Abstract
Age-related thymopoietic insufficiency has been proposed to be related to either defects in lymphohematopoietic progenitors or the thymic microenvironment. In this study, we examined whether keratinocyte growth factor (KGF), an epithelial cell–specific growth factor, could increase thymopoietic capacity in aged mice by restoration of the function of thymic epithelial cells (TECs). The thymic cellularity in KGF-treated aged mice increased about 4-fold compared to placebo-treated mice, resulting in an equivalent thymic cellularity to young mice. Enhanced thymopoiesis was maintained for about 2 months after a single course of KGF, and sustained improvement was achieved by administration of monthly courses of KGF. With the enhanced thymopoiesis after KGF treatment, the number of naive CD4 T cells in the periphery and T-cell–dependent antibody production improved in aged mice. KGF induced increased numbers of TECs and intrathymic interleukin-7 (IL-7) production and reorganization of cortical and medullary architecture. Furthermore, KGF enhanced thymopoiesis and normalized TEC organization in klotho (kl/kl) mice, a model of premature degeneration and aging, which displays thymopoietic defects. The result suggests that TEC damage is pathophysiologically important in thymic aging, and KGF therapy may be clinically useful in improving thymopoiesis and immune function in the elderly.
Introduction
The continued production of T cells throughout life requires the thymus to remain functional. In humans, the thymus is almost fully developed at birth. For approximately 1 year after birth, the thymus keeps increasing in size, but subsequently thymic cellularity and output decrease. During aging, the perivascular region of the thymus develops fatty changes. Although the thymus in aged individuals is still able to generate functional T cells, recovery from depletion of the mature T-cell compartment is decreased compared with young individuals.1,2 Therefore, age-related thymopoietic insufficiency affects immune reconstitution in patients with some clinical conditions, such as radiation, chemotherapy, and human immunodeficiency virus (HIV) infection.3–5 Age-related thymopoietic insufficiency results in a reduced contribution of recent thymic emigrants (RTEs) to the peripheral T-cell pool and the clonal expansion of pre-existing memory T cells to maintain the overall T-cell pool. Thus, the aged have an accumulation of memory T cells with low diversity for specific antigen recognition, even though the overall size of the T-cell pool is not significantly altered.6
Studies to elucidate the mechanism of age-related thymopoietic insufficiency have tended to emphasize either intrinsic defects of lymphohematopoietic progenitors with aging or primary defects of the thymic microenvironment.7 In support of the former, it has been suggested that decreased thymopoietic capacity is caused by the quantitative and/or qualitative alteration of progenitors with aging.8–11 However, other investigators have reported that the number of immature CD44+CD25−CD3−CD4−CD8− (“triple-negative,” TN) thymocytes is not significantly changed with aging.12 Instead, increased apoptosis of immature thymocytes caused by insufficient amounts of the stroma-derived cytokine IL-7 reduces the number of thymocytes at subsequent stages of thymic differentiation, and IL-7 treatment normalizes thymopoiesis.13,14 Furthermore, the thymic reconstitution of aged mice with marrow cells derived from young mice does not reverse impaired thymopoiesis, while thymic reconstitution of young mice with the marrow from aged mice is normal.15 These results suggest that defects in the thymic microenvironment play a crucial role in age-related thymic insufficiency.
Besides physiological aging in normal mice, accelerated aging phenotypes are observed in various mouse models including the klotho mutant mouse (kl/kl). Klotho encodes a transmembrane protein with homology to bacterial glucosidases. kl/kl mice undergo premature age-related tissue degeneration in many organs, including the thymus.16 Although the mechanism of premature aging in kl/kl mice is still poorly understood, this model suggests that age-related thymic insufficiency can be caused by degenerative mechanisms that do not require genotoxic stress or genomic instability, such as is seen in telomerase or helicase deficiency. kl/kl hematopoietic cells have normal lymphoid differentiation potential in a nonklotho (severe combined immunodeficient [scid]) thymic microenvironment, and kl/kl bone marrow stromal cells produce abnormally low amounts of IL-7. These data suggest that premature age-related thymic insufficiency in kl/kl mice might be caused by microenvironmental defects of the marrow or thymus in support of lymphoid progenitors, rather than an intrinsic defect of lymphohematopoietic cells.17
The importance of the thymic microenvironment, especially thymic epithelial cells (TECs) in thymic organogenesis and thymopoiesis, has been demonstrated by immunodeficiency caused by primary microenvironmental defects. For example, loss-of-function FoxN1 mutation results in the athymic nude phenotype marked by abnormal epithelial cell development, including that of TECs.18 Critical functions of TECs include production of cytokines (eg, IL-7 and Kit ligand) and chemokines (eg, SDF-1 and TECK), and mediation of positive and negative selection of thymocytes in the cortex and medulla, respectively.19–22 IL-7 plays a central role in the development of immature TN thymocytes by induction of proliferation and regulation of survival by induction of the antiapoptotic protein, bcl-2, and down-regulation of the proapoptotic protein, bax.23–25
A general model of TEC differentiation proposes that immature TEC progenitors, which localize at the corticomedullary junction, differentiate into medullary TECs or cortical TECs.26 The existence of clonal TEC progenitors is supported by the demonstration that each medulla is derived from a single TEC progenitor.27 Recently, TEC progenitors were identified that are able to reconstitute a functional thymic microenvironment in athymic nude recipients.28,29 Proper differentiation and proliferation of TECs requires interaction both between TECs and mesenchyme as well as between TECs and thymocytes.30,31 The processes that govern the maintenance of TECs throughout postnatal life are not well understood.
Keratinocyte growth factor (KGF; aka, Fgf7), a mesenchymally derived member of the fibroblastic growth factor family, is important for maintenance of TECs. KGF interacts with its cognate receptor, FGFR2-IIIb, which is specifically expressed by epithelial cells, including TECs.32,33 KGF treatment induces proliferation of TEC progenitors, as well as mature TECs in fetal thymic organ culture.6 In addition to KGF, FGF1, FGF3, and FGF10 interact with FGFR2-IIIb.33–36 During thymic organogenesis, FGF7 and FGF10 are required for TEC proliferation before hematopoietic cells seed the thymic primordium, and the mesenchyme is the major source of KGF and FGF10.37,38 Embryonic FgfR2-IIIb−/− mice have profound defects in thymopoiesis with the decreased thymic cellularity due to impaired proliferation and differentiation of TECs.33 The thymus of FGF10−/− mice is similar to that of FgfR2-IIIb−/−mice, albeit their thymopoietic defect is less severe than that of the receptor-deficient mice.33 Unlike FgfR2-IIIb−/− or FGF10−/− mice, KGF−/− mice display normal thymic organogenesis and postnatal thymopoiesis.39 However, KGF−/− mice have defective thymic recovery after injury (eg, sublethal irradiation), indicating that KGF is required for postnatal thymic regeneration.39 These results suggest that KGF and FGF10 have differential roles in TEC development. In a previous study, we demonstrated that KGF treatment prior to hematopoietic stem cell (HSC) transplantation (HSCT) conditioning protects TECs from cytotoxic therapy-induced damage and enhances thymopoiesis by maintaining IL-7–expressing cells in the thymus in experimental models of murine HSCT.40
Based on previous demonstrations of defective TEC number or function in aged mice, the correction of thymopoietic defects in aged mice by IL-7, and the ability of KGF treatment to improve thymic reconstitution after injury, we hypothesized that KGF treatment would restore TECs and improve thymic function in aging.
Materials and methods
Animals
Female C57BL/6J ex-breeder (8-9 months old) and young (6 weeks old) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The ex-breeders were maintained for up to one year at the CHLA animal care facility for use as aged mice (> 15 months old). Mice heterozygous for the klotho mutation (kl/+, C3H × C57BL/6J background) were bred and maintained at CHLA. Genotyping of klotho mice (kl/kl) and wild-type (wt) littermates was done as previously described.41 All protocols were approved by the Animal Care Committee (IACUC) of Childrens Hospital Los Angeles.
KGF treatment
Recombinant human KGF (Amgen, Thousand Oaks, CA) or PBS was administered to mice subcutaneously for 3 consecutive days (5 mg/kg per day). Recombinant human KGF (Amgen) or PBS was administered to each age group mice subcutaneously for 3 consecutive days (5 mg/kg/d).
Fluorescence-activated cell sorter (FACS) analyses
Single-cell suspensions of thymocytes were prepared by collagenase/dispase digestion.28 Cells were washed with ice-cold phosphate-buffered saline (PBS) and incubated with optimal concentrations of monoclonal antibodies directed against CD3, CD4, CD8, Thy1.2, CD44, and CD45RB (BD Pharmingen, San Diego, CA) for 30 minutes at 4°C.40 Ten thousand gated events/sample were acquired on a FACScalibur flow cytometer and analyzed with CellQuest software (BD Immunocytometry Systems, San Jose, CA).
Quantitative RT-PCR
cDNA was synthesized from thymic total RNA with oligo(dT).40 cDNA (100 ng/reaction) samples with 2 × TaqMan Reaction Mix (Applied Biosystems, Foster City, CA), 5 pmol of each primer (Invitrogen, Carlsbad, CA), and 10 pmol of probe (Biosource, Carlsbad, CA) were used for real-time reverse transcriptase–polymerase chain reaction (RT-PCR). Each reaction was performed in triplicate by using ABI 7700 Sequence Detector, and data were analyzed with Seq detector system (Applied Biosystems). PCR cycle condition was 95°C for 15 seconds and 60°C for 1 minute. The following primer and probe sets were used for RT-PCR. β-actin primers 5′-CAACGAGCGGTTCCGATG-3′, 5′-ATGGATGCCACAGGATTCCAT-3′, probe 5′-AGGCTCTTT;TCCAGCCTTCCTTCTTGG-3′; EVA: primers, 5′-GGCTGGCTTTCCCTGATGTAT-3′, 5′-TTAACCGAACATCTGTCCCGT-3′ and probe, 5′-AAGAGCCCCGCGCTTGTGCTTC-3′; and IL-7: primers, 5′-GGAATTCCTCCACTGATCCTTG-3′, 5′-TTCCTGTCATTTTGTCCAATTCA-3′ and probe, 5′-CTGCTGCCTGTCACATCATCTGAGTGC-3′. β-Actin was used to normalize cDNA inputs.
KLH immunization
KLH (Calbiochem, San Diego, CA) immunization with complete Freund adjuvant (Sigma, St Louis, MO) for primary immunization or incomplete Freund adjuvant (Sigma) for secondary immunization was performed subcutaneously to mice (50 μg/mouse) on days 31 and 45 following PBS or KGF treatment. Serum was collected from KLH-immunized mice 2 weeks after each immunization. Anti-KLH antibody titer of each isotype in the serum was determined by sandwich enzyme-linked immunosorbent assay (ELISA).42
Immunohistochemistry
Cryosections (5 μm) were immediately fixed with ice-cold acetone and were incubated with normal donkey serum for 10 minutes to block nonspecific binding of antibodies. Thymic sections were stained with anti–mouse keratin 5 (Tromas-1; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) and anti–keratin 8 (Covance Research Products, Berkeley, CA) in 1% donkey serum. For negative controls, normal rat and rabbit serum were used for staining instead of anti-K5 and -K8. FITC-donkey anti–rat IgG and Cy5-donkey anti–rabbit IgG (Jackson Laboratory) were used as secondary antibodies. Mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) was used to stain nuclei (Vector Laboratories, Burlingame, CA). Images were viewed with a Leica DM RXA-RF-8 fluorescence microscope (Leica, Bannockburg, IL) using 5×/0.12 NA, HC Plan 10×/0.4NA, or HC Plan 20×/0.7 NA objective lenses for 50×, 100×, and 200× magnification, respectively. A SkyVision-2/VDS-1300 12-bit digital camera (Applied Spectral Imaging, Carlsbad, Ca) with Easy FISH software (Migdal Ha'emek, Israel) was used to acquire images.
Statistical analysis
Differences in cell numbers and transcript levels between groups were analyzed by Student t test.
Results
Increased thymopoiesis in KGF-treated mice
In order to examine the effect of KGF on thymopoietic capacity, young (6-8 weeks old) and aged (15-20 months old) mice were injected with placebo (PBS) or KGF (5 mg/kg per day) for 3 days, and their thymic cellularity was analyzed 4 weeks later (Figure 1A). The thymic cellularity of untreated and placebo-treated aged mice was only 20% to 30% that of the young mice. KGF treatment significantly increased the number of thymocytes (Thy1.2+ cells) in both young and old mice. At 1 month after KGF treatment, thymic cellularity in young mice increased 2-fold, and the number of thymocytes in KGF-treated aged mice increased 5-fold, resulting in the equivalent thymic cellularity to that of normal, untreated young mice. In our previous study of KGF treatment of young HSC transplant recipients, the effect of KGF was maintained for at least 3 months, after a single 3-day course of treatment.40 To test the duration of enhanced thymopoiesis by KGF treatment in aged mice, the thymic cellularity of aged mice was serially analyzed following either a single 3-day KGF or PBS treatment (Figure 1A). Increased thymic cellularity was observed for 2 months after a single course of KGF compared with the placebo control, although the thymic cellularity reached a peak at 1 month after KGF treatment and then declined over time. By 3 months after a single course of KGF treatment, the thymic cellularity of KGF-treated mice was similar to PBS-treated mice. Thus, KGF treatment enhanced thymopoiesis in mice regardless of age (at least up to 15 months of age), and transiently increased thymic cellularity for 2 months after a single 3-day course of KGF treatment.
KGF treatment restores thymopoiesis in aged mice. The thymic cellularity of young (6 weeks old, n = 5) and aged mice (15 months old, n = 5-11) was analyzed 1 to 3 months after a single 3-day course of PBS or KGF treatment. (A) Mean absolute thymocyte numbers ± SD from either PBS- or KGF-treated young and aged mice. *P < .05, PBS versus KGF; †P < .05, 1 month versus 2 months; ‡P < .05, 2 months versus 3 months; NS indicates not significant. ⊡ indicates PBS-treated mice; □, KGF-treated mice. (B) Increases in the number of TN, DP, SP CD4, and SP CD8 thymic subsets 1 month after KGF treatment. The fold increase of the number of thymocyte subsets is shown in parenthesis. *P < .05, PBS versus KGF.
KGF treatment restores thymopoiesis in aged mice. The thymic cellularity of young (6 weeks old, n = 5) and aged mice (15 months old, n = 5-11) was analyzed 1 to 3 months after a single 3-day course of PBS or KGF treatment. (A) Mean absolute thymocyte numbers ± SD from either PBS- or KGF-treated young and aged mice. *P < .05, PBS versus KGF; †P < .05, 1 month versus 2 months; ‡P < .05, 2 months versus 3 months; NS indicates not significant. ⊡ indicates PBS-treated mice; □, KGF-treated mice. (B) Increases in the number of TN, DP, SP CD4, and SP CD8 thymic subsets 1 month after KGF treatment. The fold increase of the number of thymocyte subsets is shown in parenthesis. *P < .05, PBS versus KGF.
Impaired thymopoiesis in the aged thymus is associated with an increased percentage of apoptotic TN thymocytes.43 It was hypothesized that the limited number of immature TN thymocytes results in reduced numbers of thymic subpopulations at subsequent differentiation stages.43 To elucidate which thymic subpopulations expand in response to KGF, the number of cells in each thymic subpopulation was analyzed (Figure 1B). In young mice, there was an approximately 2-fold increase in the numbers of TN, DP, and SP CD4 and CD8 thymocytes after KGF treatment. In aged mice, the number of TN and DP thymocytes increased over 4-fold, while the number of SP CD4 and CD8 thymocytes increased 2.5- to 3-fold. These results demonstrated that in aged mice, KGF corrects a microenvironmental defect in the generation or survival of immature thymocytes, which likely leads to increased numbers of SP thymocytes.
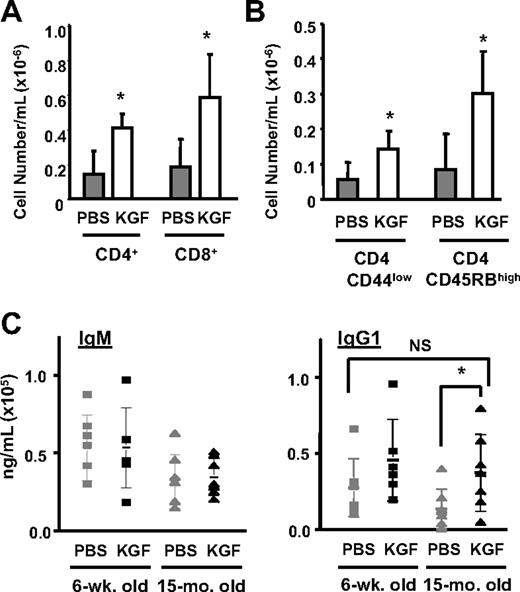
To determine whether the increased thymopoiesis induced by KGF resulted in changes in peripheral T-cell populations, the number of mature T cells in the blood was analyzed at day 28 after KGF treatment. The KGF-treated aged mice had increased numbers of mature CD4 and CD8 cells in the blood (Figure 2A). The CD4 T cells had a naive phenotype (CD4+ CD44low and CD4+ CD45RB) (Figure 2B). While homeostatic expansion of pre-existing naive T cells in the periphery cannot be excluded, the results are most consistent with the increase in mature T cells being due to increased production of naive T cells from the thymus.
KGF treatment increases the number of T cells and T-cell–dependent antibody production in the periphery. (A) Blood was drawn from young or aged mice (n = 5-7 per group) 1 month after PBS or KGF treatment, and analyzed for mature CD4+ and CD8+ T cells. ⊡ indicates PBS-treated aged mice; □, KGF-treated aged mice. *P < .05, PBS versus KGF. (B) The mean absolute cell number ± SD of naive CD4+ T cells that were CD45RBhigh and CD44low in the blood from PBS- or KGF-treated young or aged mice (n = 5-7 per group). *P < .05, PBS versus KGF. (C) KLH-specific IgM and IgG1 titer in young and aged mice. Mice were immunized with KLH at day 31 and day 45 after initiation of PBS or KGF treatment. Anti-KLH titer (mean ± SD shown) was measured 2 weeks after each immunization (n = 7 per group). *P < .05, PBS-treated aged mice versus KGF-treated aged mice; NS indicates not significant.
KGF treatment increases the number of T cells and T-cell–dependent antibody production in the periphery. (A) Blood was drawn from young or aged mice (n = 5-7 per group) 1 month after PBS or KGF treatment, and analyzed for mature CD4+ and CD8+ T cells. ⊡ indicates PBS-treated aged mice; □, KGF-treated aged mice. *P < .05, PBS versus KGF. (B) The mean absolute cell number ± SD of naive CD4+ T cells that were CD45RBhigh and CD44low in the blood from PBS- or KGF-treated young or aged mice (n = 5-7 per group). *P < .05, PBS versus KGF. (C) KLH-specific IgM and IgG1 titer in young and aged mice. Mice were immunized with KLH at day 31 and day 45 after initiation of PBS or KGF treatment. Anti-KLH titer (mean ± SD shown) was measured 2 weeks after each immunization (n = 7 per group). *P < .05, PBS-treated aged mice versus KGF-treated aged mice; NS indicates not significant.
Improved immune function in KGF-treated aged mice
Abnormal T-cell–dependent antigen-specific antibody responses (eg, after immunization) are observed in aged humans and mice.44–46 To test whether KGF-induced thymopoiesis restores functional immune responses in aged mice, placebo- or KGF-treated mice were challenged with keyhole limpet hemocyanin (KLH) as a neoantigen, and antigen-specific antibody production was analyzed. KLH immunization elicited IgM and IgG responses regardless of age. However, the overall production of KLH-specific antibodies was lower in aged mice compared with young mice. KGF treatment in aged mice specifically enhanced IgG1 production, which requires both functional T cells and B cells, but did not significantly change IgM production, which is less dependent on T-cell help (Figure 2C). The immunization results indicate that KGF treatment improved functional T-cell–dependent antigen-specific responses.
Maintenance of increased thymopoiesis by repeated KGF treatment
Since the increased thymic cellularity after a single course of KGF treatment significantly declined over time (Figure 1A), the effect of repeated KGF treatment on thymopoiesis in aged mice was tested (Figure 3). We compared the number of thymocytes in aged mice that received either a single 3-day course of KGF treatment or 3 monthly 3-day courses of KGF. Consistent with the initial result as shown in Figure 1A, thymic cellularity in aged mice 3 months after a single course of KGF was similar to that of placebo-treated mice. However, monthly administration of 3-day courses of KGF treatment maintained enhanced thymopoiesis for up to 3 months. The frequency of each thymic subpopulation was similar to that observed after a single course (Figure 1B, and data not shown).
Repeated KGF treatment (3 monthly courses of 3 days each) maintained enhanced thymopoiesis in aged mice. The thymic cellularity was analyzed after a single course or multiple courses of KGF treatment to aged mice. Mean absolute thymocyte numbers ± SD from PBS-treated young mice (▥); aged mice evaluated 3 months after PBS treatment (⊡); aged mice 3 months after a single course of KGF treatment (□); or aged mice 3 months after initiation of monthly courses (3) of KGF treatment (▧) (n = 5 in each group). *P < .05; NS indicates not significant.
Repeated KGF treatment (3 monthly courses of 3 days each) maintained enhanced thymopoiesis in aged mice. The thymic cellularity was analyzed after a single course or multiple courses of KGF treatment to aged mice. Mean absolute thymocyte numbers ± SD from PBS-treated young mice (▥); aged mice evaluated 3 months after PBS treatment (⊡); aged mice 3 months after a single course of KGF treatment (□); or aged mice 3 months after initiation of monthly courses (3) of KGF treatment (▧) (n = 5 in each group). *P < .05; NS indicates not significant.
Increased intrathymic IL-7 expression in KGF-treated mice
Decreased intrathymic IL-7 production is one of the proposed mechanisms of age-related thymic insufficiency.14,15 Although decreased intrathymic Il-7 production has been shown during aging, analyses of overall TEC number have differed, with one paper reporting no change while another demonstrated TEC decline.47,48 To determine whether KGF altered the numbers of TECs or intrathymic IL-7 production, thymuses from treated mice were analyzed for RNA levels of EVA, a constitutively expressed epithelial gene that can be used as a surrogate to quantify epithelial cells, and of IL-7.49 Thymuses were collected on day 8 after initiation of KGF treatment. Both EVA and IL-7 expression were significantly decreased with aging (Figure 4A). The reduction in both intrathymic EVA and IL-7 expression in aged mice is consistent with the loss of TECs causing reduced intrathymic IL-7 production. KGF treatment increased both intrathymic EVA and IL-7 expression in relatively equal proportion. The level of intrathymic IL-7 expression in KGF-treated aged mice was comparable with that of placebo-treated young mice. Since in vitro treatment with KGF (100 ng/mL × 3 days) did not induce EVA expression by a cortical TEC clone in vitro (data not shown), the proportional increase in EVA and IL-7 expression in the KGF-treated aged mice suggests that KGF increased the numbers of IL-7–producing TECs. KGF treatment of IL-7−/− mice failed to increase thymopoietic capacity even in young mice (data not shown), indicating that either IL-7 is essential for the effects of KGF on thymopoiesis or the profound defect of IL-7−/− mice cannot be overcome by KGF. The results are most consistent with KGF-induced increases in the number of IL-7–producing TECs leading to enhanced thymopoiesis.
KGF treatment normalized intrathymic IL-7 expression, thymic architecture, and TEC organization in aged mice. TEC organization in aged mice was compared 8 days or 1 month after initiation of a single 3-day course of PBS or KGF treatment. (A) Total RNA from whole thymus on day 8 after initiation of PBS or KGF treatment was analyzed for the epithelium-specific transcript EVA and IL-7 by quantitative RT-PCR (n = 5 per group). Each transcript level was normalized to β-actin expression. *P < .05; NS indicates not significant. (B) H&E staining of thymic sections from normal young PBS- or KGF-treated aged mice. (C) Immunofluorescence staining for K5 and K8, and DAPI nuclear staining. The thymus in aged mice displayed indistinct corticomedullary junctions and decreased TEC populations with areas that were completely devoid of keratin-positive TECs (asterisk). KGF treatment normalized TEC organization in aged mice. The magnification was 200 ×.
KGF treatment normalized intrathymic IL-7 expression, thymic architecture, and TEC organization in aged mice. TEC organization in aged mice was compared 8 days or 1 month after initiation of a single 3-day course of PBS or KGF treatment. (A) Total RNA from whole thymus on day 8 after initiation of PBS or KGF treatment was analyzed for the epithelium-specific transcript EVA and IL-7 by quantitative RT-PCR (n = 5 per group). Each transcript level was normalized to β-actin expression. *P < .05; NS indicates not significant. (B) H&E staining of thymic sections from normal young PBS- or KGF-treated aged mice. (C) Immunofluorescence staining for K5 and K8, and DAPI nuclear staining. The thymus in aged mice displayed indistinct corticomedullary junctions and decreased TEC populations with areas that were completely devoid of keratin-positive TECs (asterisk). KGF treatment normalized TEC organization in aged mice. The magnification was 200 ×.
Restoration of TEC organization in KGF-treated age mice
Aging results in abnormalities of thymic architecture marked by loss of corticomedullary distinction and areas of absent TECs, and in humans, infiltration of the perivascular space by fat cells.50–52 To study whether KGF altered the thymic microenvironment, we analyzed thymic architecture histologically and by analyses of cortical and medullary TEC subsets. The cortex in the aged thymus was notably hypoplastic and corticomedullary junctions were less distinct, compared with the young thymus (Figure 4B).
Immunofluorescent staining for keratin 8 (K8) and keratin 5 (K5) as markers of cortical and medullary TECs, respectively, was used to characterize TEC localization and organization. The thymus from young mice demonstrated a well-organized cortex with the K5−K8+ TEC subset, K5+K8− TECs clustered in distinct medullary regions, and TECs in a dense meshlike structure in both cortical and medullary regions (Figure 4C). Consistent with the hematoxylin-eosin staining, the thymus of aged mice had disorganized compartmentalization of cortical and medullary TECs, increase in the numbers of K5+K8+ immature TECs, and extensive loss of TEC populations marked by epithelium-free areas with no cytokeratin staining, a finding previously reported in the aged murine thymus.50 To determine the effect of KGF on the microenvironmental architecture of aged mice, thymic organization was analyzed 8 days after initiation of a single 3-day course of KGF treatment. KGF treatment normalized TEC organization in the aged thymus. The thymus from the KGF-treated mice displayed a distinctive cortical and medullary structure with a widespread meshlike distribution of TECs and lacked the epithelium-free areas seen in the placebo-treated group (Figure 4C). Because the defects in TEC organization were more profound in 20-month-old mice, with sparse localization of K5−K8+ TECs in the cortex and predominant K5+K8− TECs, the effects of KGF in these mice was also examined. KGF normalized TEC organization in 20-month-old mice, an effect that was observed even one month after a single course of KGF treatment (Figure 4C). KGF-treated 20-month-old mice displayed well-organized TEC compartments comparable with the thymus of young mice. Therefore, KGF not only increases TEC numbers, but also normalizes TEC organization in aged mice.
Improved thymopoiesis after KGF treatment in the Klotho premature aging model
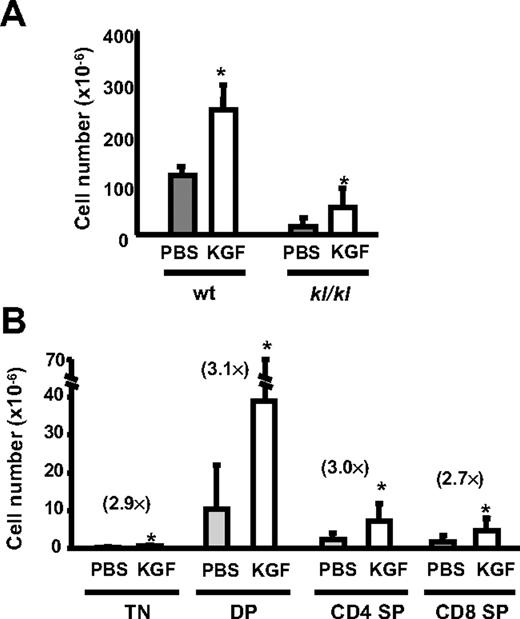
To test whether KGF reverses thymic involution due to deficiency of klotho, kl/kl mice and wt littermates were treated with PBS or KGF. Since kl/kl mice have a short life span (8-10 weeks), KGF was injected into 2-week-old kl/kl mice, and the thymic cellularity was analyzed 4 weeks later. KGF resulted in a 2-fold increase of thymic cellularity in kl/kl mice as well as wt littermates (Figure 5A). Similar to the results seen with chronologically aged wt mice (Figure 1B), KGF had a somewhat greater effect on the numbers of TN and DP thymocytes than of SP thymocytes. Despite these improvements, the KGF-treated kl/kl mice still exhibited reduced thymic cellularity compared with wt littermates. Because of the rapid acceleration of aging in kl/kl mice, the inability to fully correct thymopoiesis with KGF may be due to global declines in the animals' health. The data indicate that KGF also enhances thymopoiesis in a genetic model of age-related degeneration.
KGF treatment increases thymic cellularity and intrathymic IL-7 expression in kl/kl mice. wt littermates (2 weeks old, n = 5) or kl/kl mice (2 weeks old, n = 12) were treated with PBS or KGF for 3 days, and the thymic cellularity was analyzed on day 31. (A) The total thymic cellularity and (B) the absolute numbers of TN, DP, CD4 SP, and CD8 SP thymic subpopulations are shown; results are ± SD. The fold increase of the number of thymocyte subsets is shown in parenthesis. *P < .05, PBS versus KGF.
KGF treatment increases thymic cellularity and intrathymic IL-7 expression in kl/kl mice. wt littermates (2 weeks old, n = 5) or kl/kl mice (2 weeks old, n = 12) were treated with PBS or KGF for 3 days, and the thymic cellularity was analyzed on day 31. (A) The total thymic cellularity and (B) the absolute numbers of TN, DP, CD4 SP, and CD8 SP thymic subpopulations are shown; results are ± SD. The fold increase of the number of thymocyte subsets is shown in parenthesis. *P < .05, PBS versus KGF.
To determine the mechanism of KGF-induced improvements in thymopoiesis in kl/kl mice, we analyzed intrathymic EVA and IL-7 expression on day 8 after initiation of KGF treatment (Figure 6A). The previously reported reduced IL-7 expression by bone marrow stromal cells in kl/kl mice suggested that the thymic defect in kl/kl mice may be also caused by IL-7 deficiency.17 Intrathymic IL-7 expression in 2-week-old kl/kl mice was comparable with that of wt littermates. On day 8 after initiation of KGF treatment of 2-week-old kl/kl mice, both EVA and IL-7 expression were significantly higher than that of either PBS-treated kl/kl or untreated wt (littermate) mice. However, intrathymic IL-7 expression was significantly decreased in 6-week-old kl/kl mice, and KGF treatment of 6-week-old kl/kl mice failed to induce TEC proliferation and intrathymic IL-7 expression (data not shown). These results indicate that as age-related thymic degeneration progresses in the kl/kl mice, an irreversible stage is reached where KGF can no longer restore the number of IL-7–producing TECs.
Premature thymic involution in kl/kl mice is related to disruption of thymic microenvironmental organization, which is restored by KGF. (A) The intrathymic levels of EVA, an epithelial cell–specific marker, and IL-7 transcripts in wt littermates or kl/kl mice (2 weeks old, n = 5 per group) were analyzed by quantitative RT-PCR on day 8 after initiation of PBS or KGF treatment. Each transcript level was normalized to β-actin expression. *P < .05; NS indicates not significant. (B)Thymic architecture of wt and kl/kl mice was compared 1 month after PBS or KGF treatment by H&E staining and immunofluorescence staining of K8, K5, and DAPI for nuclear staining. Arrows indicate cysts observed in the kl/kl thymus. The magnification was 100 × for H&E staining and 200 × for immunofluorescence staining.
Premature thymic involution in kl/kl mice is related to disruption of thymic microenvironmental organization, which is restored by KGF. (A) The intrathymic levels of EVA, an epithelial cell–specific marker, and IL-7 transcripts in wt littermates or kl/kl mice (2 weeks old, n = 5 per group) were analyzed by quantitative RT-PCR on day 8 after initiation of PBS or KGF treatment. Each transcript level was normalized to β-actin expression. *P < .05; NS indicates not significant. (B)Thymic architecture of wt and kl/kl mice was compared 1 month after PBS or KGF treatment by H&E staining and immunofluorescence staining of K8, K5, and DAPI for nuclear staining. Arrows indicate cysts observed in the kl/kl thymus. The magnification was 100 × for H&E staining and 200 × for immunofluorescence staining.
Thymic organization in the kl/kl mice was analyzed by H&E staining and immunohistochemistry for K8 and K5 (Figure 6B). At 2 weeks of age, before the thymus underwent progressive degeneration, the kl/kl thymus resembled normal young thymus with distinctive corticomedullary junctions, compartmentalization of K5−K8+ and K5+K8− TECs in cortex and medulla, respectively, and no epithelium-free areas. However, thymic organization was completely disrupted in placebo-treated 6-week-old kl/kl mice. The thymic disorganization was more severe than that of chronologically aged wt mice. The mutant thymus had an aberrant compartmentalization with intermingled K5−K8+ cortical and K5+K8− medullary TECs. In addition, the K5+K8− medullary TECs were more predominant in the kl/kl mice, and an increased frequency of immature K5+K8+ TECs and epithelium-free areas and cysts was also observed. Thymic organization in kl/kl mice was dramatically improved by KGF treatment even though the kl/kl thymus was still hypoplastic compared with the wt thymus. KGF-treated kl/kl mice displayed increased TEC populations with distinct corticomedullary junctions. These results implicate KGF-sensitive degeneration of the TEC compartment in the thymopoietic defect in kl/kl mice.
Discussion
We have demonstrated improved thymopoiesis induced by KGF in 2 murine aging models—chronological aging and that induced by the loss of function of klotho. The studies demonstrated that KGF treatment increased thymopoietic capacity, resulting in increased numbers of mature T cells in the periphery. Furthermore, KGF treatment improved T-cell–dependent antibody responses to KLH with increased class-switching and amplification. We have also demonstrated that a likely mechanism for age-related decline in thymic function is the loss of IL-7–producing TECs. KGF not only increases the number of TECs but also has morphogenic effects with correction of structural abnormalities such as loss of corticomedullary distinction and development of epithelium-free areas. These data suggest that TEC progenitors persist despite thymic aging, and that with appropriate stimulation, such progenitors can regenerate the thymic microenvironment. Although the effects of KGF treatment appear to be transitory, sustained improvements in thymic function were achieved by repeated KGF administration.
The experimental results support the concept that microenvironmental defects, rather than defects of lymphohematopoietic progenitors alone underlie age-related thymic involution. KGF treatment reversed age-related thymic involution as well as restored thymic architecture in the mice, even though the mice still had their own aged hematopoietic stem cells. KGF is unlikely to have direct effects on lymphohematopoietic progenitors since they do not express the epithelial-specific FGFR2-IIIb receptor. The data do not exclude the possibility that aging of HSCs or lymphohematopoietic progenitors contributes to thymic involution. A previous study of aged HSCs suggested a loss of the ability to home and engraft recipient marrow, but the ability to form immature thymocyte progenitors was undiminished.53 However, studies of the numbers and lymphoid differentiation potential of aged progenitors have shown quantitative decreases with aging.10,11 It is not known whether these observed changes with aging are primary to HSCs or their lymphoid progeny versus secondary to microenvironmental changes. The changes in HSCs and progenitors may affect transplantability of aged HSCs, which would explain why isolated aged lymphohematopoietic progenitors have shown decreased capacity to restore thymopoiesis in some transplant or thymic culture experiments.8–12 Whether changes in engraftment characteristics of HSCs or lymphoid progenitors affect normal steady-state thymopoiesis during aging is not known.
KGF has been shown to have cytoprotective effects on various epithelial cells, including TECs. In both preclinical animal models of injury from cytotoxic agents (radiation, chemotherapy) and clinical trials, KGF administration prior to the cytotoxic agent results in decreased damage to skin and gut epithelium.54–60 Because of these cytoprotective effects, KGF (palifermin) has been approved as an adjunct to protect epithelial tissues from injury caused by radiation and chemotherapy used in cancer chemotherapy and bone marrow transplantation.61,62 In a previous study, we demonstrated that KGF administration before HSCT corrects thymopoietic defects by protecting IL-7–producing TECs from cytotoxic effects of irradiation and/or chemotherapy.40 Recently, it was reported that KGF−/− mice have normal steady-state thymopoiesis, but impaired thymic recovery after sublethal irradiation.39 The same report indicated that 16-month-old KGF−/− mice had levels of thymic cellularity that were comparable with normal aged controls. Thus, KGF signaling appears to be necessary for recovery from cytotoxic agents, but endogenous production neither prevents age-related thymic involution nor is it necessary to prevent accelerated thymic aging.
Besides the restoration of TEC numbers and intrathymic IL-7 production in aged mice (Figures 4A, 6A), KGF has potent effects on thymic architecture. Previous studies of mice with loss of FGFR2-IIIb signaling demonstrated severe abnormalities in thymic development, but as noted in the paragraph above, loss of KGF did not appear to affect thymic development.33,39 Thus, it is likely that at least one of the other ligands for FGFR2-IIIb, most likely FGF10, is complementary for thymic development.63 The thymus of mice with loss of FGFR2-IIIb or Fgf10 resulted in an amorphous histologic appearance lacking corticomedullary distinction, with a paucity of thymocytes, and abnormal differentiation of cortical TECs.33 A feature of thymic aging is the development of abnormal architecture with loss of corticomedullary distinction and the appearance of areas devoid of epithelial cells.50 KGF administration rapidly (within 8 days) improved the structural abnormalities seen during aging. Cortical and medullary regions became more distinct, and epithelium-free zones decreased in size and number. A crucial question that the present experiments do not resolve is whether the morphogenic effects of KGF on the aged thymic microarchitecture were due to KGF-mediated differentiation of immature TEC progenitors versus separate increases in mature cortical and medullary TECs. Fetal TEC progenitors can give rise de novo to a full thymic microenvironment after heterotopic implantation.28,29 If the regeneration of the thymic microenvironment is mediated by KGF action on TEC progenitors, then the number and differentiation potential of such progenitors could be limiting in aging. The frequency but not absolute numbers of TEC progenitors expressing the MTS24 epithelial progenitor surface marker is reduced in young adult mice. Of interest, analyses of the kl/kl mice prior to thymic involution have shown a high frequency of K5+K8+ and MTS24+ cells compared with wt littermates, suggesting a relative persistence of immature TEC progenitors (Figure 6B and data not shown). In the kl/kl mice, KGF was unable to restore the microenvironment in mice older than 6 weeks. Whether this is due to depletion of KGF-responsive cells or the overall poor health of the kl/kl mice is under investigation. In the chronologically aged mice, there was no age up to 20 months that was unresponsive to KGF, suggesting that sufficient immature TEC progenitors or individual cortical or medullary TECs with regeneration potential were still present late in life.
The mechanisms of loss or dysfunction of TECs with aging are not known. The kl/kl mouse model was chosen for analysis along with chronologically aged mice because it is a model of tissue degeneration without any evidence of genomic instability, a phenomenon also observed in mice that express a truncated p53 lacking the amino-terminus.64 The thymic microenvironmental defects seen in the kl/kl and chronologically aged mice were qualitatively similar, although more severe in the kl/kl mice. Besides the klotho locus, the kl/kl mice (C3H) differed from the chronologically aged mice (C57BL/6) in their genetic background; the C57BL/6 background is associated with delayed thymic involution.65 For this reason, the results of KGF treatment of kl/kl mice were compared with the wt littermates. One reason that we were interested in degenerative models of aging is the parallels between the dermal atrophy seen in such aging models and the TEC abnormalities. Both phenotypic and functional analogies exist between skin cells and TECs.66,67 Such parallels had previously led us to test whether KGF could protect TECs from cytotoxic injuries that predominantly affected skin and mucosal epithelial cells.40 It has recently been demonstrated that klotho binds to multiple FGF receptors. Most prominent of these is the FGFR1C, which is a receptor for FGF23, a regulator of vitamin D metabolism.67 Of note, FGF23−/− mice share with kl/kl mice phenotypes of premature aging.69 It is not known whether klotho modulates FGFR2-IIIb–mediated signaling, a potential mechanism that would directly connect klotho to the TEC development pathway.
The alterations in the thymic microenvironment induced by KGF had profound effects on thymopoiesis that resulted in increased numbers of peripheral T lymphocytes and improved immune function. While the present studies were being performed, Alpdogan et al reported that KGF treatment of aged mice increased the number of thymocytes and phenotypic T lymphocytes in the periphery.39 Besides providing a mechanism for the effects of KGF on age-related thymic involution, the present studies confirm the previous results of increased peripheral T cells induced by KGF treatment, and extend the findings by demonstrating functional improvement in immunity. KGF-treated mice had antibody responses to KLH, a T-cell–dependent neoantigen. These results are highly clinically relevant. Poor immune responsiveness among the elderly results in increased susceptibility and severity of infection, as well as suboptimal responses to immunization. An example of the severity of this problem is the recent description of as many as 170 000 hospitalizations and 14 000 deaths per year in the United States among elderly patients infected with respiratory syncytial virus, a pathogen usually thought of as a childhood illness.70 While immunization is a logical approach to protect the elderly from infectious agents, the low rate of successful immunization among the elderly is a serious obstacle to vaccine-based strategies.71 Both antibody- and cell-mediated immunity are affected by aging.72 The demonstration that KGF improved the T-cell–dependent antibody response of the aged mice points to the possible use of KGF as an adjunct to immunization of the elderly.
The ability of a repeated schedule of intermittent KGF administration to sustain thymic cellularity raises the possibility that thymopoiesis could be maintained throughout life. Unlike the results from our previous study of young murine HSC transplant recipients, the effects of a single course of KGF in the old mice were not sustained. Aging may cause chronic injury to the TEC compartment that results in transient responses to KGF-mediated regeneration. Continuous in vitro stimulation of fetal thymic organ cultures with KGF resulted in decreased generation of CD4 SP thymocytes, an effect that we did not observe in vivo.37 Chronic KGF administration (7 consecutive days) to normal mice has been reported to hamper thymopoiesis by blocking thymopoiesis at the TN stage.72 However, in the present study, repeated episodic KGF treatment did not appear to adversely affect thymopoiesis.
In summary, KGF ameliorated age-related thymic insufficiency by restoring TEC organization and function. The regenerative effects of KGF on TECs may be clinically applicable to the improvement of T-cell generation and functional immune responses in the elderly.
Authorship
Contribution: D.M. designed and performed experiments, analyzed data, and wrote the paper; A.P.-M. analyzed antibody response; M.K. generated and supplied kl/kl mice, and performed analyses of klotho deficiency; G.A.H. and B.R.B. designed experiments, analyzed data, and critically reviewed the paper; K.I.W. designed experiments, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Address correspondence to: Kenneth I. Weinberg, Division of Stem Cell Transplantation, Department of Pediatrics, Stanford University, 1000 Welch Rd, Suite 301, Palo Alto, CA 94304; e-mail: kw1@stanford.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Xingchao Wang and Dr. Carolyn Lutzko of Childrens Hospital Los Angeles for assistance with preparation of tissue and image acquisition.
This work was supported by grants NIH HL70005, HL54729, HL73104 (K.I.W.); NIH HL073794 (B.R.B); Eisai Research Fund, The Ellison Medical Foundation, NIH AG19712, AG25326 (M.K.); and Swiss National Science Foundation 3100-68310.02, NIH A1057477 (G.A.H).





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal