To the editor:
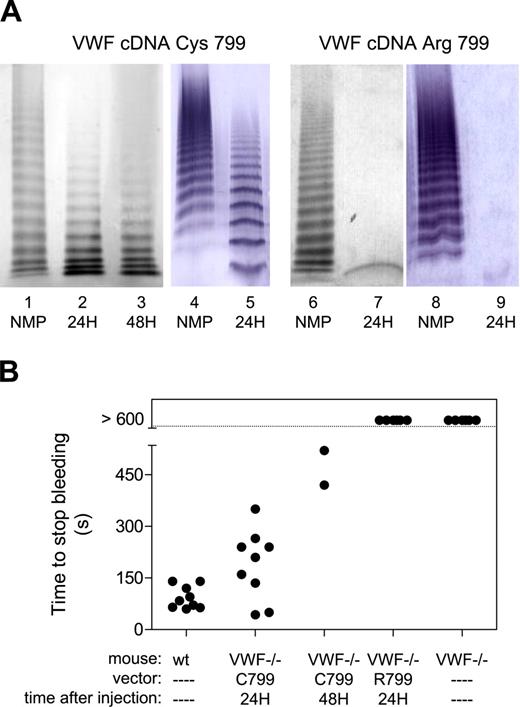
Recently, Pergolizzi et al1 reported in Blood about correction of the bleeding tendency in von Willebrand factor (VWF)–deficient mice via hydrodynamic delivery of murine VWF (mVWF) cDNA. The complete correction of the bleeding time was surprising since the multimer pattern was abnormal in these mice following treatment.1 (Fig 2) The mVWF cDNA used by Pergolizzi et al1 originates from University Medical Center (UMC) Utrecht (P.J.L., P.G.d.G) and was distributed to several laboratories shortly after completion, which took place before the cDNA was fully analyzed. In vitro analysis, however, revealed that the distributed cDNA resulted in low expression levels in stably transfected baby hamster kidney (BHK) cells and HK293T cells, with greater than 90% of intracellular retention. Moreover, purified recombinant mVWF protein displayed impaired collagen binding and lacked multimers exceeding 6-mers (not shown), suggesting that the cDNA encoded a dysfunctional VWF protein. Dysfunction was confirmed in vivo. First, intravenous injection of purified mVWF protein did not correct the bleeding time in VWF-deficient mice (> 600 s versus 93 ± 32 s for wild-type littermates). Second, no VWF multimers could be detected when expression of mVWF was established via hydrodynamic injection of 100 μg cDNA (Figure 1A, lanes 7 and 9; ie, similar conditions used by Pergolizzi et al1 ). Again, no correction of bleeding time was obtained in any of these mice (Figure 1B), despite antigen levels between 22% and 65%. These findings prompted us to compare our original cDNA sequence (Balb/c) with that of other murine strains (C57Bl6/J, A/J, and Casa/Rk mice; kindly provided by D. Motto, University of Michigan). We detected 1 striking difference, the presence of an Arg at position 799, whereas a Cys was present in the other sequences. Moreover, the presence of Cys799 (located within the D′ region) is conserved among species as well (human, pig, canine), suggesting a structural or functional role for this residue. Since the D′ region is critical for multimerization, absence of Cys799 could explain the lack of multimerization we observed. After replacing Arg799 with Cys, a new analysis was performed. In vitro expression resulted in greater than 75% of the protein being secreted as fully multimerized mVWF molecules (not shown). Multimerized mVWF was also observed upon hydrodynamic delivery of corrected VWF cDNA in VWF-deficient mice, although the balance between high and low multimers was different from normal mice (Figure 1A, lanes 2-3, 5). This approach further resulted in expression levels between 148% and 392%, 24 hours after injection, and between 146% and 281% after 48 hours, all of which are significantly higher than levels obtained with the original sequence mVWF/Arg799. Finally, expression of mVWF/Cys799 resulted in a (partial) correction of the bleeding time both at 24 hours and 48 hours after hydrodynamic delivery of 100 μg cDNA (Figure 1B), confirming that the revised sequence encodes a functional protein.
Analysis of recombinant mVWF. (A) Multimeric pattern of recombinant murine VWF after hydrodynamic delivery of 100 μg mVWF cDNA. After 24 hours (lanes 2, 5, 7, and 9) or 48 hours (lane 3), plasma was collected and examined for multimeric composition. Lanes 1, 4, 6, and 8 represent normal pooled mouse plasma (NMP). Lanes 2, 3, and 5 represent samples from mice injected with vector mVWF/Cys799, whereas lanes 7 and 9 represent those injected with vector mVWF/Arg799. For comparison, data of 2 laboratories have been included: lanes 1-3 and 6-7 originate from the Institut National de la Santé et de la Recherche Médicale (INSERM) Unité (U) 770 (Le Kremlin-Bicêtre, France), whereas lanes 4-5 and 8-9 originate from the Laboratory for Thrombosis Research (Kortrijk, Belgium). (B) Mice were examined for their bleeding time at 24 hours or 48 hours after hydrodynamic delivery of 100 μg mVWF cDNA using a tail-cut bleeding model. For wild-type mice, bleeding stopped at 93 ± 32 seconds (n = 9). Twenty-four hours after injection with 100 μg mVWF/Cys799-cDNA, the bleeding time was 188 ± 101 seconds. Bleeding did not stop (> 600 seconds) in noninjected mice or mice injected with 100 μg mVWF/Arg799-cDNA.
Analysis of recombinant mVWF. (A) Multimeric pattern of recombinant murine VWF after hydrodynamic delivery of 100 μg mVWF cDNA. After 24 hours (lanes 2, 5, 7, and 9) or 48 hours (lane 3), plasma was collected and examined for multimeric composition. Lanes 1, 4, 6, and 8 represent normal pooled mouse plasma (NMP). Lanes 2, 3, and 5 represent samples from mice injected with vector mVWF/Cys799, whereas lanes 7 and 9 represent those injected with vector mVWF/Arg799. For comparison, data of 2 laboratories have been included: lanes 1-3 and 6-7 originate from the Institut National de la Santé et de la Recherche Médicale (INSERM) Unité (U) 770 (Le Kremlin-Bicêtre, France), whereas lanes 4-5 and 8-9 originate from the Laboratory for Thrombosis Research (Kortrijk, Belgium). (B) Mice were examined for their bleeding time at 24 hours or 48 hours after hydrodynamic delivery of 100 μg mVWF cDNA using a tail-cut bleeding model. For wild-type mice, bleeding stopped at 93 ± 32 seconds (n = 9). Twenty-four hours after injection with 100 μg mVWF/Cys799-cDNA, the bleeding time was 188 ± 101 seconds. Bleeding did not stop (> 600 seconds) in noninjected mice or mice injected with 100 μg mVWF/Arg799-cDNA.
As derived from their note added in proof, the cDNA used by Pergolizzi et al1 encoded VWF/Arg799, which was found by all of us independently to be associated with impaired multimerization and lack of in vivo functionality. The reason for these discrepancies is unclear, but we strongly recommend other investigators to perform future experiments employing mVWF comprising Cys799 instead of Arg799.
Authorship
Correspondence: P. J. Lenting, Laboratory for Thrombosis and Haemostasis, G.03.550, Department of Clinical Chemistry & Haematology, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands; e-mail: p.j.lenting@umcutrecht.nl.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal