng/mL 12-O-tetradecanoyl phorbol 13-acetate (TPA; Sigma-Aldrich, St Louis, MO) for 3 days, as previously described.24 Virus particles were purified by density centrifugation on Optiprep self-forming gradient (Sentinel, Milan, Italy), at 70 000g for 3.5 hours at 4°C. Prior to use, virus stock was treated with DNase-I and RNase-A to eliminate free viral nucleic acids possibly present in the preparation. Quantitation of virus genomes present in the stock preparation was obtained by real-time polymerase chain reaction (rtPCR), as previously described.27 HHV-8 DNA standard was obtained by serial dilutions of a plasmid (pCR-ORF26) containing a cloned fragment of HHV-8 DNA (ORF26, nucleotides 47 127 to 47 556). Human gDNA was quantified by amplification of the β-actin gene. The purified cell-free virus inoculum contained an average number of 4.7 × 105 copies of viral DNA/μL. Infective titers were determined by counting the number of cells positive for the HHV-8 K8.1 protein by immunofluorescence at 36 hours after infection. Inactivated virus was obtained by irradiating the purified inoculum with UV rays (200 mJ/cm2) for 30 minutes. The efficiency of UV inactivation was assessed on the basis of total absence of viral transcripts on infection of HUVECs.
For virus infection, HUVECs were seeded at a density of 2 × 105 cells/well in 6-well plates. After 24 hours cells were washed once with PBS and infected with 10 μL/mL/well of purified infectious or UV-inactivated virus preparation. After 3 hours of absorption at 37°C, the viral inoculum was removed and cells were washed twice with PBS and incubated in fresh medium. Cells and culture supernatants were harvested at specific time points for subsequent analysis. To analyze the infectivity of newly released virions, supernatants collected from infected cells at different times after infection were filtered through 0.5-μm pore membranes and used to infect target HUVEC cultures. Virus transmission was assessed by PCR and rtPCR analyses performed on total DNA and RNA extracted from target infected cells at 1, 3, and 7 days after infection.
PCR and RT-PCR
The presence and the level of transcription of HHV-8 in HUVECs were analyzed by PCR and reverse transcription PCR (RT-PCR) amplification of the following genes: ORF26, ORF50, ORF73 (LANA), and ORF74 (vGPCR). PCR amplification was performed using, respectively, 100 ng total DNA or 200 ng total RNA extracted from infected cells, as previously described.28 Amplification of the housekeeping β-actin gene was used as a control. Specific primers and PCR conditions were described previously.24 PCR was also used to detect newborn virus release in the supernatant of infected cells. In this case, the presence of viral DNA was determined by analyzing ORF50 levels after sample pretreatment with DNAse to exclude contamination by free unencapsidated viral DNA. The absence of the β-actin gene in the same samples excluded that positivity could be due to the presence of DNA released from lysed cells.
For analysis of transcription of NF-κB target genes, total RNA was extracted using RNA-Bee reagent (Invitrogen). RT-PCR was performed (1 μg RNA) according to the manufacturer's protocols (Invitrogen). The sequences of TNF-α, IL-8, RANTES, MCP-1, and β-actin primers (MWG-Biotech, Ebersberg, Germany) and amplification parameters were described previously.29,30 PCR products were electrophoresed alongside DNA Molecular Weight Marker IX (Roche, Basel, Switzerland) in 2% agarose gels and then stained with ethidium bromide.
MCP-1 mRNA transcription was also determined by quantitative rtPCR TaqMan assay. Total RNA was extracted using the TRIzol RNA isolation kit (Invitrogen) according to the manufacturer's instructions. After reverse-transcription using ImProm reverse transcriptase (Promega, Madison, WI), the relative amounts of MCP-1 and β-actin mRNAs were determined by rtPCR. Amplification was carried out in a 25-μL reaction mix containing 12.5 μL 2 × TaqMan universal PCR master mix (Applied Biosystems, Foster City, CA), and 1.25 μL primer and FAM dye-labeled probe (Assays-on-demand, Applied Biosystem). rtPCR assays were performed in triplicate on a 7500 rtPCR system (Applied Biosystems) with the following program: 40 cycles at 95°C for 20 seconds and at 60°C for 1 minute. Analysis of rtPCR data were performed with the 2-ΔΔCt method using the Relative Quantitation Study software (Applied Biosystems). Quantification of MCP-1 cDNA was normalized in each reaction according to the internal β-actin control. Levels of MCP-1 mRNA were expressed as fold difference of HHV-8–infected cells as compared to mock-infected cells (calibrator sample).
Immunofluorescence analysis
Uninfected or HHV-8–infected HUVECs grown onto collagenated culture slides were fixed with cold methanol/acetone (50:50) at different times after infection. After rehydration with PBS, slides were incubated with anti-K8.1 antibodies (ABI, Columbia, MD) diluted 1:100 in PBS, and then with fluorescein-conjugated anti–mouse IgG (Sigma-Aldrich) diluted 1:200. Fluorescence was detected using a Nikon Eclipse TE2000-S microscope (Nikon, Tokyo, Japan).
Electrophoretic mobility shift assay
Whole-cell extracts (8 μg protein/sample) of mock-infected or HHV-8–infected HUVECs were incubated with 32 P-labeled κB DNA probe,31 followed by analysis of DNA-binding activity by electrophoretic mobility shift assay (EMSA). Binding reactions were performed as described.32 Complexes were analyzed by nondenaturing 4% polyacrylamide gel electrophoresis. Specificity of protein-DNA complexes for NF-κB was verified by immunoreactivity with polyclonal antibodies specific for p65, as well as by competition reactions, incubating the cell extracts with 25-fold excess unlabeled oligonucleotide for 30 minutes at room temperature prior to the addition of the probe. Quantitative evaluation of NF-κB/κB complex formation was determined by Molecular Dynamics PhosphoImager (MDP) analysis (Typhoon 8600, Molecular Dynamics, Amersham Pharmacia Biotech, Piscataway, NJ).
Kinase assay
Whole-cell extracts obtained as described were immunoprecipitated with anti-IKKα antibodies (Cell Signaling Technology, Beverly, MA) in the presence of 15 μL protein A-Sepharose (Sigma-Aldrich) at 4°C for 12 hours. After extensive washing, endogenous IKK activity was determined using GST-IκBα (1-54) as a substrate, as described.33 Western blot analysis for IKK was performed as kinase loading control.
ELISA
For chemokine and cytokine analysis, supernatants from HHV-8–infected or mock-infected HUVECs were collected at different times after infection, cleared by centrifugation to eliminate cell debris, and stored at −80°C. After thawing, samples were analyzed for the presence of MCP-1, TNF-α, IL-8, and RANTES using specific quantitative enzyme-linked immunosorbent assay (ELISA) kits (Biosource International, Camarillo, CA). Each sample was tested in triplicate.
Cord formation on culture basement membrane extract
In vitro capillary-like structure formation assay was performed using an extracellular basement membrane extract (BME; Cultrex; Trevigen, Gaithersburg, MA), as described.34 HUVECs suspended in EGM-2 medium were seeded on solidified BME (5 × 104 cells/well) in the presence or absence of anti–MCP-1 antibodies (R&D Systems, Minneapolis, MN) and incubated at 37°C. Tube formation on BME was analyzed at different times after cell seeding by optical microscopy.
Plasmids, cell transfection, and reporter assays
The MCP-1 promoter-luciferase constructs containing the proximal promoter (between −107 and +60) and distal enhancer (between −2742 and −2513) of MCP-1 (pGLM-ENH), the proximal promoter and the distal enhancer in which one (pGLM-MA1, pGLM-MA2) or both (pGLM-MA1A2) NF-κB sites (A1 and A2) are mutated, or the proximal promoter only (pGLM-PRM; kindly provided by Dr T. Yoshimura, National Cancer Institute, Frederick, MD) have been previously described.35 To analyze the transcriptional activation of MCP-1, HUVECs were cotransfected by nucleofection with 2 μg of each of the MCP-1 promoter-luciferase constructs together with 1 μg pRL-SV40 vector (3705 bp; Promega) as an internal transcriptional control, using the Nucleofector method (Amaxa, Cologne, Germany) following the manufacturer's instructions. Efficiency of transfection determined in parallel samples by transfection with pmax-GFP plasmid (Amaxa) was found to be approximately 45%. Transfected cells were seeded on collagenated 24-well plates and then were either infected with HHV-8 (10 μL/mL) or treated with TPA (100 ng/mL). At 30 hours after infection. cells were lysed in lysis buffer (Dual-Glo Luciferase Assay System, Promega) and cell lysates were analyzed for luciferase levels using a Berthold Microplate Luminometer, according to the manufacturer's instructions (Promega). For NF-κB inhibition experiments, HUVECs were cotransfected by nucleofection with 2 μg of either the pGLM-PRM or the pGLM-ENH constructs together with 1 μg of the pRL-SV40 vector, in the presence or absence of 2 μg IκBα super-repressor (IκBα-AA) or “empty” vectors.29,32
Results
HHV-8 infection in endothelial cells
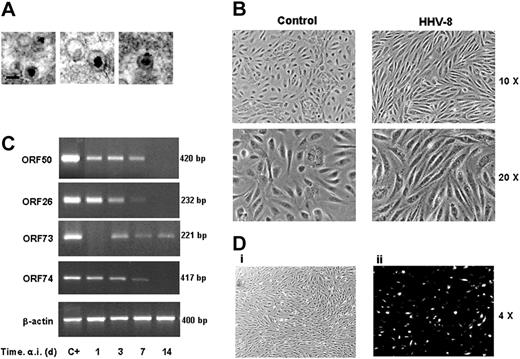
To study the effect of HHV-8 infection in endothelial cells, primary HUVECs were infected in vitro with a cell-free virus inoculum (3 × 103 infecting particles/μL) obtained by gradient purification of viral particles produced in TPA-induced BC-3 cells. Purity and integrity of viral particles was confirmed by electron microscopy (Figure 1A). HUVEC monolayers were infected with the purified viral suspension for 3 hours at 37°C. After the adsorption period, the virus inoculum was removed and fresh medium was added to the cells. As shown in Figure 1B, HHV-8 infection resulted in a marked modification of cell phenotype, visible 24 hours after infection. Infected HUVECs developed a characteristic spindle shape, markedly different from control uninfected or mock-infected cells.
Characterization of HHV-8 acute infection in HUVECs. (A) Electron micrographs of HHV-8 particles produced and purified as described in “Materials and methods” (original magnification, × 50 000; Hitachi H-800 electron microscope; Hitachi, High Technology Europe, Krefeld, Germany). (B) Subconfluent HUVEC monolayers were mock infected (control) or infected with HHV-8 (HHV-8). Microscopic observation of HHV-8 infected HUVECs was performed at 48 hours after infection (a.i.) by a Nikon Eclipse TE2000-S microscope equipped for phase-contrast observation (10×/0.25 and 20×/0.45 NA Fluor objective lenses), using a DS camera head DS-5M and a DS camera control unit DS-L; Nikon). (C) Results of RT-PCR amplification of the indicated HHV-8 genes using RNA extracted from infected cells at different days after infection. β-Actin levels were determined in the same samples as control. Positive controls of amplification of the genes analyzed (C+) are shown. (D) The presence of the HHV-8 late K8.1 protein at 36 hours after infection was detected in HHV-8–infected HUVECs grown onto collagenated culture slides by immunofluorescence analysis using a Nikon Eclipse TE2000-S microscope equipped for fluorescence observation. Immunofluorescence micrograph (ii) and the corresponding bright-field image (i) are shown at original magnification × 4 (pictures were taken with a 4×/0.13 NA Fluor objective). All images were processed by Nero Photosnap Viewer 7 (Nero ACT, Karlsbad, Germany). Under the conditions described, approximately 20% of cells were positive for the K8.1 protein.
Characterization of HHV-8 acute infection in HUVECs. (A) Electron micrographs of HHV-8 particles produced and purified as described in “Materials and methods” (original magnification, × 50 000; Hitachi H-800 electron microscope; Hitachi, High Technology Europe, Krefeld, Germany). (B) Subconfluent HUVEC monolayers were mock infected (control) or infected with HHV-8 (HHV-8). Microscopic observation of HHV-8 infected HUVECs was performed at 48 hours after infection (a.i.) by a Nikon Eclipse TE2000-S microscope equipped for phase-contrast observation (10×/0.25 and 20×/0.45 NA Fluor objective lenses), using a DS camera head DS-5M and a DS camera control unit DS-L; Nikon). (C) Results of RT-PCR amplification of the indicated HHV-8 genes using RNA extracted from infected cells at different days after infection. β-Actin levels were determined in the same samples as control. Positive controls of amplification of the genes analyzed (C+) are shown. (D) The presence of the HHV-8 late K8.1 protein at 36 hours after infection was detected in HHV-8–infected HUVECs grown onto collagenated culture slides by immunofluorescence analysis using a Nikon Eclipse TE2000-S microscope equipped for fluorescence observation. Immunofluorescence micrograph (ii) and the corresponding bright-field image (i) are shown at original magnification × 4 (pictures were taken with a 4×/0.13 NA Fluor objective). All images were processed by Nero Photosnap Viewer 7 (Nero ACT, Karlsbad, Germany). Under the conditions described, approximately 20% of cells were positive for the K8.1 protein.
Infection of HUVECs with HHV-8 was verified by PCR and RT-PCR detection of immediate early, late, and latent viral genes (ORF50, ORF26, and ORF73, respectively). In addition, the expression of vGPCR/ORF74, which was shown to induce NF-κB activity when overexpressed in cultured cells,19,22 was also determined. The results, summarized in Figure 1C, show that HHV-8 caused a productive infection in endothelial cells that lasted for 7 days, as shown by the expression of ORF50 and ORF26 lytic genes. No viral transcripts were found in mock-infected cells at all times tested (data not shown). The establishment of a productive infection was also verified by immunofluorescence analysis (IFA), which showed that infected cells express the lytic late envelope glycoprotein encoded by ORFK8.1 (Figure 1D). The occurrence of productive replication of HHV-8 in HUVECs was confirmed by detection of newborn virus released in culture supernatants as determined by PCR. Virus DNA was detectable in culture supernatants at 3 and 7 days after infection. Furthermore, the newborn virions produced and released by HUVECs were infectious, as determined by using filtered supernatants to infect target HUVEC cultures (data not shown).
At day 7 after infection, the virus entered the latent phase, as shown by the disappearance of the lytic transcripts accompanied by the appearance of the latent ones (ORF73; Figure 1C), and the absence of newborn virions in the supernatant (data not shown).
HHV-8 acute infection induces NF-κB activation via IκB kinase stimulation
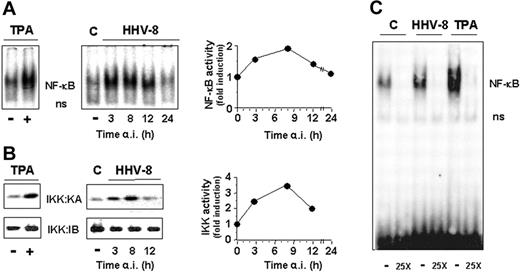
Several reports have shown that transfection with different HHV-8 genes results in NF-κB activation in different types of cells.18-23 To investigate whether HHV-8 acute infection was able to trigger NF-κB activation in endothelial cells, HUVECs were infected with HHV-8 or treated with 20 ng/mL TPA as a positive control. Mock-infected cells were treated identically. At different times after infection, cells were harvested and whole-cell extracts were prepared and analyzed for NF-κB activation by EMSA. The level of NF-κB DNA binding activity was quantified by MDP analysis. As shown in Figure 2A, HHV-8 induced NF-κB activity in endothelial cells at levels comparable to those reached after TPA treatment, starting at early times (3 hours) after infection. NF-κB activation was transient, reaching maximal levels at 8 hours after infection and then declining to control values at 24 hours after infection.
HHV-8 acute infection induces IKK and NF-κB activities in HUVECs. HUVEC monolayers were mock infected (control) or infected with HHV-8 or treated with TPA (20 ng/mL). At different times after infection, whole-cell extracts were prepared and analyzed for NF-κB activation by EMSA (A) or for IKK activity and recovery by kinase assay (KA) and immunoblotting (IB), respectively (B). Sections of fluorograms from native gels are shown (right panels). Position of NF-κB-DNA (NF-κB) and nonspecific protein-DNA (ns) complexes are indicated. The levels of NF-κB and IKK activity in HHV-8–infected cells were quantified by MDP analysis and expressed as fold induction of the levels detected in uninfected control cells (left panels). Results of a representative experiment of 3 with similar results are shown. (C) HUVEC monolayers were mock infected (C), infected with HHV-8 (HHV-8), or treated with 20 ng/mL TPA. At 4 hours after infection whole-cell extracts were subjected to competition EMSA by using the oligonucleotide containing the consensus NF-κB element as a probe in the absence (−) or the presence (25X) of 25-fold excess unlabeled consensus oligonucleotide.
HHV-8 acute infection induces IKK and NF-κB activities in HUVECs. HUVEC monolayers were mock infected (control) or infected with HHV-8 or treated with TPA (20 ng/mL). At different times after infection, whole-cell extracts were prepared and analyzed for NF-κB activation by EMSA (A) or for IKK activity and recovery by kinase assay (KA) and immunoblotting (IB), respectively (B). Sections of fluorograms from native gels are shown (right panels). Position of NF-κB-DNA (NF-κB) and nonspecific protein-DNA (ns) complexes are indicated. The levels of NF-κB and IKK activity in HHV-8–infected cells were quantified by MDP analysis and expressed as fold induction of the levels detected in uninfected control cells (left panels). Results of a representative experiment of 3 with similar results are shown. (C) HUVEC monolayers were mock infected (C), infected with HHV-8 (HHV-8), or treated with 20 ng/mL TPA. At 4 hours after infection whole-cell extracts were subjected to competition EMSA by using the oligonucleotide containing the consensus NF-κB element as a probe in the absence (−) or the presence (25X) of 25-fold excess unlabeled consensus oligonucleotide.
To investigate whether HHV-8 induced NF-κB activity by stimulating IKK function, parallel samples were analyzed for IKK activity and recovery by kinase assay and immunoblot analysis, respectively. The level of IκBα phosphorylation was quantified by MDP analysis. The results shown in Figure 2B demonstrate that HHV-8 is a potent inducer of IKK activity during acute infection of endothelial cells. Similarly to the kinetics of NF-κB activation shown above, IKK function was induced early (3 hours) after infection, and it reached levels 3- to 4-fold higher than control cells at 8 hours after infection, to decline thereafter.
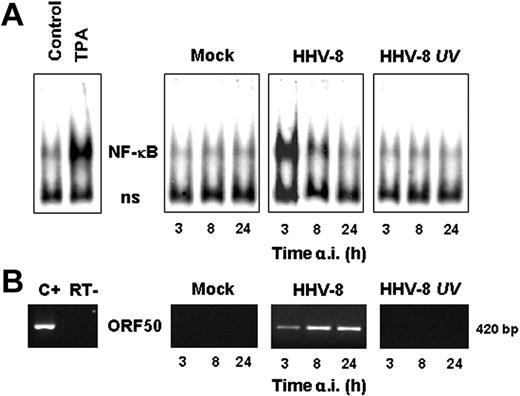
To determine whether NF-κB activation was dependent on active viral replication, HUVECs were infected with HHV-8 or UV-inactivated HHV-8. Mock-infected cells were treated identically. At different times after infection cells were harvested and whole-cell extracts were prepared and analyzed for NF-κB activation by EMSA. The presence of lytic gene expression was demonstrated by RT-PCR detection of ORF50 mRNA in parallel samples. As shown in Figure 3, UV-inactivated HHV-8 is not able to induce NF-κB, indicating that signaling for IKK activity is not triggered by the binding of HHV-8 virions to membrane receptors, but an early event of the virus replication cycle has to occur to activate the nuclear factor.
HHV-8 replication is required for NF-κB induction in HUVECs. HUVECs were mock infected (mock), infected with HHV-8 or UV-inactivated HHV-8 (HHV-8 UV), or treated with TPA (20 ng/mL). At different times after infection, whole-cell extracts were prepared and assayed for NF-κB activation by EMSA. (A) Sections of fluorograms from native gels are shown. Position of NF-κB-DNA (NF-κB) and nonspecific protein-DNA (ns) complexes are indicated. (B) Results of RT-PCR amplification of the HHV-8 ORF50 gene using RNA extracted from mock-infected (mock), HHV-8–infected, or UV-inactivated HHV-8–infected cells. Positive control of ORF50 amplification (C+) and control for DNA contamination, performed by direct amplification of RNA without retrotranscription (RT−) are shown.
HHV-8 replication is required for NF-κB induction in HUVECs. HUVECs were mock infected (mock), infected with HHV-8 or UV-inactivated HHV-8 (HHV-8 UV), or treated with TPA (20 ng/mL). At different times after infection, whole-cell extracts were prepared and assayed for NF-κB activation by EMSA. (A) Sections of fluorograms from native gels are shown. Position of NF-κB-DNA (NF-κB) and nonspecific protein-DNA (ns) complexes are indicated. (B) Results of RT-PCR amplification of the HHV-8 ORF50 gene using RNA extracted from mock-infected (mock), HHV-8–infected, or UV-inactivated HHV-8–infected cells. Positive control of ORF50 amplification (C+) and control for DNA contamination, performed by direct amplification of RNA without retrotranscription (RT−) are shown.
HHV-8 acute infection induces MCP-1 expression in endothelial cells
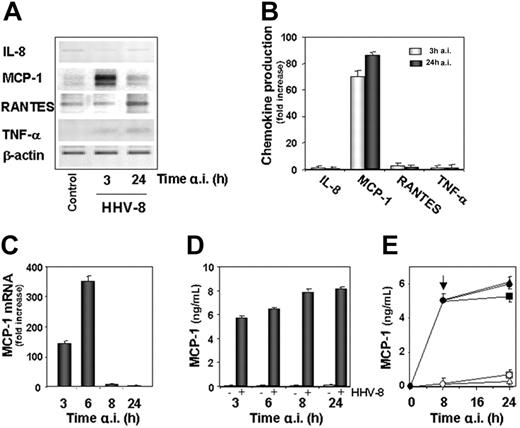
NF-κB is known to control the expression of a variety of proinflammatory genes.7 To determine whether the IKK-mediated NF-κB activity during acute HHV-8 infection may result in triggering the expression of proinflammatory proteins, we investigated the level of transcription of a panel of NF-κB–dependent inflammatory cytokines, including TNF-α, IL-8, RANTES, and MCP-1, in uninfected and HHV-8–infected HUVECs at different times after infection. RT-PCR analysis of the different proinflammatory cytokines tested indicated that HHV-8 infection selectively induced an increase in MCP-1 mRNA levels, whereas it did not affect the expression of the other cytokines analyzed (Figure 4A). Selective transcriptional activation of the MCP-1 gene in HHV-8–infected cells was confirmed by ELISA determination of the proinflammatory proteins released in culture medium at 3 and 24 hours after infection. As shown in Figure 4B, HHV-8 infection induced the production of very high levels of MCP-1 in the supernatant of infected cells, whereas it did not affect the levels of the other chemokines tested.
Induction of MCP-1 expression by HHV-8 infection in endothelial cells. (A) HUVECs were mock infected (control) or infected with HHV-8 (HHV-8). At 3 and 24 hours after infection total RNA was extracted and analyzed by RT-PCR for the expression of IL-8, MCP-1, RANTES, and TNF-α. β-Actin levels are shown as control. (B) In the same experiment culture supernatants were collected at 3 and 24 hours after infection and analyzed for the release of the indicated proinflammatory mediators by standard quantitative ELISAs. Levels of chemokines are expressed as fold induction of the levels detected in mock-infected control cells. Bars represent the mean ± SD of triplicate samples. (C) Mock-infected and HHV-8–infected HUVECs were harvested at the indicated times, and MCP-1 and β-actin mRNA levels were analyzed by TaqMan rtPCR. MCP-1 mRNA levels normalized for β-actin levels in the same samples are expressed as fold induction of the levels detected in mock-infected cells at the same time points. Bars represent the mean ± SD of triplicate samples. (D) In the same experiment MCP-1 levels in culture supernatants from mock-infected (−) and HHV-8–infected (+) cells were determined by ELISA. The results represent the mean ± SD of triplicate samples. (E) Cycloheximide and monensin (Sigma-Aldrich) were used to inhibit protein synthesis and secretion, respectively. Mock-infected (empty symbols) or HHV-8–infected (filled symbols) HUVECs were treated with 5 μM cycloheximide (□, ▪), 5 μM monensin (▴, ▵), or control diluent (○, •) 8 hours after infection (indicated by arrow). MCP-1 levels in culture supernatants were determined 24 hours after infection by ELISA. Bars represent the mean ± SD of triplicate samples. All experiments were performed 3 times with similar results.
Induction of MCP-1 expression by HHV-8 infection in endothelial cells. (A) HUVECs were mock infected (control) or infected with HHV-8 (HHV-8). At 3 and 24 hours after infection total RNA was extracted and analyzed by RT-PCR for the expression of IL-8, MCP-1, RANTES, and TNF-α. β-Actin levels are shown as control. (B) In the same experiment culture supernatants were collected at 3 and 24 hours after infection and analyzed for the release of the indicated proinflammatory mediators by standard quantitative ELISAs. Levels of chemokines are expressed as fold induction of the levels detected in mock-infected control cells. Bars represent the mean ± SD of triplicate samples. (C) Mock-infected and HHV-8–infected HUVECs were harvested at the indicated times, and MCP-1 and β-actin mRNA levels were analyzed by TaqMan rtPCR. MCP-1 mRNA levels normalized for β-actin levels in the same samples are expressed as fold induction of the levels detected in mock-infected cells at the same time points. Bars represent the mean ± SD of triplicate samples. (D) In the same experiment MCP-1 levels in culture supernatants from mock-infected (−) and HHV-8–infected (+) cells were determined by ELISA. The results represent the mean ± SD of triplicate samples. (E) Cycloheximide and monensin (Sigma-Aldrich) were used to inhibit protein synthesis and secretion, respectively. Mock-infected (empty symbols) or HHV-8–infected (filled symbols) HUVECs were treated with 5 μM cycloheximide (□, ▪), 5 μM monensin (▴, ▵), or control diluent (○, •) 8 hours after infection (indicated by arrow). MCP-1 levels in culture supernatants were determined 24 hours after infection by ELISA. Bars represent the mean ± SD of triplicate samples. All experiments were performed 3 times with similar results.
To further characterize the effect of HHV-8 infection on MCP-1 expression in endothelial cells, the kinetics of MCP-1 mRNA appearance following acute infection was investigated using quantitative rtPCR. HUVEC monolayers were infected with HHV-8 as described above, and total RNA was extracted from cell lysates at 3, 6, 8, and 24 hours after infection. MCP-1 messenger levels were quantified in relation to the β-actin mRNA expression in each sample. At the same time, MCP-1 protein levels were determined in the supernatant of HHV-8–infected or control cells by ELISA. As shown in Figure 4C, HHV-8 acute infection resulted in a great enhancement of MCP-1 mRNA expression (approximately 150-fold) at 3 hours after infection, reaching maximal levels (350-fold increase) at 6 hours after infection, and declining thereafter. Low constitutive levels of MCP-1 protein (ranging from 70 to 150 pg/mL) were detected in the supernatants of uninfected HUVECs at all times tested. As shown in Figure 4D, HHV-8 infection induced a dramatic increase of MCP-1 release already at 3 hours after infection (approximately 80-fold), and MCP-1 levels remained elevated throughout the course of infection. The elevated MCP-1 protein levels at times when MCP-1 mRNA expression was not detected suggested that HHV-8–induced MCP-1 was stable for several hours in infected cell supernatants. To verify this hypothesis, HUVEC monolayers were infected with HHV-8 and treated with 5 μM cycloheximide or monensin immediately after the adsorption period or 8 hours after infection, and MCP-1 levels were determined in culture supernatants at 8 and 24 hours after infection by ELISA. Whereas cycloheximide or monensin treatment soon after virus adsorption completely blocked MCP-1 synthesis or secretion or both (data not shown), addition of either inhibitor at 8 hours after infection, at which time high levels of MCP-1 protein were detected, had no effect on MCP-1 accumulation in the supernatant of infected cells at 24 hours after infection (Figure 4E), indicating that the synthesis and secretion of MCP-1 occur for the most part early during infection. UV inactivation of viral inoculum resulted in the complete loss of MCP-1 induction, and levels of MCP-1 mRNA and secreted protein were similar to those detected in mock-infected cells (data not shown).
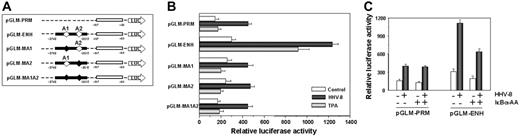
To investigate whether HHV-8 was directly affecting MCP-1 transcription, HUVEC monolayers were cotransfected with different plasmids containing the reporter luciferase gene cloned downstream the MCP-1 promoter35 together with the pRL-SV40 vector as an internal transcriptional control. MCP-1 promoter luciferase reporter plasmids used included: pGLM-PRM, which contains the proximal promoter region of the MCP-1 gene, pGLM-ENH, which contains both the proximal promoter and the distal enhancer regions, and pGLM-MA1, pGLM-MA2 and pGLM-MA1A2, where one or both NF-κB binding sites (A1 and A2) in the enhancer region were mutated (Figure 5A). Twelve hours after transfection, cells were mock infected, infected with HHV-8, or treated with TPA (100 ng/mL) as a control of NF-κB activation. TPA treatment resulted in a significant transcriptional activation in cells transfected with the pGLM-ENH plasmid, in which both the MCP-1 promoter and enhancer regions are present (Figure 5A-B). Mutation in either κB sites resulted in loss of TPA-induced activation. HHV-8 infection resulted in an increase in luciferase expression in cells transfected with the pGLM-PRM vector, which lacks a functional MCP-1 enhancer region, whereas it was more effective than TPA in inducing luciferase expression in cells transfected with the pGLM-ENH plasmid (Figure 5B). As for TPA, mutation in either κB sites resulted in a marked loss of HHV-8–induced transcriptional activation, indicating that the κB sites in the enhancer region are needed for optimal viral stimulation.
Analysis of HHV-8–induced MCP-1 transcriptional activation. (A) Schematic representation of human MCP-1 reporter constructs.35 The proximal promoter region and distal enhancer region are indicated by open and closed boxes, respectively. The position of NF-κB sites (A1 and A2) in the enhancer region is indicated by open diamonds (⋄). Mutated NF-κB sites are indicated by filled diamonds (♦). (B) HUVECs were cotransfected with the pRL-SV40 vector together with one of the following plasmids: pGLM-PRM, pGLM-ENH, pGLM-MA1, pGLM-MA2, and pGLM-MA1A2, described in panel A. After 12 hours transfected cells were mock infected (control), infected with HHV-8, or treated with TPA (20 ng/mL). Dual luciferase activity was determined 30 hours after infection. The data, expressed as relative luciferase activity, represent the mean ± SD of quadruplicate samples from 2 independent experiments. (C) HUVEC monolayers were triple-transfected with the pRL-SV40 vector and the pGLM-PRM or pGLM-ENH reporter constructs together with “empty” (−) or IκBα-AA (+) vectors. After 12 hours, transfected cells were mock infected (− HHV-8) or infected with HHV-8 (+ HHV-8). Luciferase activities were determined 30 hours after infection as described in panel B. The data, expressed as relative luciferase activity, represent the mean ± SD of quadruplicate samples from 2 independent experiments.
Analysis of HHV-8–induced MCP-1 transcriptional activation. (A) Schematic representation of human MCP-1 reporter constructs.35 The proximal promoter region and distal enhancer region are indicated by open and closed boxes, respectively. The position of NF-κB sites (A1 and A2) in the enhancer region is indicated by open diamonds (⋄). Mutated NF-κB sites are indicated by filled diamonds (♦). (B) HUVECs were cotransfected with the pRL-SV40 vector together with one of the following plasmids: pGLM-PRM, pGLM-ENH, pGLM-MA1, pGLM-MA2, and pGLM-MA1A2, described in panel A. After 12 hours transfected cells were mock infected (control), infected with HHV-8, or treated with TPA (20 ng/mL). Dual luciferase activity was determined 30 hours after infection. The data, expressed as relative luciferase activity, represent the mean ± SD of quadruplicate samples from 2 independent experiments. (C) HUVEC monolayers were triple-transfected with the pRL-SV40 vector and the pGLM-PRM or pGLM-ENH reporter constructs together with “empty” (−) or IκBα-AA (+) vectors. After 12 hours, transfected cells were mock infected (− HHV-8) or infected with HHV-8 (+ HHV-8). Luciferase activities were determined 30 hours after infection as described in panel B. The data, expressed as relative luciferase activity, represent the mean ± SD of quadruplicate samples from 2 independent experiments.
To further investigate whether HHV-8–induced MCP-1 expression was dependent on NF-κB activation, we used the IκBα super-repressor IκBα-AA, in which Ser-32/36 residues critical for phosphorylation by IKK have been replaced by alanine.29 HUVEC monolayers were cotransfected with either the pGLM-PRM or the pGLM-ENH MCP-1 promoter-luciferase constructs together with the pRL-SV40 vector, in the presence or absence of the IκBα super-repressor expression vector. Twelve hours after transfection, cells were mock-infected or infected with HHV-8. As shown in Figure 5C, expression of the dominant-negative form of IκBα resulted a marked inhibition of luciferase expression in cells transfected with the pGLM-ENH construct, further confirming that HHV-8–induced NF-κB activation is crucial for optimal MCP-1 expression.
Effects of HHV-8 infection on Matrix-induced tube formation
MCP-1 is known to play an important role in inflammatory diseases and in vascular remodeling36,37 ; in addition, a direct role of MCP-1 in promoting angiogenesis has been recently shown in vitro and in vivo.38,39 Angiogenesis is a complex process, typically consisting of cell proliferation and alignment to form tubular structures.40 Because of the selective induction of MCP-1 expression in HHV-8–infected endothelial cells, we investigated the ability of HHV-8 acute infection to induce cell capillary-like structure formation using a BME morphogenesis assay, as described in “Materials and methods.” Mock-infected or HHV-8–infected HUVECs were seeded on BME at 24 hours after infection. Surprisingly, HHV-8 infection was found to strongly promote the formation of hollow tubelike structures, which were clearly evident as early as 4 hours after cell plating (Figure 6). The formation of well defined capillary-like structures was evident up to 24 hours after seeding; however, at this time spontaneous tubule formation, though to a minor extent, was also found in uninfected control (data not shown).
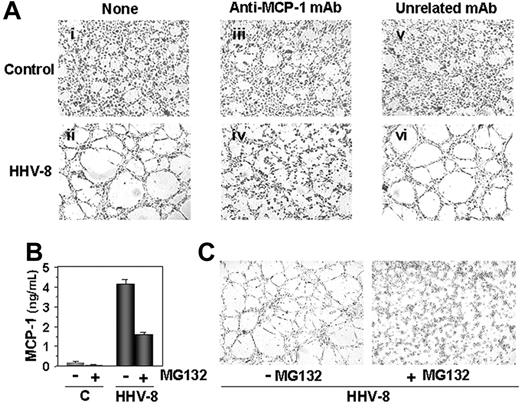
HHV-8 acute infection induces MCP-1–dependent capillary-like structure formation. Mock-infected (control) and HHV-8–infected HUVECs were harvested 24 hours after infection and plated on BME (5 × 104 cell/well). Cells were incubated at 37°C in complete medium (i-ii) or medium containing 1 μg/mL anti–MCP-1 mAb (iii-iv), or unrelated mAb (v-vi). After 4 hours cells were observed by optical microscopy (Leica DM IRB, Wetzlar, Germany) and photographed with CCD optics (Hitachi Denshi Color Camera KP-D50E/K; Rodgau, Germany) using a digital analysis system (QWIN LITE version 2.3; Leica). Tubelike structure formation is evident in HHV-8–infected cells (compare panels i and ii). The addition of anti–MCP-1 antibody completely blocks the formation of capillary-like structures in HHV-8–infected HUVECs (compare panels ii and iv), whereas the unrelated mAb has no effect (compare panels iv and vi). Photographs (4×/0.10 NA Plan objective lens) from a representative experiment of 3 with similar results are shown; mAb indicates monoclonal antibody. (B) HUVECs were treated with 5 μM MG132 (carbobenzoxy-l-leucyl-l-leucyl-l-leucynal; Sigma-Aldrich; +) or control diluent (−). After 1 hour, cells were infected with HHV-8 or mock infected (C) and incubated in the presence or absence of MG132. At 4 hours after infection, MCP-1 levels in culture supernatants were determined by ELISA. Data represent the mean ± SD of triplicate samples. (C) In a parallel experiment, HHV-8–infected HUVECs were cultured in the presence (+) or absence (−) of MG132 (1 μM) for 24 hours, and then plated on BME (5 × 104 cell/well) in fresh medium in the absence of MG132. Cells were observed under microscope for capillary-like structure formation at 4 hours after seeding. Photographs (original magnification × 40) from a representative experiment are shown. MG132 treatment during infection completely prevented endothelial cell alignment and tubule formation.
HHV-8 acute infection induces MCP-1–dependent capillary-like structure formation. Mock-infected (control) and HHV-8–infected HUVECs were harvested 24 hours after infection and plated on BME (5 × 104 cell/well). Cells were incubated at 37°C in complete medium (i-ii) or medium containing 1 μg/mL anti–MCP-1 mAb (iii-iv), or unrelated mAb (v-vi). After 4 hours cells were observed by optical microscopy (Leica DM IRB, Wetzlar, Germany) and photographed with CCD optics (Hitachi Denshi Color Camera KP-D50E/K; Rodgau, Germany) using a digital analysis system (QWIN LITE version 2.3; Leica). Tubelike structure formation is evident in HHV-8–infected cells (compare panels i and ii). The addition of anti–MCP-1 antibody completely blocks the formation of capillary-like structures in HHV-8–infected HUVECs (compare panels ii and iv), whereas the unrelated mAb has no effect (compare panels iv and vi). Photographs (4×/0.10 NA Plan objective lens) from a representative experiment of 3 with similar results are shown; mAb indicates monoclonal antibody. (B) HUVECs were treated with 5 μM MG132 (carbobenzoxy-l-leucyl-l-leucyl-l-leucynal; Sigma-Aldrich; +) or control diluent (−). After 1 hour, cells were infected with HHV-8 or mock infected (C) and incubated in the presence or absence of MG132. At 4 hours after infection, MCP-1 levels in culture supernatants were determined by ELISA. Data represent the mean ± SD of triplicate samples. (C) In a parallel experiment, HHV-8–infected HUVECs were cultured in the presence (+) or absence (−) of MG132 (1 μM) for 24 hours, and then plated on BME (5 × 104 cell/well) in fresh medium in the absence of MG132. Cells were observed under microscope for capillary-like structure formation at 4 hours after seeding. Photographs (original magnification × 40) from a representative experiment are shown. MG132 treatment during infection completely prevented endothelial cell alignment and tubule formation.
To investigate the role of MCP-1 in HHV-8–induced capillary-like structure formation, an anti–MCP-1–neutralizing antibody was used to specifically inhibit MCP-1 in the BME morphogenesis assay. Mock-infected or HHV-8–infected HUVECs were seeded in the presence of 1 μg/mL anti–MCP-1 antibody, or an unrelated antibody, and tube formation was observed 4 hours after seeding. As shown in Figure 6A, tube formation was completely inhibited in HHV-8–infected HUVECs seeded in the presence of the antibody, suggesting that MCP-1 plays a key role in the HHV-8–induced capillary-like structure formation.
Finally, to investigate the role of NF-κB on HHV-8–induced MCP-1 production and tube formation, NF-κB induction in HHV-8–infected HUVECs was blocked by treatment with the proteasome inhibitor MG132, which blocks NF-κB activation by preventing IκB degradation.28 HUVEC monolayers were treated with 5 μM MG132 for 1 hour and then infected with HHV-8 or mock infected. MCP-1 levels were determined in the culture supernatants at 4 hours after infection. As shown in Figure 6B, treatment with MG132 greatly inhibited MCP-1 production by HHV-8–infected cells. In a parallel experiment, untreated and MG132-treated HHV-8–infected HUVECs were seeded on BME 24 hours after infection, and tube formation was observed 4 hours after plating. As shown in Figure 6C, treatment with the NF-κB inhibitor completely prevented tube formation in HHV-8–infected HUVECs. These results further suggest that NF-κB plays a key role in MCP-1 production and in capillary-like structure formation induced by HHV-8 infection in endothelial cells.
Discussion
The nuclear factor NF-κB has a central role in regulating cellular metabolic events. NF-κB–binding sites have been identified in the promoter region of more than 300 cellular genes whose expression depends on a sophisticated multilevel control.9,10 As anticipated in “Introduction,” NF-κB target genes include genes involved in the control of cell proliferation and survival, as well as genes regulating the host immune and inflammatory responses such as cyclooxygenase 2 (COX-2), the inducible form of nitric oxide synthase (iNOS), receptors required for neutrophil adhesion and transmigration across blood vessel walls, and a plethora of cytokines and chemokines including MCP-1.7,11,12
A variety of viruses, including several human pathogens such as HIV-1, HTLV-1, influenza virus, hepatitis B and C viruses as well as herpesviruses have evolved different strategies to modulate the NF-κB pathway.9 Among herpesviruses, it has been shown that herpes simplex viruses, as well as the β-herpesvirus CMV and the γ-herpesvirus EBV, are potent inducers of NF-κB activation (for a review, see Santoro et al9 ).
In the case of HHV-8, NF-κB has been shown to be constitutively activated in PEL-derived cell lines, chronically infected with the virus.14 Evidence based on in vitro transient transfection of isolated viral genes indicate that several viral products are able to induce NF-κB activation in different cell types. NF-κB is induced by vFLIP, a latency-associated viral gene (also known as K13), which binds to and activates NF-κB by interaction with the IKK complex.18,20,41 Likewise, overexpression of the viral G protein-coupled receptor (vGPCR, ORF74)6,19,22,23 and of the K15 membrane protein21 have also been shown to induce NF-κB activation. Interestingly, HHV-8 encodes also the K1 protein which, conversely, has been shown to abolish TPA-induced NF-κB activation.42 In addition, the viral interferon regulatory factor 3 (vIRF3), encoded by ORF K10.5, has recently been shown to down-regulate NF-κB activity by inhibiting IKKβ.43 All together, these results indicate that the levels of NF-κB are tightly regulated in cells chronically infected with HHV-8 and depend on a fine balance between activating and inhibitory factors expressed in these cells. Therefore, rather than examining the effect of isolated genes, it appears important to establish the events regulating NF-κB activity and function after cell exposure to infectious virions. In addition, little is known on NF-κB activation during acute HHV-8 infection.
In the present report we investigated the effect of HHV-8 on NF-κB activity and function using a recently established in vitro model of HHV-8 acute productive infection in endothelial cells.24 In this model HHV-8 was found to rapidly induce NF-κB activity at levels comparable to those reached after treatment with the potent NF-κB inducer TPA. Differently from HSV-1, which has been shown to induce persistent NF-κB activation in a variety of human cells,32,44,45 HHV-8–induced NF-κB activity was transient, reaching maximal levels at 8 hours after infection and then declining to control values at 24 hours after infection. Activation of NF-κB is mediated by HHV-8–induced stimulation of IKK activity, which reached levels 3- to 4-fold higher than control cells between 3 and 8 hours after infection, to decline thereafter. This is the first time that IKK activation by HHV-8 is observed in the context of acute viral infection. In addition, we show that HHV-8–induced NF-κB activity depends on virus replication because it does not occur after infection with UV-inactivated HHV-8.
Because of the critical role of NF-κB in activating inflammatory processes, we investigated the ability of HHV-8 to induce the expression of NF-κB–dependent inflammatory and chemotactic cytokines in endothelial cells. In particular, we analyzed the effect of HHV-8 activation on a panel of NF-κB target genes, including TNF-α, IL-8, RANTES, and MCP-1, at different times after infection. We surprisingly found that HHV-8 infection selectively triggers the expression and secretion of high levels of MCP-1, whereas it does not affect the transcription and production of the other cytokines tested. MCP-1 mRNA transcription started at 3 hours after infection and peaked at 6 hours after infection. MCP-1 levels peaked at 8 hours after infection (90-fold increase as compared to uninfected controls) and remained stable for at least 24 hours after infection. This is particularly relevant in view of the recent observation that HHV-8 infection inhibits host gene expression, by accelerating the global cellular mRNA turnover.46
Transcriptional activation of the MCP-1 gene after HHV-8 infection of endothelial cells was confirmed in transient transfection experiments, using plasmids containing the reporter luciferase gene cloned downstream of the MCP-1 promoter. Levels of HHV-8–induced MCP-1 promoter-driven luciferase expression were found to be higher than the ones obtained after TPA stimulation.
The human MCP-1 gene contains 2 NF-κB–binding sites in the enhancer region, and the presence of one site in the proximal promoter region has been suggested.35,47 In addition to NF-κB sites, AP-1– and SP-1–binding sites have been located in MCP-1 enhancer and promoter regions.47-49 Diverse signal transduction pathways, including AKT/protein kinase B,50 phospholipase C,51 p60 Src-Ras,52 protein kinase C, and tyrosine kinases53 have been shown to be involved in the expression of the MCP-1 gene in response to a variety of stimuli such as mitogens, cytokines, or shear stress. It has been hypothesized that some of the pathways are activated either concomitantly or selectively according to the stimulating agent. The functional analysis of the MCP-1 promoter has demonstrated that κB-binding sites in the enhancer region are required for TPA-induced expression.35
In the case of HHV-8 infection we have now shown that the IKK/NF-κB pathway is involved in the enhancement of MCP-1 expression and is required for maximal production of the chemokine. However, mutations in both κB sites in the enhancer region did not result in the complete loss of promoter induction in HHV-8–infected HUVECs, suggesting that multiple signaling pathways may be involved in the control of MCP-1 expression in the course of acute HHV-8 infection.
MCP-1 is a CC chemokine produced by macrophages and endothelial cells in response to diverse stimuli, including microbial pathogens.54,55 MCP-1 plays an important role in inflammatory diseases, attracting both monocytes and lymphocytes.56 In addition, MCP-1 has been recently found to be a direct mediator of angiogenesis, as measured by its ability to induce in vitro endothelial cell migration and endothelial cell sprouting from aortal rings in the absence of an inflammatory response, and to stimulate in vivo angiogenesis in a matrigel plug assay.38 The ability to form networking capillary tubes is a cell-autonomous property of endothelial cells, which need permissive but not instructive signals from the microenvironment.57,58 Using a BME morphogenesis assay, we have now shown that HHV-8 infection induces a dramatic response consisting in a rearrangement of endothelial cells, which align and form capillary-like structures, as early as 3 to 4 hours after seeding. This phenomenon was abolished by anti–MCP-1–neutralizing monoclonal antibodies, suggesting a prominent and direct role of this chemokine in the herpesvirus-mediated angiogenic process. In addition, the block of HHV-8–induced NF-κB activation by treatment with the proteasome inhibitor MG132 inhibited MCP-1 production and prevented the formation of tubular networks by infected cells, suggesting that the nuclear factor may play a key role in HHV-8–induced angiogenesis.
Taken together, the data presented in this study provide compelling evidence that during acute infection of endothelial cells, HHV-8 triggers IKK-mediated NF-κB activation and drives a strong selective induction of MCP-1 synthesis. The results also suggest that HHV-8–induced MCP-1 may, in turn, play an important role in the inflammation and pathogenic angiogenesis typical of HHV-8–associated lesions by recruiting macrophages and other effector cells to the site of infection as well as by directly stimulating vascular remodeling.
Authorship
Contribution: E.C. performed viral infections and transfection experiments; C.A. performed research on the NF-κB pathway; S.F. performed research on cytokine expression and the in vitro morphogenesis assays; and D.D., A.C., and M.G.S. participated in designing the study and writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: M. Gabriella Santoro, Department of Biology, University of Rome Tor Vergata Via della Ricerca Scientifica, 00133 Rome, Italy; e-mail: santoro@bio.uniroma2.it.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Italian Ministry of University and Scientific Research (PRIN projects) and Istituto Superiore di Sanità (ISS; AIDS project).
We thank Dr T. Yoshimura (Laboratory of Immunobiology, National Cancer Institute, Frederick, MD) for providing the MCP-1 promoter-luciferase reporter plasmids.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal