Abstract
The mechanism of commencement of hematopoiesis in blood islands of the yolk sac and the aorta-gonad-mesonephros (AGM) region during primate embryogenesis remains elusive. In this study, we demonstrated that VE-cadherin+CD45− endothelial cells derived from nonhuman primate embryonic stem cells are able to generate primitive and definitive hematopoietic cells sequentially, as revealed by immunostaining of floating erythrocytes and colony-forming assay in cultures. Single bipotential progenitors for hematopoietic and endothelial lineages are included in this endothelial cell population. Furthermore, hemogenic activity of these endothelial cells is observed exclusively in the α4-integrin+ subpopulation; bipotential progenitors are 4-fold enriched in this subpopulation. The kinetics of this hemogenic subpopulation is similar to that of hemogenic endothelial cells previously reported in the yolk sac and the AGM region in vivo in that they emerge for only a limited time. We suggest that VE-cadherin+CD45−α4-integrin+ endothelial cells are involved in primitive and definitive hematopoiesis during primate embryogenesis, though VE-cadherin−CD45−α4-integrin+ cells are the primary sources for primitive hematopoiesis.
Introduction
During mammalian embryogenesis, hematopoietic system development undergoes 2 distinct phases, primitive hematopoiesis and definitive hematopoiesis. The phases are distinguished from each other by 2 characteristics. First, primitive hematopoiesis originates exclusively in the extraembryonic yolk sac and is transient, whereas definitive hematopoiesis occurs in the intraembryonic aorta-gonad-mesonephros (AGM) region, shifts to the liver, spleen, and bone marrow, and persists for life. Evidence also indicates that the yolk sac serves as a source of initial definitive hematopoietic progenitors in humans, as it does in mice.1,2 Second, in primates, primitive erythrocytes are larger and primarily synthesize embryonic globin chains (ζ, ϵ), whereas definitive erythrocytes are smaller and synthesize fetal/adult globin chains (α, γ, and β).3,4 Because the embryonic–fetal globin switch (ζ → α and ϵ → γ) occurs gradually in the fetal liver, embryonic and fetal globins are expressed during the transition from primitive to definitive hematopoiesis. However, adult β-globin is predominantly expressed in definitive erythrocytes and is only marginally expressed, if at all, in primitive erythrocytes
The existence of the hemangioblast, the common precursor of hematopoietic and endothelial lineages, has been discussed for many years. Histologically, hematopoietic and endothelial cells develop from the same clusters of mesoderm in yolk sac blood islands.5,6 In addition to the shared expression of several markers, gene-targeting experiments on vascular endothelial growth factor receptor-2 (VEGFR-2) disclose a common developmental pathway between both cell types.7,8 Furthermore, a single common precursor generates both cell types during in vitro differentiation of mouse embryonic stem cells (ESCs).9 Recent evidence shows that intraembryonic hematopoiesis originates from the ventral endothelial walls of the dorsal aorta and the umbilical and vitelline arteries, challenging the concept of common progenitors.10–16 Endothelial cells capable of generating hematopoietic cells are designated “hemogenic endothelium.”17,18 Earlier studies using mouse embryos demonstrate that endothelial cells in the yolk sac are able to generate hematopoietic cells, which is also suggested by some reports on human embryos.12,15,16 The embryos were used, however, at the stage after vascular connection between the yolk sac and the embryo proper. Hence, though it is established that definitive hematopoiesis in the AGM region originates at least in part in endothelial cells, the origin of primitive/definitive hematopoiesis in the yolk sac is still unclear.
The aims of this study were to investigate the relationship between hemogenic endothelium and primitive/definitive hematopoiesis in primates and to identify markers of the hemogenic endothelium. Analyses using primate materials are necessary because a number of differences occur in hematopoietic development between mice and primates (human and monkey). These studies are difficult to perform because of the poor availability of primate embryos and the ethical limitations involved in their use. Recently established primate ESC lines19–22 are promising alternative tools in developmental biology and regenerative medicine. We previously showed the development of hematopoietic and endothelial cells when cynomolgus monkey ESCs were cocultured with OP9 stromal cells, which was enhanced by exogenous vascular endothelial growth factor (VEGF).23,24 In our coculture system, the transition from primitive to definitive hematopoiesis was induced, as confirmed by globin switching.25 Here, we examined the hematopoietic potential of endothelial cells in our coculture system and demonstrated that isolated VE-cadherin+CD45− endothelial cells generated primitive and definitive hematopoietic cells based on morphologic and globin expression analyses. We used α4-integrin, an effective marker of the hemogenic population among endothelial cells in mouse embryos and in in vitro differentiating ESCs,26 as a candidate marker of hemogenic endothelial cells in primates. Our data show that the capacity for primitive and definitive hematopoiesis resides exclusively in the α4-integrin+ subpopulation among ESC-derived endothelial cells, though VE-cadherin−CD45−α4-integrin+ cells are primary sources for primitive hematopoiesis.
Materials and methods
Maintenance of cell lines
Antibodies
Primary antibodies used in this study included mouse anti–human CD34-phycoerythrin (PE), CD41a-allophycocyanin (APC), α4-integrin-PE, endothelial nitric oxide synthase (eNOS) monoclonal antibodies (mAbs; BD PharMingen, San Diego, CA), mouse anti–human CD31-PE (eBioscience, San Diego, CA), rabbit anti–human von Willebrand factor (VWF; Nichirei, Tokyo, Japan), mouse anti–human CD45 and CD41 mAbs (Dako, Kyoto, Japan), mouse anti–human VE-cadherin mAb (Immunotech, Marseille, France), mouse anti–human β-globin and γ-globin mAbs (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti–human hemoglobin (Hb) polyclonal Ab (Cappel, Aurora, OH), and their corresponding IgG1 isotype controls (BD PharMingen and Dako). Mouse anti–human VE-cadherin mAb (BD PharMingen) and its corresponding IgG1 isotype control were labeled with Alexa Fluor 647 monoclonal antibody labeling kit (Invitrogen, Carlsbad CA). Mouse anti–human ϵ-globin and ζ-globin and mouse anti–human VEGFR-2 mAbs were used, as reported previously.28–30 Mouse anti–human α-globin mAb was established in the laboratory of D.H.K.C. All primary antibodies against human antigens used in this study cross-reacted with cynomolgus monkey compartments.23,24 Secondary Abs included Cy3-conjugated, horseradish peroxidase (HRP)–conjugated, or alkaline phosphatase (ALP)–conjugated donkey anti–mouse IgG, fluorescein isothiocyanate (FITC)–conjugated donkey anti–rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), PE-conjugated goat anti–mouse IgG (Dako), and FITC- or APC-conjugated goat anti–mouse IgG (BD PharMingen).
In vitro differentiation of primate ESCs
Initial differentiation of ESCs and cell sorting were based on earlier experiments.23,24 Briefly, trypsin-treated undifferentiated ESCs were transferred onto OP9 cells and cultured in the presence of 20 ng/mL VEGF (R&D Systems, Minneapolis, MN).
For hematopoietic differentiation, cells sorted on day 10 were cultured in α-MEM (Gibco BRL, Grand Island, NY) containing 10% fetal calf serum (FCS; Sigma, St Louis, MO), 50 μM 2-mercaptoethanol (ME), and a mixture of 10 ng/mL G-CSF, 2 U/mL EPO, 20 ng/mL IL-3, 100 ng/mL SCF, and 10 ng/mL TPO (hematopoietic cytokine mixture; all were provided by Kirin Brewery, Tokyo, Japan). For endothelial differentiation, sorted cells were cultured on OP9 cells in α-MEM containing 10% FCS, 50 μM 2-ME, and 20 ng/mL VEGF or were seeded onto type I collagen-coated plates in medium (CS-C Complete Medium; Cell Systems, Kirkland, WA) supplemented with 20 ng/mL VEGF.
Fluorescence-activated cell sorter analysis and cell sorting
Staining procedures, FACS analysis, and cell sorting were performed as described earlier.23,24 For multicolor staining, single-cell suspensions were initially stained with unconjugated anti–CD45 mAb or its corresponding IgG1 isotype control, followed by FITC-, PE-, or APC-conjugated goat anti–mouse IgG. The cells were washed twice, incubated with robust mouse IgG to prevent redundant secondary Abs from reacting with other mouse mAbs, and stained with fluorochrome-conjugated mAbs, including CD34, VE-cadherin, and α4-integrin. Dead cells were excluded by propidium iodide (PI) staining. Samples were analyzed with the use of FACSCalibur and Cell Quest software (Becton Dickinson, San Jose, CA) or were sorted on a FACSVantage SE (Becton Dickinson).
Immunochemistry and acetylated low-density lipoprotein (Ac-LDL) uptake
May-Giemsa staining, immunostaining of floating erythrocytes and endothelial colonies, and DiI-Ac-LDL incorporation assay were performed as described previously.23,24 VE-cadherin+CD45− cells isolated on day 10 or their progeny were cytospun onto glass slides, fixed, and permeabilized in a staining procedure similar to that for hemoglobin.24 Cells were initially stained with anti–VE-cadherin mAb and Cy3-conjugated donkey anti–mouse IgG, followed by double staining with various Abs using the Vector MOM kit (Vector Laboratories, Burlingame, CA), visualized with the TSA fluorescence systems kit (PerkinElmer Life Sciences, Boston, WA), and counterstained with Hoechst 33342. Fluorescence was detected on an Olympus IX70 microscope (Olympus, Tokyo, Japan) that was equipped with 4 ×/0.13 NA, 10 ×/0.30 NA, and 20 ×/0.40 NA objectives, and images were obtained with an AxioCam photomicroscope and AxioVision software version 3.0.6 SP4 (Carl Zeiss Vision, Hallbergmoos, Germany). Images were processed using Adobe Photoshop 6.0 (Adobe Systems, San Diego, CA).
Colony-forming assays for primitive and definitive cells
Colony-forming assays were performed as described elsewhere.24,31 Briefly, for colonies consisting of primitive cells, sorted cells in each subpopulation were reseeded on OP9 layers, and the medium was replaced with methylcellulose-containing medium supplemented with 30% FCS and hematopoietic cytokine mixture on the following day. For colonies composed of definitive cells, we initially trypsinized cells and allowed OP9 stromal cells to adhere to culture dishes to exclude OP9. Resultant floating fractions were transferred to new Petri dishes with methylcellulose-containing medium supplemented with 30% FCS and hematopoietic cytokine mixture and were cultured at 37°C, 5% CO2, in a humidified incubator. Colonies were scored using an inverted microscope24,32,33 after 7 days for primitive cells and 14 days for definitive cells. Colonies were selected for cytospin and further staining. All assays were performed at a concentration of 0.5-2 × 104 cells/mL in duplicate or triplicate.
Single-cell deposition assay for hematopoietic and endothelial differentiation
Single-cell deposition assay was performed as described earlier.23 Briefly, single-sorted cells were deposited in individual wells of 96-well plates with confluent OP9 layers and were cultured in α-MEM containing 10% FCS, 50 μM 2-ME, and hematopoietic cytokine mixture for 7 days. Each well was initially stained with a mixture of anti-CD45, anti-CD41, and anti–γ-globin mAbs, followed by HRP-conjugated donkey anti–mouse IgG for hematopoietic lineage detection, and each was double stained with anti–VE-cadherin mAb using the Vector MOM kit (Vector), followed by ALP-conjugated donkey anti–mouse IgG for endothelial lineage detection.
Reverse transcription–polymerase chain reaction
We performed RNA isolation and RT-PCR according to previously established protocols.23,24 Samples were initially denatured at 94°C for 5 minutes, followed by 35 to 40 amplification reactions consisting of 94°C for 1 minute (denaturing), 60°C to 62°C for 1 minute (annealing), 72°C for 1 minute (extension), and a final extension at 94°C for 7 minutes. Primers for eNOS, SCL, GATA-2, RUNX1, and GAPDH are described elsewhere.23,34 Other primers used included α4-integrin (434 bp) (sense, 5′-AGATGGGATCTCGTCAACCTTC-3′; antisense, 5′-TGGACACCTGTATGCTTCCTG-3′), VWF (472 bp) (sense, 5′-GGGACCTTTCGGATCCTAGTG-3′; antisense, 5′-AGGAGGAATCCACCATCGTC-3′), and mouse β-actin (613 bp) (sense, 5′-ATCCTGACCCTGAAGTACCCCATT-3′; antisense, 5′-CCAAGAAGGAAGGCTGGAAAAGAG-3′). cDNA from adult cynomolgus monkey BM cells, human erythroblastic cells (K562), and human umbilical vein endothelial cells were used as positive controls, and mouse OP9 cells were used as a negative control. For semiquantitative comparison, samples were normalized by dilution to produce equivalent signals for GAPDH.
Statistical analysis
Statistical analyses were conducted using the Student t test or Fisher exact test. Statistical significance was defined as a P value below .05.
Results
Development of primate ESC-derived VE-cadherin+CD45− endothelial cells
Initially, we investigated when cells positive for hematopoietic or endothelial markers emerged during ESC differentiation. GFP-transfected ESCs were induced to differentiate by coculture with OP9 stromal cells in the presence of exogenous VEGF. Sequential FACS analysis for various surface markers was performed, and the percentage of positive cells among viable GFP+ cells was quantified (Figure 1A). Undifferentiated ESCs expressed low levels of VEGFR-2 but not other hematopoietic or endothelial markers, such as CD31, CD34, VE-cadherin, CD41a, or CD45 (Figure 1B). VEGFR-2 was down-regulated by day 4 of culture but subsequently was re-expressed on a fraction of differentiating cells. CD31+, CD34+, and VE-cadherin+ cells initially appeared on day 6, and CD41a+ and CD45+ cells appeared on day 12. Thus, cells positive for VE-cadherin, an endothelial marker, emerged in the OP9 coculture earlier than those positive for the hematopoietic marker CD41a or CD45.
ESC-derived VE-cadherin+CD45− cells have endothelial properties. Undifferentiated GFP-transfected ESCs (ES) and subsequent differentiating cells were analyzed by FACS. GFP+PI− cells were gated as ESC-derived viable cells. (A) Percentage of gated cells among the total cells is specified. (B) Sequential analysis of the percentage of cells positive for each antigen among ESC-derived viable cells. Data are presented as mean ± SD of 3 independent experiments. (C) VE-cadherin+CD45−(VEcad+CD45−) or VE-cadherin−CD45−(VEcad−CD45−) cells were sorted on day 10. Representative FACS dot plots and percentages of gated cells are shown. Purities of viable VE-cadherin+CD45− and VE-cadherin−CD45− cells are 99.2% ± 0.6% and 99.9% ± 0.1%, respectively, from at least 3 independent experiments. (D) VE-cadherin+ cells on day 10 were analyzed by FACS with various mAbs. Percentages of cells positive for each antigen among VE-cadherin+ cells are shown. Gray line indicates isotype control; black line, VE-cadherin+ cells. (E-J) Untransfected ESC-derived VE-cadherin+CD45− cells sorted on day 10 were evaluated by the DiI-Ac-LDL incorporation assay (E) or immunostaining with IgG1 (F), anti–Hb (G), VEGFR-2 (H), eNOS (I), or VWF (J) Abs. Hoechst 33342 (E-J) and anti–VE-cadherin mAb (F-J) were used to detect nuclei and endothelial cells, respectively. Original magnification × 200. Scale bar, 50μm.
ESC-derived VE-cadherin+CD45− cells have endothelial properties. Undifferentiated GFP-transfected ESCs (ES) and subsequent differentiating cells were analyzed by FACS. GFP+PI− cells were gated as ESC-derived viable cells. (A) Percentage of gated cells among the total cells is specified. (B) Sequential analysis of the percentage of cells positive for each antigen among ESC-derived viable cells. Data are presented as mean ± SD of 3 independent experiments. (C) VE-cadherin+CD45−(VEcad+CD45−) or VE-cadherin−CD45−(VEcad−CD45−) cells were sorted on day 10. Representative FACS dot plots and percentages of gated cells are shown. Purities of viable VE-cadherin+CD45− and VE-cadherin−CD45− cells are 99.2% ± 0.6% and 99.9% ± 0.1%, respectively, from at least 3 independent experiments. (D) VE-cadherin+ cells on day 10 were analyzed by FACS with various mAbs. Percentages of cells positive for each antigen among VE-cadherin+ cells are shown. Gray line indicates isotype control; black line, VE-cadherin+ cells. (E-J) Untransfected ESC-derived VE-cadherin+CD45− cells sorted on day 10 were evaluated by the DiI-Ac-LDL incorporation assay (E) or immunostaining with IgG1 (F), anti–Hb (G), VEGFR-2 (H), eNOS (I), or VWF (J) Abs. Hoechst 33342 (E-J) and anti–VE-cadherin mAb (F-J) were used to detect nuclei and endothelial cells, respectively. Original magnification × 200. Scale bar, 50μm.
VE-cadherin belongs to the cadherin family of adhesive transmembrane proteins and is expressed solely in endothelial cells.35 The protein is additionally detected in all developing vessels from early embryonic stages.36 To date, VE-cadherin has been used for the isolation of cells committed to endothelial lineage as an endothelial-specific marker.15,16,26,37 Accordingly, we used anti–VE-cadherin and CD45 mAbs to purify endothelial cells and exclude hematopoietic cells (Figure 1C). Indeed, isolated VE-cadherin+CD45− cells on day 10 exclusively coexpressed CD31 and CD34 but not CD41a (Figure 1D). Moreover, all VE-cadherin+CD45− cells on day 10 had Ac-LDL uptake capacity and expressed VEGFR-2 and eNOS but not Hb (Figure 1E-I). On the other hand, only 27.4% ± 7.8% cells expressed VWF (n = 3) (Figure 1J), suggesting that most were functionally immature. Our results indicated that VE-cadherin+CD45− cells generated on day 10, before the emergence of hematopoietic cells, had several endothelial characteristics.
VE-cadherin+CD45− population generates hematopoietic and endothelial cells
To determine the hematoangiogenic capacity of isolated VE-cadherin+CD45− cells, VE-cadherin+CD45− and VE-cadherin−CD45− cells were reseeded separately onto fresh, confluent OP9 cells and cultured with hematopoietic cytokine mixture (see “Materials and methods”) for hematopoietic differentiation or with VEGF for endothelial differentiation.
In hematopoietic differentiation cultures, adherent hematopoietic clusters initially emerged from the VE-cadherin+CD45− population at approximately day 10 + 3 (3 days after sorting on day 10) (Figure 2A) and covered large areas of OP9 stromal layers by day 10 + 21 (Figure 2B). In contrast, adherent hematopoietic clusters from the VE-cadherin−CD45− population were rare and small and disappeared by day 10 + 12 (data not shown). Under hematopoietic differentiation conditions, the numbers of CD45+ and CD45+CD34+ hematopoietic cells generated from the VE-cadherin+CD45− population were 9.5- and 16.1-fold higher, respectively, than from the VE-cadherin−CD45− population at day 10 + 7 (P < .05) (Figure 2N-O). It should be noted that VE-cadherin−CD45− cells generated a small but significant minority of hematopoietic cells at day 10 + 7.
ESC-derived VE-cadherin+CD45− cells generate hematopoietic and endothelial cells/colonies. Light micrographs of adherent hematopoietic clusters on days 10 + 5 (A) and 10 + 23 (B). (C-D) May-Giemsa staining of floating hematopoietic cells on days 10 + 6 (C) and 10 + 30 (D). (E) Immunostaining of floating hematopoietic cells on day 10 + 30 with anti–CD41 (red) and Hb (green) Abs. (F-K) Light micrographs and May-Giemsa staining of a GM (F-G), erythroid (H-I), or mixed colony (J-K) are depicted. (L) Immunostaining of an endothelial colony with anti–VE-cadherin mAb (blue). (M) Immunostaining of VE-cadherin+CD45− cell-derived cells after 7-day culture with anti–VE-cadherin (red) and VWF (green) Abs. (E, M) Nuclei were labeled with Hoechst 33342. (N, O) Numbers of CD45+ (N) or CD45+CD34+ cells (O) derived from 1 × 105 VE-cadherin+CD45− (VEcad+CD45−) or VE-cadherin−CD45− (VEcad−CD45−) cells. (P) Sequential analysis of the percentages of nucleated erythrocytes (red), enucleated erythrocytes (yellow), myeloid lineage cells (green), and megakaryocytes (blue) among floating cells. Each bar represents the mean of 3 independent experiments. (Q) Sequential analysis of the numbers of hematopoietic colonies per 1 × 104 VEcad+CD45− or VEcad−CD45− cell-derived cells. (R) Numbers of endothelial colonies per 1 × 103 VEcad+CD45− or VEcad−CD45− cells. (A-P) Data obtained from VEcad+CD45− cells are shown. Data are presented as mean ± SD of 3 independent experiments in N-O and Q-R. Each experiment was performed in triplicate in P-R. Original magnification × 40 (B, F, L), × 100 (A, H, J), and × 200 (C-E, G, I, K, M). Scale bars, 20 μm (C-E, G, I, K), 50 μm (M), and 100 μm (A-B, F, H, J, L).
ESC-derived VE-cadherin+CD45− cells generate hematopoietic and endothelial cells/colonies. Light micrographs of adherent hematopoietic clusters on days 10 + 5 (A) and 10 + 23 (B). (C-D) May-Giemsa staining of floating hematopoietic cells on days 10 + 6 (C) and 10 + 30 (D). (E) Immunostaining of floating hematopoietic cells on day 10 + 30 with anti–CD41 (red) and Hb (green) Abs. (F-K) Light micrographs and May-Giemsa staining of a GM (F-G), erythroid (H-I), or mixed colony (J-K) are depicted. (L) Immunostaining of an endothelial colony with anti–VE-cadherin mAb (blue). (M) Immunostaining of VE-cadherin+CD45− cell-derived cells after 7-day culture with anti–VE-cadherin (red) and VWF (green) Abs. (E, M) Nuclei were labeled with Hoechst 33342. (N, O) Numbers of CD45+ (N) or CD45+CD34+ cells (O) derived from 1 × 105 VE-cadherin+CD45− (VEcad+CD45−) or VE-cadherin−CD45− (VEcad−CD45−) cells. (P) Sequential analysis of the percentages of nucleated erythrocytes (red), enucleated erythrocytes (yellow), myeloid lineage cells (green), and megakaryocytes (blue) among floating cells. Each bar represents the mean of 3 independent experiments. (Q) Sequential analysis of the numbers of hematopoietic colonies per 1 × 104 VEcad+CD45− or VEcad−CD45− cell-derived cells. (R) Numbers of endothelial colonies per 1 × 103 VEcad+CD45− or VEcad−CD45− cells. (A-P) Data obtained from VEcad+CD45− cells are shown. Data are presented as mean ± SD of 3 independent experiments in N-O and Q-R. Each experiment was performed in triplicate in P-R. Original magnification × 40 (B, F, L), × 100 (A, H, J), and × 200 (C-E, G, I, K, M). Scale bars, 20 μm (C-E, G, I, K), 50 μm (M), and 100 μm (A-B, F, H, J, L).
Floating hematopoietic cells emerged from the VE-cadherin+CD45− population at approximately day 10 + 3. Their number was low until day 10 + 12 but increased exponentially afterward. Cells consisted exclusively of mature hematopoietic cells, such as erythrocytes, myeloid lineage cells, and megakaryocytes, as revealed by May-Giemsa staining and immunostaining (Figure 2C-E). Floating erythrocytes on day 10 + 6 were apparently larger than those on day 10 + 30 (Figure 2C-D). The percentage of erythrocytes among floating cells was approximately 60% to 70% throughout the experiments, which increased to some degree with time (Figure 2P). Enucleated erythrocytes accounted for less than 1% of total erythrocytes on day 10 + 6 and 6% to 10% from day 10 + 12 onward. Again, floating cells from the VE-cadherin−CD45− population were rarely observed.
We also performed a sequential standard methylcellulose colony assay to evaluate the clonogenic potential of VE-cadherin+CD45−-derived or VE-cadherin−CD45−-derived cells. As shown in Figure 2Q, few colonies were generated from VE-cadherin+CD45− cells (denoting day 10 + 0) and coculture of VE-cadherin+CD45− cells on the OP9 layer for another 12 days (day 10 + 12), consisting exclusively of granulocyte-macrophage (GM) colonies. After 18 days of coculture (day 10 + 18), other colonies, including GM (Figure 2F-G), erythroid (Figure 2H-I), and mixed (Figure 2J-K), were detected. During the experiment, few colonies were generated from the coculture of VE-cadherin−CD45− cells on the OP9 layer.
In endothelial differentiation cultures, sheetlike or cordlike VE-cadherin+ endothelial colonies were generated after 7 days almost exclusively from the VE-cadherin+CD45− population (P < .05) (Figure 2L, R). In 7-day culture, all endothelial cells had Ac-LDL uptake capacity and expressed VE-cadherin, VWF, VEGFR-2, CD31, CD34, and eNOS (Figure 2M and data not shown). Our results indicated that VE-cadherin+CD45− cells isolated on day 10 composed a population of early endothelial cells with hemogenic properties that could differentiate into mature endothelial cells.
VE-cadherin+CD45− population contains single cells with hematopoietic and endothelial capacities
We performed a single-cell deposition assay to analyze whether the VE-cadherin+CD45− population contained common progenitors for hematopoietic and endothelial lineages. Individual wells of a 96-well plate were subjected to fluorescence microscopy 24 hours after cell deposition, and wells that contained more than one GFP+ cell (ESC-derived cells) were excluded from subsequent analyses (5 of 2885 wells). Consistent with previous reports,23 when a mixture of anti–CD45, CD41, and γ-globin mAbs was used, all the round cells belonging to the hematopoietic lineage were stained positively.
Of the 2880 wells analyzed, 269 (8.6%) demonstrated clonal outgrowth consisting of endothelial progeny only (7.4%; 213 wells) (Figure S1A, available on the Blood website; see the Supplemental Figures link at the top of the online article), hematopoietic progeny only (0.17%; 5 wells) (Figure S1B), and both endothelial and hematopoietic progeny (1.1%; 31 wells) (Figure S1C). Thus, our results clearly demonstrated that the VE-cadherin+CD45− population contained common progenitors for hematopoietic and endothelial lineages.
VE-cadherin+CD45− cells generated primitive and definitive erythrocytes sequentially
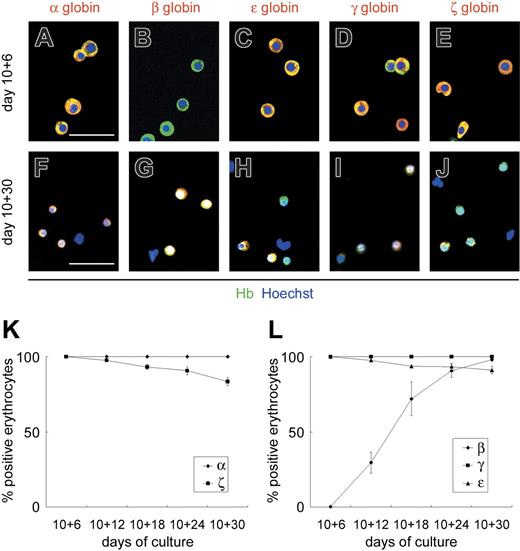
To determine whether the erythrocytes derived from the VE-cadherin+CD45− population were primitive or definitive, we analyzed the expression patterns of embryonic (ζ and ϵ), fetal (α and γ), and adult (β) globins in floating erythrocytes by sequentially immunostaining for various globin chains (Figure 3). Until day 10 + 6, all floating erythrocytes expressed ϵ- and ζ-globins, whereas β-globin expression was hardly detected (less than 1%). The percentage of floating erythrocytes positive for β-globin increased gradually from day 10 + 12, and almost all erythrocytes were positive by day 10 + 30. Meanwhile, expression of ϵ- and ζ-globins declined gradually to approximately 90% and 80% by day 10 + 30, respectively. All floating erythrocytes expressed α- and γ-globins throughout the experimental period. Others and we have found β-globin the most specific type of globin genes for the identification of definitive erythrocytes.4,25,29,30,34 Results here showed that β-globin, a specific marker for definitive erythrocytes, is up-regulated gradually in the OP9 coculture and that VE-cadherin+CD45− cells generate primitive and definitive erythrocytes sequentially.
ESC-derived VE-cadherin+CD45− cells generate primitive and definitive erythrocytes sequentially. Immunostaining of floating erythrocytes on days 10 + 6 (A-E) and 10 + 30 (F-J). Cy3 detection of erythrocytes stained with anti–α-, anti–β-, anti–ϵ-, anti–γ-, or anti–ζ-globin mAbs (red) and FITC detection with anti–Hb polyclonal Ab (green). Anti-Hb Ab, which reacts with embryonic, fetal, and adult erythrocytes, was used to detect all erythrocytes. Nuclei were labeled with Hoechst 33342. Merged images are shown. Original magnification × 200. Scale bars, 50 μm. (K-L) Sequential analysis of the proportion of erythrocytes positive for anti–α- or anti–ζ-globin mAbs (K) and anti–β-, anti–γ-, or anti–ϵ-globin mAbs (L) among the total erythrocytes. Data are presented as mean ± SD of 3 independent experiments.
ESC-derived VE-cadherin+CD45− cells generate primitive and definitive erythrocytes sequentially. Immunostaining of floating erythrocytes on days 10 + 6 (A-E) and 10 + 30 (F-J). Cy3 detection of erythrocytes stained with anti–α-, anti–β-, anti–ϵ-, anti–γ-, or anti–ζ-globin mAbs (red) and FITC detection with anti–Hb polyclonal Ab (green). Anti-Hb Ab, which reacts with embryonic, fetal, and adult erythrocytes, was used to detect all erythrocytes. Nuclei were labeled with Hoechst 33342. Merged images are shown. Original magnification × 200. Scale bars, 50 μm. (K-L) Sequential analysis of the proportion of erythrocytes positive for anti–α- or anti–ζ-globin mAbs (K) and anti–β-, anti–γ-, or anti–ϵ-globin mAbs (L) among the total erythrocytes. Data are presented as mean ± SD of 3 independent experiments.
Hematopoietic progenitors exclusively reside in the α4-integrin+ subpopulation of VE-cadherin+CD45− cells
In vivo and in vitro experiments in mice show that α4-integrin is a marker of the earliest precursor of hematopoietic cell lineage from endothelial progenitors.26 To determine whether this is applicable to primates, we sequentially traced the expression patterns of VE-cadherin, CD45, and α4-integrin in differentiating ESCs cocultured with OP9 cells. As shown in Figure 4, VE-cadherin+α4-integrin+ cells first appeared on day 6, peaked at approximately days 8 to 10, and almost disappeared by day 16. In contrast to the previously reported time-course of mouse ESC differentiation,26 VE-cadherin+ and α4-integrin+ cells simultaneously developed from monkey ESCs. CD45+ cells appeared from day 12 onward, and most coexpressed α4-integrin.
Temporal emergence of VE-cadherin+α4-integrin+ cells during ESC differentiation. As described for Figure 1, undifferentiated GFP-transfected ESCs and subsequent differentiating cells were analyzed by FACS. Percentages of cells in each quadrant among the total viable GFP+ cells are indicated. Representative results from 1 of 3 independent experiments are shown.
Temporal emergence of VE-cadherin+α4-integrin+ cells during ESC differentiation. As described for Figure 1, undifferentiated GFP-transfected ESCs and subsequent differentiating cells were analyzed by FACS. Percentages of cells in each quadrant among the total viable GFP+ cells are indicated. Representative results from 1 of 3 independent experiments are shown.
Next, we isolated VE-cadherin+α4-integrin−, VE-cadherin+α4-integrin+, and VE-cadherin−α4-integrin+ cells on day 10 after excluding dead cells and CD45+ cells to evaluate their hematopoietic and endothelial capacity (Figure 5A). Isolated cells were reseeded onto fresh confluent OP9 layers and were cultured with hematopoietic cytokine mixture. Among the VE-cadherin+CD45− population, only the α4-integrin+ subpopulation gave rise to adherent hematopoietic clusters and floating hematopoietic cells (Figure 5C, F, H). Adherent clusters grew, and the number of floating cells increased throughout the experimental period (Figure 5D, G-H). VE-cadherin+α4-integrin+ cells generated 5- to 10-fold more floating hematopoietic cells than total VE-cadherin+ cells. VE-cadherin−α4-integrin+ cells yielded more adherent clusters and floating cells than VE-cadherin+α4-integrin+ cells until day 10 + 6 (Figure 5B, E). Interestingly, however, the adherent clusters from VE-cadherin−α4-integrin+ cells disappeared by day 10 + 12, and the number of floating cells declined drastically (Figure 5H). Again, the sizes of floating erythrocytes on day 10 + 6 derived from VE-cadherin+α4-integrin+ and VE-cadherin−α4-integrin+ cells were larger than those on day 10 + 30 from VE-cadherin+α4-integrin+ cells (Figure 5E-G).
Hematopoietic progenitors exclusively reside in the α4-integrin+ subpopulation among VE-cadherin+CD45−cells. (A) VE-cadherin+α4-integrin− (VEcad+α4−), VE-cadherin+α4-integrin+ (VEcad+α4+), or VE-cadherin−α4-integrin+ (VEcad−α4+) cells were sorted on day 10 after the exclusion of PI+ and CD45+ cells. Representative FACS dot plots and percentages of gated cells are shown. Purities of viable VEcad+α4−, VEcad+α4+, and VEcad−α4+ cells are 95.0%± 3.0%, 94.1%± 0.5%, and 96.9%± 2.1%, respectively, from at least 3 independent experiments. (B-D) Light micrographs of adherent hematopoietic clusters on day 10 + 6 from VEcad−α4+ cells (B) and days 10 + 6 (C) and 10 + 18 (D) from VEcad+α4+ cells. (E-G) May-Giemsa staining of floating hematopoietic cells on day 10 + 6 from VEcad−α4+ cells (E) and days 10 + 6 (F) and 10 + 30 (G) from VEcad+α4+ cells. Original magnification × 40 (B-D) and × 200 (E-G). Scale bars, 100 μm (B-D) and 20 μm (E-G). (H) Sequential analysis of the numbers of floating viable cells per cultured 1 × 104 VE-cadherin+ (▪), VE-cadherin− (□), VEcad+α4− (○), VEcad+α4+ (•), or VEcad−α4+ (▴) cells. (I) Numbers of endothelial colonies per 1 × 103 cells in each subpopulation. (H-I) Data are presented as mean ± SD of 3 independent experiments. Each experiment was performed in triplicate. (J) RT-PCR analysis of genes associated with hematopoietic or endothelial development. Each lane contained cDNA from the following cells: adult cynomolgus monkey BM cells (lane 1), K562 erythroblastic cells (lane 2), human umbilical vein endothelial cells (lane 3), OP9 stromal cells (lane 4), total GFP+ ESC-derived cells (lane 5), VE-cadherin+α4-integrin− cells (lane 6), VE-cadherin+α4-integrin+ cells (lane 7), and VE-cadherin−α4-integrin+ cells (lane 8) sorted on day 10. Representative results from 1 of 3 independent experiments are shown.
Hematopoietic progenitors exclusively reside in the α4-integrin+ subpopulation among VE-cadherin+CD45−cells. (A) VE-cadherin+α4-integrin− (VEcad+α4−), VE-cadherin+α4-integrin+ (VEcad+α4+), or VE-cadherin−α4-integrin+ (VEcad−α4+) cells were sorted on day 10 after the exclusion of PI+ and CD45+ cells. Representative FACS dot plots and percentages of gated cells are shown. Purities of viable VEcad+α4−, VEcad+α4+, and VEcad−α4+ cells are 95.0%± 3.0%, 94.1%± 0.5%, and 96.9%± 2.1%, respectively, from at least 3 independent experiments. (B-D) Light micrographs of adherent hematopoietic clusters on day 10 + 6 from VEcad−α4+ cells (B) and days 10 + 6 (C) and 10 + 18 (D) from VEcad+α4+ cells. (E-G) May-Giemsa staining of floating hematopoietic cells on day 10 + 6 from VEcad−α4+ cells (E) and days 10 + 6 (F) and 10 + 30 (G) from VEcad+α4+ cells. Original magnification × 40 (B-D) and × 200 (E-G). Scale bars, 100 μm (B-D) and 20 μm (E-G). (H) Sequential analysis of the numbers of floating viable cells per cultured 1 × 104 VE-cadherin+ (▪), VE-cadherin− (□), VEcad+α4− (○), VEcad+α4+ (•), or VEcad−α4+ (▴) cells. (I) Numbers of endothelial colonies per 1 × 103 cells in each subpopulation. (H-I) Data are presented as mean ± SD of 3 independent experiments. Each experiment was performed in triplicate. (J) RT-PCR analysis of genes associated with hematopoietic or endothelial development. Each lane contained cDNA from the following cells: adult cynomolgus monkey BM cells (lane 1), K562 erythroblastic cells (lane 2), human umbilical vein endothelial cells (lane 3), OP9 stromal cells (lane 4), total GFP+ ESC-derived cells (lane 5), VE-cadherin+α4-integrin− cells (lane 6), VE-cadherin+α4-integrin+ cells (lane 7), and VE-cadherin−α4-integrin+ cells (lane 8) sorted on day 10. Representative results from 1 of 3 independent experiments are shown.
To clarify the difference in hematopoietic kinetics between VE-cadherin+α4-integrin+ and VE-cadherin−α4-integrin+ populations, we examined whether floating erythrocytes derived from each population were primitive or definitive. As in the VE-cadherin+CD45− population, all floating erythrocytes derived from both populations expressed α-, ϵ-, γ-, and ζ-globins but were devoid of β-globin on day 10 + 6 (Figure S2A-E, K-O), indicative of primitive erythrocytes. Moreover, almost all erythrocytes from the VE-cadherin+α4-integrin+ population were positive for β-globin by day 10 + 30, whereas the expression of ϵ- and ζ-globins declined gradually (Figure S2F-J), characteristic of definitive erythrocytes.
In endothelial differentiation cultures with VEGF, VE-cadherin+α4-integrin+ cells generated significantly more endothelial colonies than VE-cadherin+α4-integrin− cells after 7-day culture (P < .05) (Figure 5I). VE-cadherin−α4-integrin+ cells barely generated endothelial colonies.
To verify the hematopoietic and endothelial capacities of these 3 populations, gene expression profiles were investigated with the use of RT-PCR (Figure 5J). The presence of α4-integrin was confirmed specifically in the VE-cadherin+α4-integrin+ and VE-cadherin−α4-integrin+ populations. eNOS and VWF, representative endothelial proteins, were expressed in VE-cadherin+α4-integrin− and VE-cadherin+α4-integrin+ populations, whereas VWF was expressed weakly in the VE-cadherin−α4-integrin+ population. SCL and GATA-2, transcriptional factors associated with hematopoietic and endothelial development,38,39 were expressed in all 3 populations. Notably, RUNX1, a transcriptional factor associated with definitive hematopoiesis,40,41 was expressed in the VE-cadherin+α4-integrin+ and VE-cadherin−α4-integrin+ populations but not in VE-cadherin+α4-integrin− cells.
Finally, we performed a single-cell deposition assay of VE-cadherin+α4-integrin+ cells. We differentiated GFP-transfected ESCs, and single VE-cadherin+α4-integrin+ cells that were exclusively negative for CD45 were assayed on day 10. Of the 958 wells analyzed (2 of 960 wells were omitted because they contained more than 1 GFP+ cell), 106 (11.1%) demonstrated clonal outgrowth consisting of endothelial progeny only (6.3%; 60 wells), hematopoietic progeny only (0.52%; 5 wells), and both endothelial and hematopoietic progeny (4.3%; 41 wells). Hence, common progenitors for hematopoietic and endothelial lineages in the VE-cadherin+CD45− population were 4.0-fold more enriched in the α4-integrin+ subpopulation (P < .001).
Thus, these results suggested that among VE-cadherin+CD45− cells, only the α4-integrin+ subpopulation participated in primitive and definitive hematopoiesis, whereas α4-integrin+ and α4-integrin− subpopulations were involved in endothelial lineage development. Our results also showed that VE-cadherin−CD45−α4-integrin+ and VE-cadherin+CD45−α4-integrin+ cells were primary sources for primitive and definitive hematopoiesis, respectively.
Colonies consisting of primitive and definitive erythrocytes are generated from VE-cadherin+α4-integrin+ cells
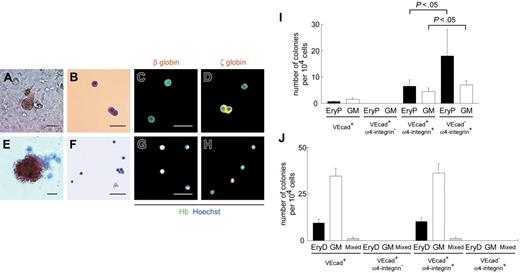
As shown, erythroid colonies were not generated from VE-cadherin+CD45− cells by day 10 + 18 with the standard methylcellulose assay (Figure 2Q). Others and we24,42 have reported the successful development of colonies consisting of primitive erythrocytes on OP9 stromal layers. Colony-forming assays were performed on OP9 layers. Colonies consisting of primitive erythrocytes were generated after 7-day coculture on OP9 cells from the VE-cadherin+, VE-cadherin+α4-integrin+, and VE-cadherin−α4-integrin+ populations but not the VE-cadherin+α4-integrin− population (Figure 6A-D, I). All erythrocytes in individual colonies from these populations were positive for ζ-globin but devoid of β-globin, indicative of primitive erythrocytes. The primitive erythroid clonogenic progenitors in the VE-cadherin+ population were 8.7-fold more enriched in the α4-integrin+ subpopulation (Figure 6I). On the other hand, the VE-cadherin−α4-integrin+ population yielded a significantly higher number of colonies consisting of primitive erythrocytes and GM than the VE-cadherin+α4-integrin+ population (each P < .05), analogous to the patterns for floating hematopoietic cells in both populations (Figure 5H). We used the standard methylcellulose assay to generate colonies consisting of definitive erythrocytes after day 10 + 18 from the VE-cadherin+ and VE-cadherin+α4-integrin+ populations but not the VE-cadherin+α4-integrin− and VE-cadherin−α4-integrin+ populations (Figure 6E-H, J). All erythrocytes in individual colonies were positive for β-globin, and some were devoid of ζ-globin, characteristic of definitive erythrocytes.
α4-Integrin+ subpopulation among VE-cadherin+CD45− cells generates hematopoietic colonies composed of primitive and definitive erythrocytes. (A-H) Light micrographs and May-Giemsa staining of colonies consisting of primitive (A-B) and definitive (E-F) erythrocytes from VE-cadherin+α4-integrin+ cells are depicted. Immunostaining data from colonies consisting of primitive (C-D) and definitive (G-H) erythrocytes from VE-cadherin+α4-integrin+ cells are also presented. Cy3 detection of erythrocytes stained with anti–β-globin (C, G) or anti–ζ-globin (D, H) mAbs (red) and FITC detection with anti-Hb polyclonal Ab (green). Nuclei were labeled with Hoechst 33342. Merged images are shown. Original magnification × 100 (A, E) and × 200 (B-D, F-H). Scale bars, 50 μm. (I-J) Numbers of hematopoietic colonies per 1 × 104 cells sorted on day 10 (I) or after 30-day coculture on OP9 layers (J) in each subpopulation. VEcad+ denotes the VE-cadherin+CD45− population. EryP and EryD represent colonies consisting of primitive and definitive erythrocytes, respectively. Data are presented as mean ± SD of 3 (I) or 2 (J) independent experiments. Each experiment was performed in duplicate (I) or triplicate (J).
α4-Integrin+ subpopulation among VE-cadherin+CD45− cells generates hematopoietic colonies composed of primitive and definitive erythrocytes. (A-H) Light micrographs and May-Giemsa staining of colonies consisting of primitive (A-B) and definitive (E-F) erythrocytes from VE-cadherin+α4-integrin+ cells are depicted. Immunostaining data from colonies consisting of primitive (C-D) and definitive (G-H) erythrocytes from VE-cadherin+α4-integrin+ cells are also presented. Cy3 detection of erythrocytes stained with anti–β-globin (C, G) or anti–ζ-globin (D, H) mAbs (red) and FITC detection with anti-Hb polyclonal Ab (green). Nuclei were labeled with Hoechst 33342. Merged images are shown. Original magnification × 100 (A, E) and × 200 (B-D, F-H). Scale bars, 50 μm. (I-J) Numbers of hematopoietic colonies per 1 × 104 cells sorted on day 10 (I) or after 30-day coculture on OP9 layers (J) in each subpopulation. VEcad+ denotes the VE-cadherin+CD45− population. EryP and EryD represent colonies consisting of primitive and definitive erythrocytes, respectively. Data are presented as mean ± SD of 3 (I) or 2 (J) independent experiments. Each experiment was performed in duplicate (I) or triplicate (J).
Thus, the VE-cadherin+α4-integrin+ population displayed primitive and definitive erythroid clonogenic activity. Our data showed that hemogenic endothelial cells are not only the sole progenitor population for definitive hematopoiesis, they are deeply involved in primitive hematopoiesis.
Discussion
Despite several similarities, a number of differences were observed between mouse and primate hematopoietic development, such as the pattern of globin switching during the shift of hematopoietic sites. To clarify the pathogenesis and treatment of hematologic disorders in humans, it was important to investigate hematopoietic development using primate materials. In the near future, it may be necessary to apply human ESC-derived products to nonhuman primates as preclinical models for cell transplantation, ahead of their use in clinical settings. However, relatively little is known about hematopoiesis during primate embryogenesis compared with mouse embryogenesis, partly because of poor availability and ethical limitations of primate embryos. Thus, primate ESCs are more promising for studies on primate embryogenesis. In addition, coculture with the OP9 stromal cells has been used successfully for hematopoietic differentiation of mouse and primate ESCs.23,24,31,42,43 In this report, we used the primate ESC and OP9 coculture system and demonstrated for the first time that α4-integrin+ hemogenic endothelial cells are deeply involved in primitive and definitive hematopoiesis in primates.
Sequential development of primitive and definitive hematopoiesis from ESC-derived endothelial cells
We showed that VE-cadherin+CD45− endothelial cells derived from nonhuman primate ESCs generate primitive and definitive erythrocytes. To date, several studies demonstrate hematopoietic differentiation of human and nonhuman primate ESCs.23,24,34,43–46 Previous in vivo and in vitro experiments in humans indicate that at least a proportion of hematopoietic cells originate in vascular endothelial cells.12,45 However, whether primitive hematopoiesis and definitive hematopoiesis originate in hemogenic endothelium in primates remains to be elucidated. Data obtained in mice are controversial. Numerous investigators report that only multilineage definitive, but not primitive, hematopoietic progenitors arise from endothelial cells,47 whereas others show that VE-cadherin+ endothelial cells derived from mouse ESCs generate primitive and definitive hematopoietic cells.48 Here, we demonstrate that primitive and definitive hematopoietic cells are, at least in part, generated from a subset of endothelial cells in primates. Because primitive hematopoiesis occurs only in the yolk sac, we hypothesized that ESC-derived VE-cadherin+CD45− endothelial cells are equivalent to those in yolk sac blood islands and possibly in the AGM region in vivo.
VE-cadherin is a specific endothelial lineage marker,15,16,26,37 whereas CD45 is widely accepted as a specific hematopoietic lineage marker except in erythroid and megakaryocytic lineage cells. Based on reports that VE-cadherin+CD45+ intermediate cells exist in mouse embryos,49,50 we isolated VE-cadherin+CD45− cells as definitive endothelial, but not hematopoietic, cells. In addition, our immunochemistry and FACS analyses demonstrated that VE-cadherin+CD45− cells on day 10 of culture coexpress other endothelial markers, such as CD31, CD34, VEGFR-2, and eNOS, and take up Ac-LDL but that they lack mature endothelial properties, including VWF expression (Figure 1D-J). These results are consistent with the established multiparameter criteria for defining endothelial cells.45,51 Furthermore, VE-cadherin+CD45− cells are devoid of hematopoietic specific marker expression, such as hemoglobin, CD45, and CD41a. Thus, the VE-cadherin+CD45− cells in this study are confirmed as endothelial, albeit immature, cells.
Studies show that β-globin is the most specific type of globin gene for the identification of definitive erythrocytes during human embryogenesis and primate ESC differentiation.4,25,29,30,34 In our experiments, VE-cadherin+CD45− cells initially produced larger, nucleated erythrocytes almost with no β-globin expression and later generated smaller, partly enucleated, erythrocytes expressing β-globin (Figures 2C-D, P, 3). This result is morphologically supported by the finding that human ESC-derived erythroblasts devoid of β-globin expression are megaloblastic and similar to primitive erythroid cells found in 4- to 5-week-old human embryos.44 On the other hand, our results showed that the high proportion of β-globin+ cells on day 10 + 30 also expressed embryonic globins (ϵ and ζ). This is consistent with previous reports that the embryonic globins and β-globin are expressed in early definitive hematopoietic cells.29,30 Hence, VE-cadherin+CD45− endothelial cells isolated on day 10 generated primitive and definitive erythrocytes sequentially.
Clonal analysis disclosed that 1.1% of the single VE-cadherin+CD45− cells yielded endothelial and hematopoietic cells (Figure S1). Our results are in agreement with previous data,45 and the characteristics of endothelial cells isolated by both groups are similar. Given that VE-cadherin−CD45− cells almost never generated endothelial colonies, even under endothelial culture conditions (Figure 2R), we suggest that bipotential cells among the VE-cadherin+CD45− population are not the contaminating cells during cell sorting.
α4-Integrin is a marker of the hemogenic endothelial cells in primates
The differences between hemogenic and nonhemogenic endothelial cells and how a subset of endothelial cells acquires hemogenic capacity during early embryogenesis in primates remain unclear. Here, we used α4-integrin as a candidate marker of hemogenic endothelial cells. To our knowledge, there are no reports on the expression or function of α4-integrin during early primate embryogenesis. Developmentally, in mice, α4-integrin is expressed on yolk sac blood islands and all hematopoietic cells in the fetal liver.52,53 It is essential for the maintenance of efficient development of multilineage progenitors in the fetal liver54 and is a marker of the earliest precursor of the hematopoietic cell lineage from endothelial progenitors in vivo and in vitro.26 We show that the α4-integrin+, not the α4-integrin− subpopulation among ESC-derived endothelial cells, yields hematopoietic cells. Except for the generation of primitive hematopoiesis, this is consistent with previous findings in mice.26 In our study, α4-integrin+ hemogenic endothelial cells generated primitive and definitive hematopoietic cells, as confirmed by immunostaining of erythroid cells/colonies (Figures 6, S2), though it remains to be clarified whether primitive and definitive hematopoiesis have common precursors.
In human embryos, yolk sac blood islands are observed from gestational days 16 to 24, and intraaortic hematopoietic cell clusters are observed in the AGM region from days 27 to 40.55,56 The frequency of hemogenic endothelial cells in vivo reflects the actual blood-forming activity of these hematopoietic tissues as a function of developmental age.12 Interestingly, α4-integrin+ endothelial cells were detected for a limited period of time in this study (Figure 4), but primitive and definitive hematopoietic cells emerged sequentially, suggesting that precursors of primitive and definitive hematopoiesis arise simultaneously but that the definitive precursors required a maturation phase (on OP9) before they differentiated into hematopoietic cells. This may recapitulate the hemogenic activity of endothelial cells in and temporal lag of hematopoiesis between yolk sac blood islands and the AGM region in vivo.
The transcriptional factor Runx1 is required for definitive hematopoiesis.40,41 Runx1 is expressed in endothelial cells where definitive hematopoietic cells emerge, specifically the yolk sac, vitelline and umbilical arteries, and ventral wall of the dorsal aorta in the AGM region but not in endothelial cells elsewhere in mouse embryos.57 In addition, Runx1 is reportedly required for the formation of intraaortic hematopoietic clusters and the emergence of hematopoietic stem cells.57–59 Our RT-PCR data show that RUNX1 is expressed in the hemogenic α4-integrin+ subpopulation, but not the nonhemogenic α4-integrin− subpopulation, among ESC-derived endothelial cells (Figure 5J). RUNX1 is additionally expressed in the VE-cadherin−α4-integrin+ population generating mainly primitive erythrocytes, consistent with the finding that primitive erythrocytes in the yolk sac express Runx1 8 days after coitus in mice.57
Based on the results from this study, we propose a model of primitive/definitive hematopoietic and endothelial lineage development from primate ESCs (Figure 7). We suggest that VE-cadherin−CD45−α4-integrin+ and VE-cadherin+CD45−α4-integrin+ cells contain a subset with hemogenic capacity and that these are primary sources for primitive and definitive hematopoiesis, respectively. Moreover, we hypothesize that in primates, hemogenic endothelial cells are located in the yolk sac and in the AGM region, generate primitive and definitive hematopoietic cells, and are marked by the temporal expression of α4-integrin. The function of α4-integrin during hematopoietic development remains to be elucidated.
Schematic representation of the differentiation pathway from ESCs into endothelial or primitive and definitive hematopoietic cell lineages on coculture with OP9 cells.
Schematic representation of the differentiation pathway from ESCs into endothelial or primitive and definitive hematopoietic cell lineages on coculture with OP9 cells.
In conclusion, we have successfully induced the differentiation of nonhuman primate ESCs into hemogenic endothelial cells, which in turn gave rise to primitive and definitive hematopoietic cells with the OP9 coculture system. Hemogenic activity exclusively resides in the α4-integrin+ subpopulation among endothelial cells. Our culture system should provide an alternative, powerful tool for understanding early hematopoietic development during primate embryogenesis, such as the processes triggering transition from endothelial cells to hematopoietic cells in the yolk sac and the AGM region or the initial emergence of hematopoietic stem cells.
Authorship
Contribution: G.S. performed all the experiments and wrote the manuscript. K.U. contributed to the study design, data analysis, and writing of the manuscript. T.H. contributed to the study design, data analysis, and writing of the manuscript. M.A. contributed to the study design, writing of the manuscript, and interpretation of the results. A.N. contributed to the study design and developed a method for colony-forming assays. F.M. supervised the flow cytometric analysis. H.S. supervised the culture of primate embryonic stem cells. H.Y.L. contributed vital antibodies. D.H.K.C. contributed vital antibodies and revised the manuscript. R.T. supervised the culture of primate embryonic stem cells. M.S. contributed vital antibodies. N.N. contributed to the study design and supervised the culture of primate embryonic stem cells. T.N. supervised the study and contributed to the study design and to the writing and revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tatsutoshi Nakahata, Department of Pediatrics, Graduate School of Medicine, Kyoto University, 54 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto 606-8507, Japan; e-mail: tnakaha@kuhp.kyoto-u.ac.jp.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants from Science Research on Priority Areas; Creative Science Research; the Japan Society for the Promotion of Science; the Ministry of Education, Culture, Sports, Science and Technology; and Tanabe Seiyaku (Osaka, Japan) for help with primate ESC preparation.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal