Abstract
What governs the increased apoptosis sensitivity of HIV-specific CD8+ T cells is poorly understood. Here, we examined the involvement of mitochondria in this apoptosis. Remarkably higher mitochondrial mass (MM) was found in HIV-specific compared with CMV-specific CD8+ T cells from HIV+ patients and this could not be attributed to their different differentiation status. MMHigh phenotype characterized those CD8+ T cells from HIV+ patients that are sensitive to spontaneous and CD95/Fas-induced apoptosis. CD38 expression did not correlate with high MM, whereas Bcl-2 levels were significantly reduced in both CD38+ and CD38− HIV-specific CD8+ T cells. Although CD38+ HIV-specific CD8+ T cells were more susceptible to apoptosis, CD38 expression does not explain on its own the selective apoptosis sensitivity of HIV-specific CD8+ T cells, as CD38− HIV-specific CD8+ T cells were more apoptotic than CD38+ CMV-specific ones. Proapoptotic HIV-specific CD8+ T cells were CD38+Bcl-2LowMMHigh. Copolarization of mitochondria with CD95/Fas capping, very early in CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells, suggests that mitochondria act as an amplification step for this apoptosis. Thus, an extensive mitochondrial network contributes to apoptosis sensitivity of CD8+ T cells and, when this occurs together with reduced levels of Bcl-2 and chronic activation, determines the proapoptotic state of HIV-specific CD8+ T cells.
Introduction
CD8+ T-cell response is a critical player for controlling HIV infection and AIDS progression.1,2 However, HIV-specific CD8+ T cells ultimately fail to control the virus. Various mechanisms related either to HIV-infected cells or to intrinsic defects in CD8+ T cells themselves have been proposed to explain this failure.3–5 Among others, apoptosis of HIV-specific CD8+ T cells has been suggested as a strategy employed by HIV to evade the immune system.6,7 What governs this apoptosis sensitivity, however, remains to be elucidated.
CD8+ T cells from HIV-infected patients are susceptible to spontaneous and CD95/Fas-induced apoptosis.8–10 We recently found HIV-specific CD8+ T cells to be highly sensitive to apoptosis11 whereas this is not the case for CMV-specific CD8+ T cells from HIV+ individuals.11 This sensitivity is associated with a remarkable down-regulation of Bcl-2 and Bcl-xL antiapoptotic molecules,12 suggesting a role of mitochondria in this apoptotic process. Several studies have revealed a mitochondrial dysfunction in T cells during HIV infection.13,14 CD95/Fas cross-linking initiates a complex signaling process involving CD95/Fas capping and formation of death-inducing signaling complex (DISC),15,16 ultimately leading to activation of the executor caspase-3 directly by caspase-8 or through mitochondrial-secreted proapoptotic factors.17 The cross-talk of these 2 pathways in apoptosis of primary T cells, particularly in CD8+ T cells from HIV+ patients, is currently unknown. Furthermore, no studies have addressed the role and impact of mitochondria in the apoptosis of HIV-specific CD8+ T cells.
HIV infection results in chronic activation of the immune system, potentially contributing to T-cell sensitization to apoptosis.18,19 Furthermore, HIV-specific CD8+ T cells were shown to be skewed toward a CD38+CD8+ phenotype, whereas spontaneous and CD95/Fas-mediated apoptosis of CD38+CD8+ T cells was significantly higher in patients with high percentages of ex vivo CD38 expression on CD8+ T cells.6 These data raise the question whether chronic activation is the mechanism priming HIV-specific CD8+ T cells to apoptosis and whether CD38 expression is a predictive factor for this process.
In this study we have investigated the involvement of mitochondria in the apoptosis sensitivity of HIV-specific CD8+ T cells. We present data describing an extensive mitochondrial network selectively found in HIV-specific CD8+ T cells. Furthermore, mitochondrial mass (MM) is independent of the maturation and activation level of HIV-specific CD8+ T cells, whereas Bcl-2 expression is regulated by multiple factors in addition to chronic activation. Thus, proapoptotic HIV-specific CD8+ T cells are characterized by increased MM and reduced Bcl-2 levels on a background of chronic activation as determined by CD38 expression.
Patients, materials, and methods
Donors
Peripheral blood was collected from HIV+ individuals (n = 40) following Drexel University Institutional Review Board approval and obtaining informed consent in accordance with the Declaration of Helsinki. All individuals were HIV+ for at least 1 year (range, 1-17 years), the median value of CD4 count was 468 cells/μL (range, 70-1178 cells/μL), and the viral load was 696 RNA copies/mL blood (range, < 50 to 100 000 RNA copies/mL blood); 27 individuals were asymptomatic and 22 were on antiretroviral treatment. Control samples were obtained from HIV− age-matched healthy individuals (n = 14).
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were freshly isolated by density centrifugation using Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden) from heparinized venous blood. Flow cytometry was performed as previously described.11 HIV- and CMV-specific CD8+ T cells were identified using tetramers of HLA class I A* 0201 loaded with either HIV-Gag p17 77-85 (SLYNTVATL), HIV-Pol 476-484 (ILKEPVHGV), or CMV p65 495-503 (NLVPMVATV) peptide and HLA class I A3 loaded with HIV-Nef 71-80 (QVPLRPMTYK) peptide, as previously described.20 The following antibody combinations were used: (1) activation, anti-CD38–FITC/anti-CD8–PE–Cy5/HIV– or CMV-specific tetramer-APC; (2) Bcl-2 and Bcl-xL expression, anti-CD38–FITC/anti-CD8–PE–Cy5/HIV– or CMV-specific tetramer-APC with Bcl-2–PE (BD Biosciences, San Jose, CA) or Bcl-xL–PE (Southern Biotechnology, Birmingham, AL) antibodies; (3) apoptosis, anti-CD38–FITC/annexin V–PE/anti-CD8–PE–Cy5/HIV– or CMV-specific tetramer-APC and MitoTracker green FM/anti-CD8-PE-Cy5/annexin V–APC. Early and late apoptosis were analyzed using annexin V–Cy5.5 and Violet Live/Dead Fixable Dead Cell Stain (Invitrogen, Carlsbad, CA) together with CD8-PE–Texas Red and Gag-tetramer APC after 6-hour culture in the presence or absence of plate-bound anti-CD95 antibodies. All monoclonal antibodies were purchased from eBioscience (San Diego, CA) unless otherwise indicated. Samples were collected on a FACSCalibur and FACSAria (BD Biosciences) and data were analyzed using FlowJo software (Treestar, San Carlos, CA). Standardization and performance of the Bcl-2 and Bcl-xL intracellular stains was done as previously described.12
Apoptosis studies
Spontaneous, CD95/Fas-induced, and treatment-specific apoptosis sensitivity were determined by annexin V positivity as previously described.11
Mitochondrial mass measurement
Incubation of PBMCs with 100 nM of MitoTracker Green FM (Molecular Probes, Eugene, OR) for 45 minutes at 37°C, 5% CO2, was followed by surface staining using the following antibody combinations: anti-CD8–PE/anti-CD38–PE–Cy5/HIV– or CMV-specific tetramer-APC, anti-CD8–PE–Texas Red/anti-CD45RA–Pacific Blue/anti-CD62L–PE–Cy7/HIV– or CMV-specific tetramer APC, or anti-CD8–PE/anti-CD45RA–PE–Cy5/anti-CD62L-APC. Fixation with 1% paraformaldehyde or incubation on ice has no effect on MitoTracker staining. Samples were collected immediately after the assay was completed. For acridine orange 10-nonyl bromide (nonyl acridine orange [NAO]; Molecular Probes) staining, cells were first stained with anti-CD3–PE/anti-CD8–Pacific Blue/HIV-specific tetramer APC, washed once, and incubated with NAO (100 nM) for 15 minutes at 37°C, 5% CO2. Unfixed samples were immediately collected on a FACSAria (BD Biosciences) operated in BSL-3 facility. Forward scatter (FSC) was used as a cell-size index.
Confocal microscopy studies
CD95/Fas capping was induced in Jurkat cells as previously described.15 Briefly, 0.5 × 106 cells were incubated with CH-11 antibody (1 μg/mL) for 1 hour in ice, transferred to 37°C for 2 minutes (controls: no heat treatment), and transferred again on ice. After incubation with secondary Rhodamine Red–X–conjugated goat anti–mouse IgM antibody (Jackson Immunoresearch Laboratories, West Grove, PA), cells were transferred by cytospin onto poly-L-lysine–coated slides (Sigma-Aldrich, St Louis, MO), mounted with mounting media (Vector Laboratories, Burlingame, CA), and visualized using a confocal microscope (Leica TCS SP2; Leica Microsystems, Heidelberg, Germany) equipped with a 63×/1.4 oil-immersion objective lens (Leica). For colocalization studies, the Z-stack feature of the software was used to obtain a library of images of various sections of cells; 3D images were acquired using Leica Confocal Software (Leica Microsystems), and Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA) was used to process them. A cell was considered “capped” when the fluorescence condensed into less than 25% of the cell surface.15 For studies with primary human CD8+ T cells, 1 × 106 positively purified CD8+ T cells (Dynal ASA, Oslo, Norway) were incubated with 5μg/mL of CH-11 antibody in 100 μL media for 1 hour at 37°C, transferred onto poly-L-lysine slides, fixed for 20 minutes with 4% PFA, and processed as described above. In costaining experiments, cells were permeabilized with PBS/Triton-X 100 (0.05%) for 5 minutes after fixation, incubated in PBS–urea 5% for 10 minutes at 95°C,21 stained first with a monoclonal antiporin antibody (Clone 20B12; Molecular Probes) in PBS–FBS 2% for 1 hour at room temperature and then with secondary antibodies in PBS–FBS 2% for 30 minutes at room temperature (antimouse IgM–Rhodamine Red–X for CD95/Fas and anti–mouse IgG-FITC for porin; Jackson Immunoresearch Laboratories). To study HIV-specific CD8+ T cells, purified CD8+ T cells were first treated with anti-Fas antibody as described above, stained with tetramer, fixed (1% PFA), sorted (FACSAria; BD Biosciences), and transferred onto slides as described above. The purity of sorted HIV-specific CD8+ T cells was more than 92%. To minimize the loss of cells, a second fixation step for 20 minutes with 4% PFA was performed. CD95/Fas and porin costaining was carried out as described above.
Statistical analysis
Statistical analysis was performed using Student t test and regression analysis. P values less than .05 were considered significant. The JMP statistical analysis program was used (SAS Institute, Cary, NC).
Results
HIV-specific CD8+ T cells express high MM
Here we sought to examine the possible involvement of mitochondria in apoptosis of HIV-specific CD8+ T cells. As a first step, we measured ex vivo the MM of freshly isolated T lymphocytes from HIV+ patients and healthy donors. We used a mitochondrial-specific dye (MitoTracker Green FM)22,23 that binds mitochondrial membrane independently of the membrane potential, and thus staining intensity has been considered an index of mitochondrial mass.23–25
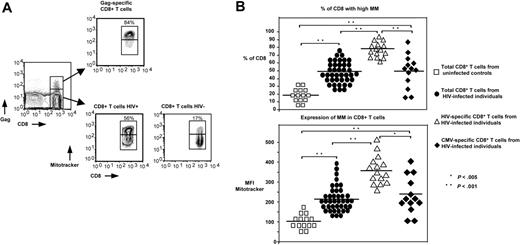
When gating on the CD8+ T cells, 2 distinct populations can be observed: one exhibiting high MM and another with low MM (Figure 1A). The percentage of HIV-specific CD8+ T cells expressing high MM (77% ± 7.2%, n = 15) was found to be significantly higher compared with CMV-specific (50% ± 5.9%, n = 13, P < .001) and total CD8+ T cells from HIV+ patients (48.5% ± 2%, n = 40, P < .001) independently of the HIV epitopes examined (A2-gag, A2-pol, A3-nef). This difference was even higher when HIV-specific CD8+ T cells were compared with total CD8+ T cells from healthy donors (21.5% ± 6.7%, n = 14, P < .001; Figure 1B). Furthermore, our analysis revealed a greatly increased mean fluorescence intensity (MFI) of MM, an index of the MM per cell, in HIV-specific CD8+ T cells (MFI = 353 ± 20) compared with CMV-specific (MFI = 244 ± 29, P < .005) and total CD8+ T cells from HIV+ individuals (MFI = 212 ± 13, P < .001) and healthy donors (Figure 1B). It should be noted that similar data were obtained when the MM was examined only in patients that exhibited both HIV- and CMV-specific CD8+ T cells. Again, the percentages of HIV-specific CD8+ T cells expressing high MM (77% ± 3%, n = 10) were found to be significantly higher compared with CMV-specific CD8+ T cells (44% ± 7.8%, n = 8, P < .001). Similarly, a significantly higher level of MM was observed in HIV-specific (MFI = 343 ± 29) compared with CMV-specific CD8+ T cells (MFI = 209 ± 33, P < .01). By using the FSC as a cell-size index, no difference was found between the low and high MM populations in all groups tested (data not shown). No correlation was found between MM and viral load, MM and CD4 counts, or MM and CD8 counts, whereas a nonsignificant trend was found between MM and disease duration (data not shown). No difference in MM was found between HIV-specific CD8+ T cells from patients under antiretroviral treatment (n = 8) compared with those from untreated patients (n = 7; 79% ± 3.8% vs 76% ± 4% of MMHigh HIV-specific CD8+ T cells from treated and untreated HIV+ patients, respectively). However, significantly lower percentages of CD8+ T cells expressing high MM were found in total CD8+ T cells from patients under antiretroviral treatment (n = 22) compared with those without treatment (n = 18; 44% ± 2.5 vs 53% ± 3.2% of MMHigh CD8+ T cells from treated and untreated HIV+ patients, respectively, P < .04). Treated HIV+ patients, however, still had significantly higher levels compared with controls (44% ± 2.5% vs 21.5% ± 6.7% of MMHigh CD8+ T cells from HIV+ [n = 22] and healthy donors [n = 14], respectively, P < .001). Further studies are needed to clarify whether measurement of MM could be used as a prognostic factor for disease progression. When NAO was used to measure MM in unfixed cells, HIV-specific CD8+ T cells again had significantly increased MM compared with CD8+ T cells from healthy controls (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). However, MM levels of HIV-specific CD8+ T cells in this assay were not higher than total CD8+ T cells from HIV+ patients. This is most likely due to the reduced sensitivity and range the NAO stain on unfixed cells has compared with MitoTracker. The standard NAO assay requires ethanol fixation, which cannot be combined with tetramer stains. Despite the limitations of using NAO on unfixed cells, these studies with NAO confirm our basic observation with MitoTracker that HIV-specific CD8+ T cells have increased MM. Our data revealed that an extensive mitochondrial network selectively characterizes HIV-specific CD8+ T cells, potentially affecting their biology compared with other virus-specific CD8+ T cells.
HIV-specific CD8+ T cells are selectively characterized by extensive MM. (A) Representative flow cytometry showing ex vivo MM in HIV-specific and total CD8+ T cells from 1 HIV+ patient and a healthy donor. Cells were gated first for lymphocytes by forward and side light scatter and then for HIV-specific and total CD8+ T cells by tetramer and CD8 staining. (B) Pooled data showing the percentage (%) of CD8+ T cells expressing high MM in total CD8+ T cells from healthy donors (□; n = 14), HIV+ patients (•; n = 40), and HIV-specific (▵; n = 15) and CMV-specific (♦) CD8+ T cells from HIV+ patients (n = 13) (top panel). Horizontal lines depict mean values. Analysis of corresponding MFI values of MitoTracker staining is also shown (bottom panel). The P values were calculated using Student t test.
HIV-specific CD8+ T cells are selectively characterized by extensive MM. (A) Representative flow cytometry showing ex vivo MM in HIV-specific and total CD8+ T cells from 1 HIV+ patient and a healthy donor. Cells were gated first for lymphocytes by forward and side light scatter and then for HIV-specific and total CD8+ T cells by tetramer and CD8 staining. (B) Pooled data showing the percentage (%) of CD8+ T cells expressing high MM in total CD8+ T cells from healthy donors (□; n = 14), HIV+ patients (•; n = 40), and HIV-specific (▵; n = 15) and CMV-specific (♦) CD8+ T cells from HIV+ patients (n = 13) (top panel). Horizontal lines depict mean values. Analysis of corresponding MFI values of MitoTracker staining is also shown (bottom panel). The P values were calculated using Student t test.
MMHigh CD8+ T cells from HIV+ patients are sensitive to apoptosis
Next, we sought to verify whether CD8+ T cells from HIV-infected patients exhibiting MMHigh phenotype are more sensitive to apoptosis compared with those that were MMLow. Measurement of CD8+ T-cell apoptosis, by using annexin V staining after overnight culture, revealed that the vast majority of CD8+ T cells undergoing spontaneous and CD95/Fas-induced apoptosis are of MMHigh origin (Figure 2A). Indeed, MMHigh CD8+ T cells were found to be more sensitive to spontaneous apoptosis (14.8% ± 1.4% annexin V+, n = 9) compared with MMLow ones (3% ± 0.4% annexin V+, n = 9, P < .001; Figure 2B). This difference was also found to be statistically significant in CD95/Fas-induced apoptosis (41.4% ± 4% annexin V+ [n = 9] versus 8.2% ± 1.7% annexin V+ [n = 9] for MMHigh and MMLow CD8+ T cells, respectively, P < .001; Figure 2B). Furthermore, treatment-specific CD95/Fas-induced apoptosis was found significantly higher in MMHigh (26.6% ± 3.2% annexin V+) compared with MMLow CD8+ T cells (5.1% ± 1.3% annexin V+, P < .001; Figure 2B). Such increased high mitochondrial mass was also found in early apoptotic cells after 6-hour anti-CD95 antibody treatment (Figure S2). We found no difference in MM when early and late apoptotic Gag-specific and total CD8+ T cells were compared. Our data clearly demonstrate that increased MM is linked to apoptosis sensitivity of CD8+ T cells from HIV+ patients.
MMHigh CD8+ T cells from HIV+ patients are highly sensitive to apoptosis. (A) Representative plots showing the gating strategies for lymphocytes, and histograms showing MM and annexin V positivity in total CD8+ T cells after culturing PBMCs from an HIV+ patient for 14 hours in the absence or presence of anti-CD95/Fas antibody. Apoptosis was measured in MMHigh and MMLow CD8+ T cells by annexin V staining. (B) Pooled data showing the percentage (%) of annexin V+ CD8+ T cells in MMHigh and MMLow CD8+ T cells from HIV+ individuals (n = 9). Bars depict means ± SE. The P values were calculated using Student t test.
MMHigh CD8+ T cells from HIV+ patients are highly sensitive to apoptosis. (A) Representative plots showing the gating strategies for lymphocytes, and histograms showing MM and annexin V positivity in total CD8+ T cells after culturing PBMCs from an HIV+ patient for 14 hours in the absence or presence of anti-CD95/Fas antibody. Apoptosis was measured in MMHigh and MMLow CD8+ T cells by annexin V staining. (B) Pooled data showing the percentage (%) of annexin V+ CD8+ T cells in MMHigh and MMLow CD8+ T cells from HIV+ individuals (n = 9). Bars depict means ± SE. The P values were calculated using Student t test.
MM is independent of the differentiation level of HIV-specific CD8+ T cells
Contrary to CMV-specific CD8+ T cells, HIV-specific CD8+ T cells exhibit a skewed maturation toward the preterminally differentiated (CD45RA−CCR7−CD62L−) effector memory compartment.11,26 Therefore, the observed differences in MM between the 2 virus-specific CD8+ T populations could be attributed, at least in part, to their different maturation level. We therefore directly investigated the levels of MM in relation to maturation status of virus-specific CD8+ T cells. Comparable percentages of MMHigh cells were found between the CD45RA−CD62L− (73.1% ± 6%, n = 7) and CD45RA+CD62L− (67.6% ± 6.1%, n = 7) memory compartments of HIV-specific CD8+ T cells (Figure 3A-B). CMV-specific CD8+ T cells also had comparable percentages of MMHigh cells in CD45RA−CD62L− (34.5% ± 14.1%, n = 5) and CD45RA+CD62L− (31.9% ± 14.5%, n = 5) subsets. Therefore differences of MM between HIV-specific and CMV-specific CD8+ T cells from HIV+ patients cannot be attributed to differences in their maturation status.
No relationship between differentiation status and MM levels in HIV- and CMV-specific CD8+ T cells from HIV+ patients. (A) Representative flow cytometry showing MM and memory populations in HIV- and CMV-specific CD8+ T cells from an HIV+ patient. Memory populations were identified by CD45RA and CD62L staining. Histograms depict the MM levels in CD45RA−CD62L− and CD45RA+CD62L− memory subsets of HIV- and CMV-specific CD8+ T cells. (B) Pooled data showing the percentage of MMHigh cells in CD45RA−CD62L− and CD45RA+CD62L− memory HIV-specific (n = 7) and CMV-specific (n = 5) CD8+ T cell populations. Horizontal bars depict means.
No relationship between differentiation status and MM levels in HIV- and CMV-specific CD8+ T cells from HIV+ patients. (A) Representative flow cytometry showing MM and memory populations in HIV- and CMV-specific CD8+ T cells from an HIV+ patient. Memory populations were identified by CD45RA and CD62L staining. Histograms depict the MM levels in CD45RA−CD62L− and CD45RA+CD62L− memory subsets of HIV- and CMV-specific CD8+ T cells. (B) Pooled data showing the percentage of MMHigh cells in CD45RA−CD62L− and CD45RA+CD62L− memory HIV-specific (n = 7) and CMV-specific (n = 5) CD8+ T cell populations. Horizontal bars depict means.
MM does not correlate with CD38 expression on HIV-specific CD8+ T cells
Next, we attempted to investigate whether the MM of HIV-specific CD8+ T cells associates with their activation status (expression of the CD38 surface marker). A significantly increased percentage of CD38+ cells was found in HIV-specific (31.9% ± 5.2%, n = 10), CMV-specific (39.9% ± 3.1%, n = 17), and total CD8+ T-cell populations (32.6% ± 2.9%, n = 35) from HIV+ patients compared with control CD8+ T cells (13% ± 1.4%, n = 14, P < .007; Figure 4A). This is in agreement with previously published data,6,27 including our own studies.11 When the cell size was considered, no difference was found between CD38+CD8+ and CD38−CD8+ T cells in all groups tested (data not shown). MM was then measured in CD38+ and CD38−CD8+ T-cell populations. Similar percentages of MMHigh CD8+ T cells were found to be expressed in both populations (89% ± 2% vs 84% ± 2.4% [n = 4]; 57% ± 19% vs 44% ± 18% [n = 6]; and 53% ± 8% vs 44% ± 10% [n = 8] for CD38− and CD38+ HIV-specific, CMV-specific, and total CD8+ T cells from HIV-infected individuals, respectively; Figure 4B). Similarly, no difference was found when the percentage of CD38+CD8+ T cells was analyzed in the MMHigh and MMLow CD8+ T cells (data not shown). Collectively, although our data reveal an increased activation status of virus-specific CD8+ T cells in HIV infection, the MM of these cells does not correlate with their activation status.
MM is independent of the activation level of HIV-specific CD8+ T cells. (A) Representative flow cytometry showing HIV- and CMV-specific CD8+ T cells from an HIV+ patient. Histograms depict ex vivo levels of CD38 in HIV-specific, CMV-specific, and CD8+ T cells from a healthy donor (left panel). Pooled data showing the ex vivo percentages (%) of CD38+ cells in HIV-specific (□; n = 10), CMV-specific (▪; n = 17), and total CD8+ T cells from HIV+ patients (♦; n = 35) and healthy donors (○; n = 14) (right panel). (B) Representative flow cytometry showing ex vivo MM in CD38+ and CD38− HIV-specific CD8+ T cells from an HIV+ patient (left panel). Pooled data showing the ex vivo percentages (%) of MMHigh cells in CD38+ and CD38− HIV-specific (n = 4), CMV-specific (n = 6), and total CD8+ T cells from HIV+ patients (n = 8) and healthy individuals (n = 4). Bars depict means ± SE (right panel). The P values were calculated using Student t test.
MM is independent of the activation level of HIV-specific CD8+ T cells. (A) Representative flow cytometry showing HIV- and CMV-specific CD8+ T cells from an HIV+ patient. Histograms depict ex vivo levels of CD38 in HIV-specific, CMV-specific, and CD8+ T cells from a healthy donor (left panel). Pooled data showing the ex vivo percentages (%) of CD38+ cells in HIV-specific (□; n = 10), CMV-specific (▪; n = 17), and total CD8+ T cells from HIV+ patients (♦; n = 35) and healthy donors (○; n = 14) (right panel). (B) Representative flow cytometry showing ex vivo MM in CD38+ and CD38− HIV-specific CD8+ T cells from an HIV+ patient (left panel). Pooled data showing the ex vivo percentages (%) of MMHigh cells in CD38+ and CD38− HIV-specific (n = 4), CMV-specific (n = 6), and total CD8+ T cells from HIV+ patients (n = 8) and healthy individuals (n = 4). Bars depict means ± SE (right panel). The P values were calculated using Student t test.
Expression of CD38 alone does not explain the selective apoptosis of HIV-specific CD8+ T cells
A significant correlation was observed between the ex vivo percentages of CD38+CD8+ T cells and spontaneous, CD95/Fas-induced, and treatment-specific CD95/Fas-induced apoptosis of total CD8+ T cells isolated from HIV+ patients (Figure 5A), in agreement with previously published data.6 We further divided our population of HIV+ patients in low-CD38 and high-CD38 groups based on the median value of percentage of CD8+ T cells expressing CD38 in all patients (median value 32%), as previously described.6 These 2 groups presumably represent patients with low and high levels of chronic non–antigen-specific activation, respectively. Comparable spontaneous apoptosis was observed in both patient groups for HIV- and CMV-specific CD8+ T cells (data not shown). Similar levels of CD95/Fas-induced apoptosis were found in HIV-specific CD8+ T cells (37.6% ± 5.9% vs 36.4% ± 6.6% annexin V+ for the high-CD38 [n = 10] and low-CD38 group [n = 8], respectively), whereas higher CD95/Fas-induced apoptosis was found in CMV-specific CD8+ T cells from the high-CD38 (n = 11; 21.3% ± 4.2% annexin V+) compared with the low-CD38 group (n = 11; 13.9% ± 3.1% annexin V+). Comparison of both virus-specific CD8+ T cells revealed significantly higher CD95/Fas-induced apoptosis in HIV-compared with CMV-specific CD8+ T cells in both the high-CD38 and low-CD38 group (P < .004). When the apoptosis sensitivity of CD38+ compared with CD38− HIV-specific CD8+ T cells (Figure 5B-C) was analyzed, no difference was found in spontaneous apoptosis of CD38+ compared with CD38− HIV-specific CD8+ T cells (Figure 5C). In contrast, CD38+ HIV-specific CD8+ T cells exhibited significantly higher CD95/Fas-induced apoptosis (57.9% ± 6% annexin V+, n = 6) compared with CD38− HIV-specific CD8+ T cells (37.2% ± 6.6% annexin V+, P = .04) and this was also true for specific CD95/Fas-induced apoptosis (Figure 5C). When CD38+ and CD38− CMV-specific CD8+ T cells were compared, higher CD95/Fas-induced apoptosis sensitivity was found in the CD38+ cells (Figure 5C). Thus CD38 expression appears to confer increased apoptosis sensitivity. However, spontaneous apoptosis was found in the CD38+ cells to be moderately higher in CD38+ HIV-specific CD8+ T cells (16.8% ± 5.3% annexin V+) compared with CD38+ CMV-specific (9.5% ± 3% annexin V+) and total CD38+CD8+ T cells (11.5% ± 1.7% annexin V+, n = 15) from HIV+ patients (Figure 5C). This difference was found to be remarkably higher when the CD95/Fas-induced apoptosis was investigated. CD38+ HIV-specific CD8+ T cells were characterized by higher sensitivity (57.9% ± 6% annexin V+) compared with CD38+ CMV-specific (30.4% ± 7.7% annexin V+, P < .02) and total CD38+CD8+ T cells (26.2% ± 3.7% annexin V+, P < .001; Figure 5C). Finally, CD38− HIV-specific CD8+ T cells exhibit comparable levels of CD95/Fas-induced apoptosis (37.2% ± 6.6% annexin V−, n = 6) compared with CD38+ CMV-specific CD8+ T cells (30.4% ± 7.7% annexin V+, n = 7). Thus although CD38+ HIV-specific CD8+ T cells exhibit more apoptosis than CD38− HIV-specific CD8+ T cells, these latter cells have comparable apoptosis to CD38+ CMV-specific CD8+ T cells. Therefore CD38 expression on virus-specific CD8+ T cells does not necessarily predict apoptosis sensitivity. Our data indicate that in addition to CD38 expression, other factors also contribute to the selective sensitivity of HIV-specific CD8+ T cells to apoptosis.
CD38 expression, although a correlate of apoptosis, does not explain on its own the selective apoptosis of HIV-specific CD8+ T cells. (A) The correlation between the percentage (%) of spontaneous, anti-CD95/Fas–induced, and treatment-specific CD8+ T-cell apoptosis and ex vivo percentages (%) of CD38+ cells (top left, middle, and right panels, respectively) in total CD8+ T cells from HIV+ patients (n = 21). (B) Representative flow cytometry showing HIV-specific and CD8+ T cells from an HIV+ patient. Histograms depict CD38 levels and annexin V positivity in HIV-specific CD8+ T cells after culturing PBMCs for 14 hours in the absence or presence of anti-CD95/Fas antibody. (C) Pooled data presenting annexin V positivity in CD38+ and CD38− HIV-specific (n = 6) and CMV-specific (n = 7) CD8+ T cells from HIV+ patients. Open symbols represent CD38+ whereas closed ones represent CD38−CD8+ T cells. (D) Pooled data showing the ex vivo MFI of Bcl-2 staining for CD38+ and CD38− HIV-specific (triangles; n = 8), CMV-specific (diamonds; n = 13), and total CD8+ T cells from HIV+ patients (circles; n = 30) and healthy donors (squares; n = 9). Horizontal lines depict mean values. The P values were calculated using Student t test.
CD38 expression, although a correlate of apoptosis, does not explain on its own the selective apoptosis of HIV-specific CD8+ T cells. (A) The correlation between the percentage (%) of spontaneous, anti-CD95/Fas–induced, and treatment-specific CD8+ T-cell apoptosis and ex vivo percentages (%) of CD38+ cells (top left, middle, and right panels, respectively) in total CD8+ T cells from HIV+ patients (n = 21). (B) Representative flow cytometry showing HIV-specific and CD8+ T cells from an HIV+ patient. Histograms depict CD38 levels and annexin V positivity in HIV-specific CD8+ T cells after culturing PBMCs for 14 hours in the absence or presence of anti-CD95/Fas antibody. (C) Pooled data presenting annexin V positivity in CD38+ and CD38− HIV-specific (n = 6) and CMV-specific (n = 7) CD8+ T cells from HIV+ patients. Open symbols represent CD38+ whereas closed ones represent CD38−CD8+ T cells. (D) Pooled data showing the ex vivo MFI of Bcl-2 staining for CD38+ and CD38− HIV-specific (triangles; n = 8), CMV-specific (diamonds; n = 13), and total CD8+ T cells from HIV+ patients (circles; n = 30) and healthy donors (squares; n = 9). Horizontal lines depict mean values. The P values were calculated using Student t test.
Bcl-2 expression in HIV-specific CD8+ T cells is not solely dependent on their activation level
We examined the expression of the antiapoptotic Bcl-2 and Bcl-xL molecules in relation to activation status of HIV-specific CD8+ T cells, as these cells have markedly reduced levels of these molecules that may contribute to their apoptosis sensitivity.12 Expression of the activation marker CD38 was found to be associated with down-regulation of Bcl-2 in all populations tested (Figure 5D). In CD38+ cells, lower expression of Bcl-2 was found in HIV-specific (MFI = 169 ± 33, n = 8, P < .001), CMV-specific (MFI = 264 ± 26, n = 13, P < .01), and total CD8+ T cells (MFI = 275 ± 30, n = 30, P < .005) from HIV+ patients compared with CD8+ T cells from healthy donors (MFI = 404 ± 42, n = 9; Figure 5D). Bcl-2 levels were significantly lower in CD38+ HIV-specific compared with CD38+ CMV-specific CD8+ T cells from HIV+ individuals (P < .04; Figure 5D). In CD38− cells, CMV-specific (MFI = 432 ± 35) and total CD8+ T cells (MFI = 436 ± 32) from HIV+ patients had Bcl-2 levels comparable to healthy CD8+ T cells (MFI = 505 ± 59). Bcl-2 expression, however, was considerably lower in CD38− HIV-specific CD8+ T cells (MFI = 237 ± 59) compared with CD38− CMV-specific (MFI = 432 ± 35, P < .003) and total CD38−CD8+ T cells from HIV+ patients (MFI = 436 ± 32, P < .007) and healthy donors (MFI = 505 ± 59, P < .002; Figure 5D). Analysis of the Bcl-xL expression revealed higher levels, although not statistically significant, of this molecule in CD38+ CMV-specific (MFI = 70 ± 10.7, n = 4) compared with CD38+ HIV-specific CD8+ T cells (MFI = 45 ± 9.1, n = 5). Therefore, Bcl-2 down-regulation in HIV-specific CD8+ T cells may depend on activation, as CD38+ cells have lower levels than CD38− cells; however these latter cells still have lower levels than CD38+ CMV-specific CD8+ T cells, suggesting that additional factors are involved in the regulation of Bcl-2 in HIV-specific CD8+ T cells.
CD95/Fas and mitochondria copolarization in early steps of CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells
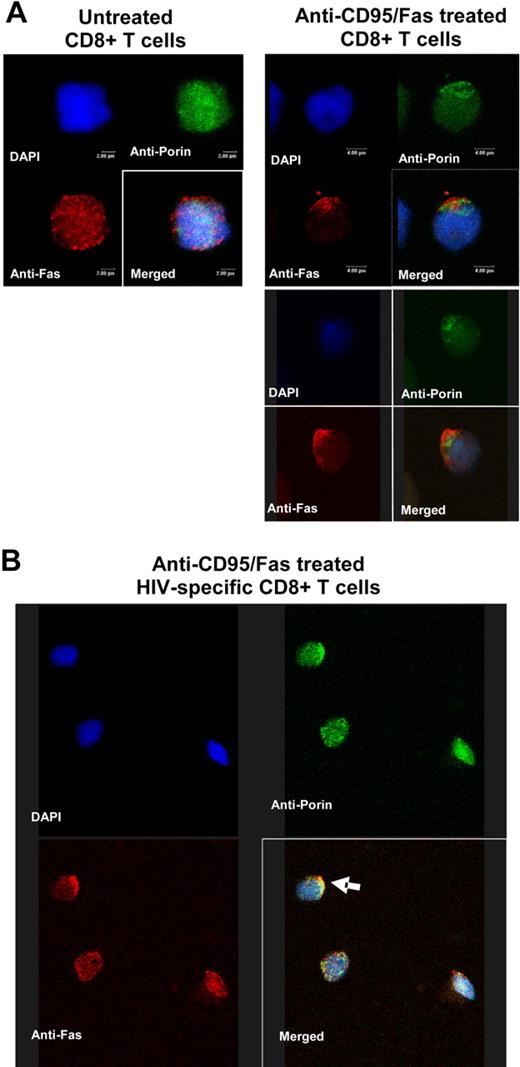
The active involvement of mitochondria in apoptosis of HIV-specific CD8+ T cells has not been yet investigated. To this end, we asked whether mitochondria localization is changed in early steps of CD95/Fas-induced apoptosis, indicative of their active role in this process.28,29 Jurkat cells were used as a positive control in order to set up the in vitro CD95/Fas-capping assay (Figure 6A). In untreated cells, a homogeneous surface staining was observed, whereas the anti-CD95/Fas treatment resulted in aggregation of CD95/Fas into less than 25% to 30% of the cell surface (Figure 6A). Next, a modified CD95Fas-capping assay was performed in purified CD8+ T cells from HIV+ patients and healthy donors. Similarly to Jurkat cells, anti-CD95Fas treatment induced aggregation of CD95/Fas receptor in CD8+ T cells from HIV+ patients (n = 7) but not in those from healthy donors (n = 4; Figure 6B). CD95/Fas capping was detected after 30 to 60 minutes incubation with anti-CD95Fas antibody (Figure 6B). Like in human lymphoblastoid CD4+ T cells (CEM cell line),30 CD95/Fas capping in human primary CD8+ T cells from HIV+ patients was accompanied by the formation of uropod-like structures that protrude from the cell surface at various extents (Figure 6C). To investigate the localization of mitochondria under CD95/Fas-capping conditions, a double-staining protocol was developed and applied after treatment of purified CD8+ T cells from HIV+ patients with anti-CD95Fas antibody. Preliminary experiments were carried out in Jurkat cells by using either anti-CD95Fas or antiporin antibody alone in order to test the specificity of the staining (data not shown). Antiporin staining revealed a cytoplasmic localization of mitochondria in the form of clusters (Figure 7A). No effect was also observed on anti-CD95Fas staining when cells were treated with urea or Triton-X 100 (data not shown). Untreated purified CD8+ T cells from HIV+ patients (n = 3) exhibited a uniform distribution of CD95/Fas on the cell surface, whereas mitochondria appeared as cytoplasmic clusters (Figure 7A). Upon anti-Fas treatment, a translocation aggregation of mitochondria into the proximal area of CD95/Fas capping was observed (Figure 7A). Most importantly, such colocalization between CD95/Fas capping and mitochondria was also observed when sorted HIV-specific CD8+ T cells were examined under CD95/Fas-capping conditions (Figure 7B). Taken together, our data point to an active involvement of mitochondria, possibly as a potent amplifier, in the very early steps of CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells.
Anti-CD95/Fas treatment induces CD95/Fas capping in purified CD8+ T cells from HIV+ patients but not from healthy donors. (A) Confocal imaging analysis showing CD95/Fas distribution in untreated (left panel) or treated (right panel) Jurkat cells under CD95/Fas-capping conditions. Merged images (blue for DAPI and green for anti-CD95/Fas staining) of representative cells are shown at a single section (top row) or 3D projection (bottom row). (B) CD95/Fas distribution in purified CD8+ T cells from an HIV+ individual and a healthy donor under treatment with anti-CD95/Fas antibody for 5 minutes or 1 hour at 37°C. White arrows point at CD95/Fas-capping cells. Zoomed images of selected fields are also shown. (C) Representative imaging showing the accumulation of CD95/Fas in uropod-like structures extending from the cell surface. Zoomed images of selected fields are also shown. These results are representative of CD8+ T cells from 7 HIV+ patients and 4 healthy donors.
Anti-CD95/Fas treatment induces CD95/Fas capping in purified CD8+ T cells from HIV+ patients but not from healthy donors. (A) Confocal imaging analysis showing CD95/Fas distribution in untreated (left panel) or treated (right panel) Jurkat cells under CD95/Fas-capping conditions. Merged images (blue for DAPI and green for anti-CD95/Fas staining) of representative cells are shown at a single section (top row) or 3D projection (bottom row). (B) CD95/Fas distribution in purified CD8+ T cells from an HIV+ individual and a healthy donor under treatment with anti-CD95/Fas antibody for 5 minutes or 1 hour at 37°C. White arrows point at CD95/Fas-capping cells. Zoomed images of selected fields are also shown. (C) Representative imaging showing the accumulation of CD95/Fas in uropod-like structures extending from the cell surface. Zoomed images of selected fields are also shown. These results are representative of CD8+ T cells from 7 HIV+ patients and 4 healthy donors.
CD95/Fas and mitochondria copolarization in early steps of CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells. (A) Purified CD8+ T cells from an HIV+ individual were left untreated or treated with a mouse anti-CD95/Fas antibody for 1 hour at 37°C. Following fixation, a double-staining protocol was performed. Localization of CD95/Fas and mitochondria was analyzed by confocal microscopy. Representative 3D images are shown from 1 of 3 experiments performed. Untreated cells are shown on the left; right panels depict 2 cells showing copolarization. (B) Representative 3D confocal image showing CD95/Fas and mitochondria localization in sorted HIV-specific CD8+ T cells treated with anti-CD95/Fas antibody. CD95/Fas capping is accompanied with copolarization of mitochondria (arrow).
CD95/Fas and mitochondria copolarization in early steps of CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells. (A) Purified CD8+ T cells from an HIV+ individual were left untreated or treated with a mouse anti-CD95/Fas antibody for 1 hour at 37°C. Following fixation, a double-staining protocol was performed. Localization of CD95/Fas and mitochondria was analyzed by confocal microscopy. Representative 3D images are shown from 1 of 3 experiments performed. Untreated cells are shown on the left; right panels depict 2 cells showing copolarization. (B) Representative 3D confocal image showing CD95/Fas and mitochondria localization in sorted HIV-specific CD8+ T cells treated with anti-CD95/Fas antibody. CD95/Fas capping is accompanied with copolarization of mitochondria (arrow).
Discussion
We report here that HIV-specific CD8+ T cells are characterized by considerably increased MM when compared with other virus-specific cells such as CMV-specific CD8+ T cells from HIV+ individuals. We used a mitochondria-specific dye that binds mitochondrial membrane independently of membrane potential and thus is considered as an index of their MM.23–25 To the best of our knowledge, this is the first time that this parameter in HIV-specific CD8+ T cells or primary human CD8+ T cells in general has been investigated. MM was found to be significantly increased in HIV-specific CD8+ T cells whereas CMV-specific CD8+ T cells from HIV+ patients possess similar MM levels to total CD8+ T cells, indicating that the MMhigh phenotype is not a general characteristic of virus-specific CD8+ T cells in HIV infection but specifically characterizes HIV-specific CD8+ T cells. The MMhigh phenotype of HIV-specific CD8+ T cells could not be attributed to their known skewed CD45RA−CCR7−CD62L− memory phenotype,11,26 as similar levels of MM were found in both CD45RA−CD62L− and CD45RA+CD62L− in HIV- and CMV-specific CD8+ T cells, indicating that this biologic parameter is independent of their differentiation status. Using this flow-cytometry–based assay, however, one cannot distinguish whether high MM corresponds to higher numbers of mitochondria or larger organelles, both situations which would result in higher levels of staining. Further experiments such as electron microscopy studies are needed to clarify this issue.
Our data demonstrate that MMhigh CD8+ T cells are sensitive to apoptosis, directly linking mitochondria to the apoptosis sensitivity of HIV-specific CD8+ T cells, which are almost all MMHigh. Mitochondria act as an amplification loop for CD95/Fas-induced apoptotic signals under certain experimental conditions.31,32 We hypothesize that under increased MM conditions, as a result of either more or larger organelles, this amplification loop is intensified, further enhancing the apoptosis sensitivity of HIV-specific CD8+ T cells. It is of particular notice that MM is higher in HIV-specific compared with CMV-specific CD8+ T cells from HIV+ patients, and this correlates with their increased apoptosis sensitivity.11 Increased MM in HIV-specific CD8+ T cells compared with healthy CD8+ T cells was also found using NAO, a dye that binds cardiolipin and has been used as a marker of mitochondrial mass.33 Contrary to MitoTracker, no difference was observed between HIV-specific and total CD8+ T cells from HIV+ donors using NAO. This may be due to the reduced sensitivity and range of the NAO stain when performed on unfixed cells, since the standard NAO assay requires ethanol fixation.34,35 Unfortunately, ethanol fixation cannot be combined with tetramer stains. Increased production of reactive oxygen species, which has been described in T cells from HIV+ donors,36 could induce cardiolipin oxidization—something that would influence NAO but not MitoTracker staining. One can hypothesize that such a mechanism may be increased in HIV-specific CD8+ T cells, resulting in less sensitive recognition of cardiolipin by NAO.37 Overall, despite the limitations of using NAO on unfixed cells, these studies with NAO confirm our basic observation with MitoTracker that HIV-specific CD8+ T cells have increased MM. An important question yet to be addressed is whether this increased MM is associated with an altered functional state of these organelles (ie, defective regulation of cellular Ca++ homeostasis).38
CD38 expression has been considered as a poor prognostic factor for HIV-infected patients.39,40 We found a strong correlation between ex vivo expression of CD38 and in vitro spontaneous as well as CD95/Fas-induced apoptosis of total CD8+ T cells from HIV-infected patients. Analysis of virus-specific populations revealed that both HIV- and CMV-specific CD8+ T cells express high ex vivo percentages of CD38+ cells, in agreement with previous studies.11,27 Whether this represents a generalized, bystander activation of the immune system or a virus-specific activation is an open question. However, CMV-specific CD8+ T cells, even from patients expressing high ex vivo percentages of CD38+CD8+ T cells, were characterized by moderate apoptosis sensitivity compared with HIV-specific CD8+ T cells, in agreement with our previous data.11 Furthermore, our data revealed that indeed the presence of CD38 favors CD95/Fas-induced apoptosis, as indicated by the increased sensitivity of CD38+ compared with CD38− HIV-specific CD8+ T cells. The presence of CD38 had no effect in spontaneous apoptosis, implying a specific interaction between this surface molecule and CD95/Fas-induced signaling. CD38+ HIV-specific CD8+ T cells, however, are characterized by significantly higher apoptosis sensitivity compared with CD38+ CMV-specific and total CD38+CD8+ T cells from HIV+ patients, challenging a potentially principal role of CD38 in this process. In this case, one should expect comparable sensitivity among the CD38+CD8+ T-cell populations tested, irrespective of virus specificity. Therefore, CD38 expression cannot explain on its own the selective apoptosis sensitivity of HIV-compared with CMV-specific CD8+ T cells from HIV-infected individuals. CD38+ HIV-specific CD8+ T cells exhibited dramatically lower ex vivo levels of the antiapoptotic factor Bcl-2 compared with CD38+ CMV-specific CD8+ T cells, indicating an additional selective defect of HIV-specific CD8+ T cells. Surprisingly, CD38− HIV-specific CD8+ T cells also had significantly down-regulated ex vivo Bcl-2 levels, and this was not observed in CD38− CMV-specific CD8+ T cells. Thus CD38− HIV-specific CD8+ T cells have lower Bcl-2 levels and exhibit higher apoptosis compared with CD38− CMV-specific CD8+ T cells. These data indicate that although CD38 expression confers increased apoptosis sensitivity to HIV-specific CD8+ T cells, it is not the sole factor controlling this death, as CD38− HIV-specific CD8+ T cells also exhibit high apoptosis.
A critical issue in order to understand the molecular mechanism(s) governing the apoptosis sensitivity of HIV-specific CD8+ T cells is whether the commitment to death takes place upstream or downstream of mitochondria, the main organelle where Bcl-2 family molecules exert their antiapoptotic function.41 To this end, the role of mitochondria in early steps of CD95/Fas-induced apoptosis of HIV-specific and total CD8+ T cells from HIV+ individuals is unexplored. In vitro HIV studies based on ΔΨm measurement may not be sufficient to definitively answer this question. A very early requisite step during CD95/Fas-induced apoptosis is the formation of CD95/Fas capping.16 Therefore, early molecular events can be monitored under CD95/Fas-capping experimental conditions. Special effort was undertaken to develop an assay allowing us to detect such a capping in HIV-specific CD8+ T cells. Subsequently, a double-staining protocol was established for detection of mitochondrial localization under capping conditions. Translocation clustering of mitochondria upon death-receptor apoptosis induction has been previously described.28,29,42 Of note, this perinuclear accumulation precedes the release of cytochrome c and ΔΨm loss28 or production of mitochondrial reactive oxygen species (ROS),29 whereas it correlates with apoptosis sensitivity.29 To the best of our knowledge, this is the first time the colocalization between CD95/Fas and mitochondria upon CD95/Fas induction of apoptosis of primary HIV-specific and total CD8+ T cells from HIV-infected patients has been shown. This colocalization further supports the active role of mitochondria as amplifiers of early CD95/Fas-induced signaling in these cells. Since T-cell cytoplasm represents a thin layer around the nucleus, we cannot however distinguish whether this mitochondrial translocation clustering is perinuclear or proximal to cytoplasmic membrane, which may bring the mitochondria closer to the CD95/Fas receptor. What governs the translocation clustering of mitochondria in CD8+ T cells is not known. Disruption of microtubules by nocodazole proved to be toxic itself for CD8+ T cells from HIV+ patients (data not shown), preventing further investigation of this process in the apoptosis of HIV-specific CD8+ T cells. An interesting question is whether this is also the case for CD4+ T-cell depletion, the hallmark of HIV infection. Furthermore, longitudinal studies following the apoptosis sensitivity along with changes related to mitochondria during the course of HIV infection could further shed light on the involvement of mitochondria in the biology and apoptosis of T cells.
Differences in MM of total CD8+ T cells or HIV-specific CD8+ T cells did not correlate with viral load or CD4 or CD8 counts, although a trend was found between MM and disease duration (data not shown) and a small but significant increase in percentages of high MM CD8+ T cells was found in patients under antiretroviral treatment. This raises the intriguing question of whether measurement of MM could be used as a prognostic factor for disease progression, and further studies are needed to address this.
Overall our data show that HIV-specific CD8+ T cells are selectively characterized by extensive MM. Furthermore, expression of high MM is independent of their maturation and activation level. Interestingly, the remarkable down-regulation of Bcl-2 was also found to be independent of CD38 expression in HIV-specific CD8+ T cells. CD38 expression, although a correlate of apoptosis sensitivity, does not on its own explain the selective apoptosis of HIV-specific CD8+ T cells. We therefore suggest that the combination of extensive MM with low expression of antiapoptotic factors on an activated background selectively primes HIV-specific CD8+ T cells to apoptosis. Thus, mitochondria seem to be critical regulators of HIV-specific CD8+ T apoptosis, as their colocalization with CD95/Fas aggregates also suggest. Therapeutic interventions targeting these organelles may be considered for strategies aiming at immune-system reconstitution in HIV infection.
Authorship
Contribution: C.P. and Y.M.M. designed, performed, and analyzed experiments and wrote the manuscript; I.D.D., S.R.A., and A.B. participated in performing the experiments and collecting data; P.S. and K.C.M. contributed patient samples and participated in analyzing the clinical data; J.D.A. contributed to experimental design and reagents; and P.D.K. designed experiments and wrote the manuscript. All authors discussed the results and contributed to the manuscript writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter D. Katsikis, Department of Microbiology and Immunology and Institute for Molecular Medicine and Infectious Disease, Drexel University College of Medicine, 2900 Queen Lane, Philadelphia, PA, 19129; e-mail: peter.katsikis@drexelmed.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported in part by National Institutes of Health grants R01 AI46719, R01 AI52005, and R01 AI62437 (P.D.K.).
The authors would like to thank Peter Pitsakis and the staff of the Partnership Comprehensive Care Practice of the HIV/AIDS Medicine Division of Drexel University College of Medicine for patient recruitment. The authors want to express their gratitude to Dr David Ambrozak at Vaccine Research Center (VRC), National Institute of Allergy and Infectious Diseases (NIAID)/NIH, for his help with the BSL-3 flow-cytometry studies.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal