Abstract
Helios is a zinc-finger protein belonging to the Ikaros family of transcriptional regulators. It is expressed, along with Ikaros, throughout early stages of thymocyte development where it quantitatively associates with Ikaros through C-terminal zinc-finger domains that mediate heterodimerization between Ikaros family members. To understand the role of Helios in T-cell development, we used a retroviral vector to express full-length Helios or a Helios isoform that lacked the N-terminal DNA-binding domain in hematopoietic progenitor cells of reconstituted mice. Constitutive expression of full-length Helios resulted in an inhibition of T-cell development at the double-negative stage within the thymus. Although expression of the DNA-binding mutant of Helios did not contribute to developmental abnormalities at early times after transplantation, 60% of animals that expressed the Helios DNA-binding mutant developed an aggressive and transplantable T-cell lymphoma 4 to 10 months after transplantation. These results demonstrate a vital function for Helios in maintaining normal homeostasis of developing T cells and formally show that non–DNA-binding isoforms of Helios are lymphomagenic if aberrantly expressed within the T-cell lineage.
Introduction
The Ikaros family of zinc-finger DNA-binding proteins plays a vital role in the generation of all lymphoid-derived hematopoietic cell lineages.1,2 Members of this highly conserved family of proteins that are expressed predominantly in lymphoid cells include Ikaros, Helios, and Aiolos. Each protein is highly homologous throughout an N-terminal DNA-binding domain comprised of 4 Krüppel-like zinc-finger domains of the C2H2 type. Each protein also contains a C-terminal region comprised of 2 zinc-finger domains that mediate dimerization between family members.3–10 Extensive alternative mRNA splicing of exons within the DNA-binding domain of Ikaros, Helios, and Aiolos results in the expression of multiple, functionally distinct isoforms of each protein that are differentially expressed in unique subsets of hematopoietic cells.11–14 All mRNA splice variants characterized to date retain the C-terminal multimerization domain, therefore permitting the association of isoforms that lack the DNA-binding domain with isoforms that retain the ability to bind DNA. Association of non–DNA-binding isoforms of Ikaros with full-length Ikaros proteins has been shown to inhibit DNA-binding and transactivation potential of Ikaros in in vitro assays.5
Inhibition of Ikaros activity by non–DNA-binding Ikaros isoforms is further supported by the phenotype of animals with a targeted deletion of exons encoding the Ikaros DNA-binding domain (Ikaros DN)1 and in animals with a point mutation in one of the zinc-finger exons that disrupts DNA binding without affecting multimerization.15 Heterozygous Ikaros DN animals express similar levels of the dominant-negative and wild-type Ikaros alleles and develop T-cell lymphoma and leukemia with 100% penetrance soon after 3 months of age.16 Prior to the development of lymphoma, thymocytes within the heterozygous animals displayed increased proliferative responses to T-cell receptor (TCR)–mediated signals, which suggests that non–DNA-binding isoforms of Ikaros suppress the antiproliferative activity of full-length Ikaros isoforms during thymocyte development. Lymphoma in the animals was characterized by a loss of heterozygosity at the Ikaros locus, which indicates that the balance of different Ikaros mRNA splice variants is crucial for normal homeostasis of developing thymocytes. The increased proliferative response of B lymphocytes to B-cell receptor stimulation observed in animals with a targeted disruption of the Aiolos DNA-binding domain17 or in transgenic animals that express full-length Helios in the B-cell lineage18 suggests that higher order complexes comprised of Ikaros family members function as key regulators of proliferative responses in both T and B lymphocytes.
Consistent with the role of Ikaros family members as tumor suppressor genes in lymphocytes are observations of increased expression of non–DNA-binding isoforms of Ikaros, Aiolos, and Helios in cases of acute lymphocytic leukemia (ALL). An increase in non–DNA-binding isoforms of Ikaros was observed in childhood and adult pre-B–cell ALL and in childhood T-cell ALL.19–23 Expression of non–DNA-binding isoforms of Helios have been noted in adult cases of T-cell ALL.24–26 To date, a loss-of-function mutation in the Helios gene has not been described so it remains unclear as to whether Helios is also functioning as a tumor suppressor in lymphocytes in a manner analogous to Ikaros and Aiolos.
In addition to the function of Ikaros family members in the regulation of lymphoproliferation, Ikaros has also been shown to regulate early developmental decisions within the hematolymphoid system. Mice homozygous for the Ikaros DN mutation died soon after birth and completely lacked all B-, T-, and NK-cell lineages.1 In contrast, mice homozygous for an Ikaros null mutation (targeted deletion of the C-terminal dimerization domain) had no B, NK, or fetal T cells and severely reduced numbers of specific γδ T-cell subsets and thymic dendritic cells.2 Postnatal αβ T-cell development proceeded with an aberrant skewing to CD4+ thymocytes in the absence of Ikaros. The more severe phenotype of the Ikaros DN mutation in the T-cell lineage suggests that the dominant-negative Ikaros isoform is able to interact or interfere with another critical factor(s) that regulates the earliest stage of αβ T-cell development.
Because Helios is expressed in early double-negative (DN) thymocytes and quantitatively associates with Ikaros in developing T cells,7,8,27 we wished to determine whether Helios might be functioning as a critical regulator of αβ T-cell development. In these experiments, we have used a retroviral expression system to express wild-type Helios or a Helios mutant lacking the DNA-binding domain in hematopoietic progenitor cells used to reconstitute lethally irradiated mice. In contrast to our initial expectation, mice receiving transplants of cells expressing full-length Helios exhibited a partial block in αβ T-cell development and showed increased frequencies of γδ T cells and NK cells in peripheral lymphoid organs. Animals reconstituted with cells expressing the Helios DNA-binding mutant or the control GFP vector had no detectable abnormality in the development of T, B, or NK cells. However, 9 of 15 mice expressing the Helios DNA-binding mutant developed T-cell lymphoma 4 to 10 months after transplantation. These results suggest that isoforms of Helios (and Ikaros) that lack a DNA-binding domain may primarily function in regulating lymphocyte proliferation, whereas unique combinations of the DNA-binding isoforms of Ikaros, Helios, and Aiolos would have the additional function of regulating developmental decisions within the lymphoid lineage.
Materials and methods
Construction of Helios retroviral vectors
A cDNA encoding full-length Helios (kindly provided by Dr Stephen Smale, UCLA, Los Angeles, CA) was cloned into the MSCV-IRES-GFP retroviral vector28 and orientation was confirmed by DNA sequencing. A FLAG epitope tag (DYKDDDDK) was cloned at the 3′ end of the DNA-binding Helios mutant using polymerase chain reaction (PCR). Primers used to generate the Helios DNA-binding mutant, Helios Δ49-285, were as follows: Helios 1, 5′-gccgccatggactacaaggacgacgatgacaaggaagcagacgcaattgatggc-3′; Helios 2, 5′-gcttgtcatgtgacttggct-3′; Helios 3, 5′-ggggaaaagcttatgcgattc-3′; Helios 4, 5′-ttatcactatacctttctcttcttttttggatgtcgccatccgtgggaagg-3′. PCR products generated by amplification from the full-length Helios cDNA using Helios 1 and 2 primers in one reaction and Helios 3 and 4 primers in another reaction were then blunt-end ligated and cloned into MSCV upstream of the IRES sequence. Verification of the cloned product was confirmed by DNA sequencing.
Retroviral transduction and transplantation
Bone marrow cells were isolated from C57BL/6-Ly-5.1 mice injected intraperitoneally 4 days previously with 5-fluorouracil (150 mg/kg body weight). Transductions were done by coculturing bone marrow on transiently transfected BOSC23 retroviral producer cells for 48 hours. Approximately 1 to 2 million transduced cells were then transplanted into lethally irradiated congenic C57BL/6-Ly-5.2 recipients as previously described. 29
Immunoprecipitation
BOSC23 cells were transfected with MSCV plasmids expressing Helios Δ49 or IK4 or both and lysed 24 hours later in lysis buffer (1% Triton X-100, 50 mM Tris-HCl, pH 7.4, 300 mM NaCl, 5 mM EDTA). Each lysate was incubated with anti-FLAG antibody (M2; Sigma-Aldrich, St Louis, MO) bound to protein G-Sepharose for 2 hours at 4°C. Immune complexes were sedimented by centrifugation, washed 3 times with wash buffer (0.1% Triton X-100, 50 mM Tris-HCl, pH 7.4, 300 mM NaCl, 5 mM EDTA), resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and analyzed by Western blotting using an anti-Helios goat polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or an anti-Ikaros rabbit polyclonal antisera (gift from Dr Stephen Smale, UCLA).
Western analysis of Helios and Ikaros expression in thymocytes
Lysates from 2 × 106 fluorescence-activated cell sorting (FACS)–sorted thymocytes or from transiently transfected BOSC23 cells were analyzed by Western blotting using goat polyclonal antibodies against N-terminal (M-20) and C-terminal (G-20) Helios peptides (Santa Cruz Biotechnology), a rabbit polyclonal antibody against Ikaros (gift from Dr Stephen Smale, UCLA), or a mouse monoclonal antibody against β-actin (Sigma-Aldrich). Donkey anti–goat, goat anti–rabbit, and goat anti–mouse polyclonal IgG (each conjugated to horseradish peroxidase) were used as secondary reagents for enhanced chemiluminescence (ECL) detection (Amersham Biosciences, Piscataway, NJ). For β-actin, blots originally probed for Ikaros or Helios were stripped and reprobed with the β-actin antibody.
Antibodies and FACS analysis
Bone marrow, spleen, thymus, and Peyer patches were collected, homogenized, and treated to remove red blood cells by hypotonic shock. Monoclonal antibodies used in immunofluorescence staining were reactive against Thy-1.1 (19XE5), c-Kit (2B8), CD3 (KT31.1), CD4 (GK1.5), CD5 (53-7.3), CD8 (53-6.7), Ter119, B220 (6B2), Gr-1 (8C5), Mac-1 (M1/70), CD43 (S7), CD44 (1M7), CD25 (3C7), IL-7Rα (CD127, SB/199), IgM (Jackson ImmunoResearch, West Grove, PA), CD45.2 (104), γδ receptor (GL3), and NK1.1 (PK136). Nonspecific binding of antibody to Fc receptor was blocked by first incubating cells with an Fc-blocking antibody, 2.4G2 (BD PharMingen, San Diego, CA), prior to incubation with specific antibodies.
T-cell proliferation assay: SNARF labeling
A single-cell suspension was generated from the spleens of chimeric mice. Erythrocytes were lysed by hypotonic shock, and the remaining cells were labeled with 6 μM SNARF-1 (Molecular Probes, Eugene, OR) for 10 minutes at 37°C. The staining reaction was quenched with fetal calf serum, and the cells were washed and plated at a density of 300 000 cells/200 μL media in a 96-well round-bottom plate in RPMI 1640 plus 10% fetal calf serum (Hyclone, Logan, UT). Cells were stimulated with 3 μg/mL anti-CD3 (145-2C11) and 3μg/mL anti-CD28 (37.51). Control cells were left unstimulated. Cells were harvested daily for 4 days following stimulation and stained with anti-CD4 (GK1.5) or anti-CD8 (53-6.7) and analyzed for SNARF-1 dilution on a FACSCalibur flow cytometer (BD Biosciences, San Diego, CA).
T-cell proliferation: tritiated thymidine incorporation
Ecotropic packaging cells (Phoenix-E) were transfected with retroviral constructs using Lipofectamine (Life Technologies, Bethesda, MD). Retrovirus-containing cell supernatant was collected 48 hours after transfection and used to transduce C57BL/6 CD4+ splenic T cells in the presence of polybrene (8 mg/mL). CD4+ T cells were purified using CD4 microbeads (Miltenyi Biotec, Auburn, CA; catalog no. 130-049-201), and then were activated by plate-bound anti-CD3 and anti-CD28 stimulation (2 μg/mL each). Retroviral supernatant was added 24 hours after T-cell activation. GFP+CD4+ T cells were sorted after 2 days of transduction. Proliferative responses were measured by culturing 1 × 105 sorted GFP+ T cells in the presence of irradiated antigen-presenting cells (APCs) and varying concentrations of anti-CD3 (1.5 and 2.0 mg/mL) for 48 hours. Each concentration was set up in quadruplicate wells. Cells were then pulsed with 3H-thymidine for 16 hours and then harvested for counting on a beta plate scintillation counter (Wallac, Turku, Finland).
Real-time PCR
Each of the DN, DP, and SP thymocyte subsets underwent FACS once and then were resorted to ensure a final sort purity of more than 98% for each subset. DN1 thymocytes were sorted as CD4−CD8−CD25−B220−Mac-1−IL-7Rα−CD44+c-Kit+. Total RNA was extracted from the sorted populations using RNA STAT-60 (Tel-Test B, Friendswood, TX). cDNA was transcribed from isolated RNA using High Capacity cDNA Archive Kit (Applied Biosystems, Weiterstadt, Germany). Gene-specific primer sequences were generated with the aid of ABI Prism Primer Express software and are as follows: helios forward 5′-TAAGCTCAGCTTATTCTCAGGTCTATCA -3′and helios reverse 5′-ATGTTGTTTTCGTGACTATCAGATGTT-3′; hprt forward 5′-CTGGTGAAAAGGACCTCTCG-3′ and hprt reverse 5′-TGAAGTACTCATTATAGTCAAGGGCA-3′. Real-time PCR was carried out in a 20-μL volume using 2 × SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer's instructions and run on a 7900HT Sequence Detection System (Applied Biosystems).
Image acquisition
Images were acquired at 160× magnification using a Leica DMRB upright microscope (Wetzlar, Germany), PL FluoroTar 16× objective with 0.5 numerical aperture and a Hamamatsu C5810 CCD camera. Acquisition software was IPLab version 3.6 (Signal Analytics Corp, BD Biosciences, San Jose, CA). Sections were stained with hematoxylin/eosin and visualized using immersion oil (Leica).
Results
Generation of Helios mutants
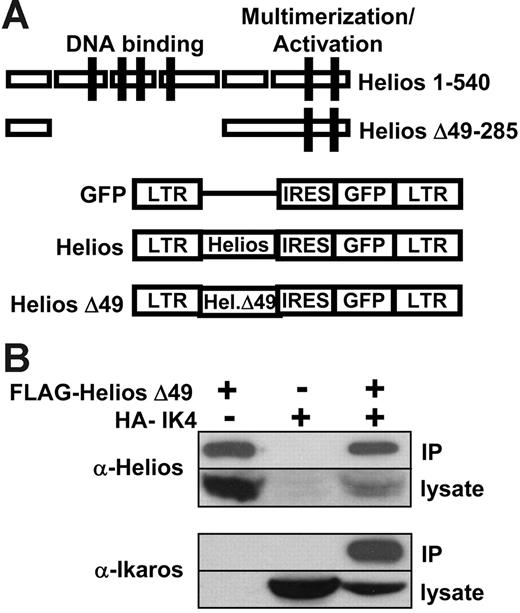
To assess the function of Helios in early lymphoid-lineage development and leukemia, we generated retroviral constructs that expressed either full-length Helios (amino acids 1-540) or a Helios mutant that lacked the 4 N-terminal zinc finger domains that mediate Helios DNA binding (Δ49-285; Figure 1A). In both cases, the Helios variants were coexpressed with a green fluorescent protein (GFP) reporter that was translated from an internal ribosome entry sequence (IRES) in the MSCV retroviral vector.28 The Δ49-285 variant also contained a FLAG epitope tag at the C-terminus to allow for molecular characterization of the mutant protein. Western blot analysis of lysates from transiently transfected BOSC23 cells30 using an N-terminal Helios polyclonal antibody indicated that both Helios variants were stably expressed from the MSCV retroviral vector (see Western data described in “Loss of Ikaros is not required for lymphoma in moribund Δ49-285 animals”).
Full-length and mutant Helios constructs in MSCV-IRES-GFP retroviral vector. (A) Schematic representation of MSCV-IRES-GFP and 2 Helios constructs. Zinc-finger domains comprising the DNA-binding and multimerization domains of Helios are indicated by the solid boxes. The Helios Δ49-285 variant contained a C-terminal FLAG tag. LTR indicates long terminal repeat; IRES, internal ribosomal entry site; GFP, green fluorescent protein. (B) Coimmunoprecipitation of FLAG-Helios Δ49-285 with HA-IK4. An anti-FLAG monoclonal antibody was used to immunoprecipitate FLAG-Helios Δ49-285 from BOSC23 cell lysates that were transiently transfected with the indicated retroviral constructs. The presence of Helios or Ikaros in the immune complexes was detected by Western blot using anti-Helios or anti-Ikaros polyclonal antibodies.
Full-length and mutant Helios constructs in MSCV-IRES-GFP retroviral vector. (A) Schematic representation of MSCV-IRES-GFP and 2 Helios constructs. Zinc-finger domains comprising the DNA-binding and multimerization domains of Helios are indicated by the solid boxes. The Helios Δ49-285 variant contained a C-terminal FLAG tag. LTR indicates long terminal repeat; IRES, internal ribosomal entry site; GFP, green fluorescent protein. (B) Coimmunoprecipitation of FLAG-Helios Δ49-285 with HA-IK4. An anti-FLAG monoclonal antibody was used to immunoprecipitate FLAG-Helios Δ49-285 from BOSC23 cell lysates that were transiently transfected with the indicated retroviral constructs. The presence of Helios or Ikaros in the immune complexes was detected by Western blot using anti-Helios or anti-Ikaros polyclonal antibodies.
To determine whether deletion of the DNA-binding domain in the Δ49-285 Helios variant would alter its ability to interact with Ikaros through the C-terminal multimerization domain, we performed an immunoprecipitation experiment using FLAG-tagged Helios Δ49-285 and an HA-tagged Ikaros isoform (IK4).5 Nuclear extracts from BOSC23 cells transiently transfected with the indicated constructs were incubated with an anti-FLAG monoclonal antibody and immunoprecipitates were then used for Western analysis using an anti-Helios (Figure 1B top panel) or an anti-Ikaros polyclonal antibody (Figure 1B bottom panel). IK4 was only immunoprecipitated in the presence of FLAG-Helios Δ49-285, which indicates that the Δ49-285 Helios variant was capable of interacting with Ikaros. The reciprocal experiment using an anti-HA monoclonal antibody to immunoprecipitate IK4 and FLAG-Helios gave similar results (data not shown).
Full-length Helios expression partially blocks αβ T-cell development in the thymus
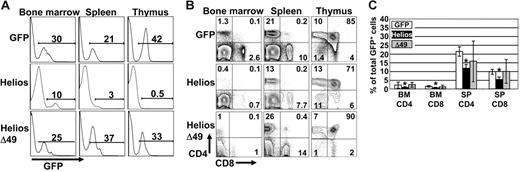
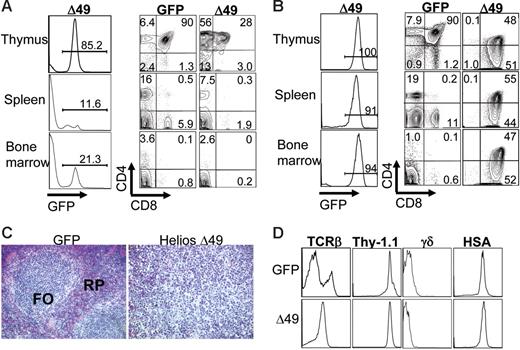
To investigate the function of Helios on early lymphocyte development, lethally irradiated mice were recipients of hematopoietic progenitor cells that stably expressed each Helios construct. All GFP control and Helios-reconstituted animals showed stable levels of GFP chimerism in different hematopoietic tissues for longer than 10 months, which indicates that primitive hematopoietic stem cells were transduced with the retroviral constructs (Figure 2A). One observation that was immediately apparent was the extremely low levels of GFP chimerism in the thymus of all animals reconstituted for more than 10 weeks with cells that expressed full-length Helios (Figure 2A; mean percentage of GFP+ thymocytes was 1.7% for full-length Helios, n = 16, and 25.8% for GFP control animals, n = 8). Bone marrow chimerism averaged 13.3% and 19.8% for full-length Helios and GFP control-reconstituted animals, respectively. This indicates that the severe reduction in GFP+ thymocytes expressing full-length Helios was not due to loss of stem cell activity in the presence of Helios. Further analysis showed an approximate 5-fold increase in the frequency of GFP+ DN thymocytes in all full-length Helios-expressing animals compared with GFP control and Helios Δ49-285–reconstituted animals (Figure 2B). The overall reduction in GFP+ thymocyte production in full-length Helios animals resulted in an approximate 50% reduction in both CD4 and CD8 SP (GFP+) cells in both the bone marrow and spleen of reconstituted animals (Figure 2C). No gross abnormalities in thymopoiesis were initially observed in animals reconstituted with the Helios Δ49-285 mutant.
T-cell development is partially blocked by enforced expression of full-length Helios. (A) Representative FACS analyses of bone marrow, spleen, and thymus from mice reconstituted with bone marrow transduced with the indicated retroviral vectors at 12 to 16 weeks after transplantation. Numbers on the histograms show the percentage of GFP+ cells in the indicated organs. (B) Cells were gated for GFP expression and then analyzed for CD4 versus CD8 expression by FACS analysis. (C) Frequency of CD4+ and CD8+ cells among the total GFP+ cells in bone marrow (BM) and spleen (SP) at 12 to 16 weeks after transplantation (GFP, n = 8; Helios, n = 12; Δ49, n = 7). Asterisks indicate statistically significant differences between control and Helios mice (spleen, P < .01; bone marrow, P < .05; for Helios versus GFP control mice). Error bars represent standard deviation from the mean.
T-cell development is partially blocked by enforced expression of full-length Helios. (A) Representative FACS analyses of bone marrow, spleen, and thymus from mice reconstituted with bone marrow transduced with the indicated retroviral vectors at 12 to 16 weeks after transplantation. Numbers on the histograms show the percentage of GFP+ cells in the indicated organs. (B) Cells were gated for GFP expression and then analyzed for CD4 versus CD8 expression by FACS analysis. (C) Frequency of CD4+ and CD8+ cells among the total GFP+ cells in bone marrow (BM) and spleen (SP) at 12 to 16 weeks after transplantation (GFP, n = 8; Helios, n = 12; Δ49, n = 7). Asterisks indicate statistically significant differences between control and Helios mice (spleen, P < .01; bone marrow, P < .05; for Helios versus GFP control mice). Error bars represent standard deviation from the mean.
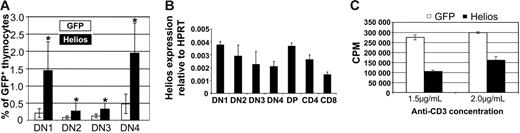
To characterize the increased frequency of GFP+ DN thymocytes in full-length Helios animals, we analyzed CD44 and CD25 expression, which divided the CD4−CD8−B220−Mac-1− population into 4 distinct subsets. In full-length Helios animals, we noted significant increases in the frequencies of all DN stages compared with GFP control animals (Figure 3A). Even though the frequency of all DN subsets was increased, the absolute numbers of the DN2, DN3, and DN4 subsets were reduced approximately 3- to 5-fold. There was a 2-fold reduction in the absolute numbers of GFP+ DN1 cells. These data suggest that the significant reduction in thymopoiesis in the presence of full-length Helios (as evidenced by the extremely low GFP chimerism; Figure 2A) may partially be explained by reduced expansion within the DN populations that give rise to DP cells.
Characterization of expression and function of full-length Helios. (A) Increased frequencies of DN thymocytes among cells that express full-length Helios. Data represent mice at 10 to 16 weeks after transplantation from 6 separate experiments. Asterisks indicate statistically significant differences between GFP control (n = 17) and Helios mice (n = 16) (DN1 and DN2, P < .001; DN3 and DN4, P < .05). DN1 thymocytes were analyzed as CD4−CD8−CD25−B220−Mac-1−CD44+ (B) Real-time reverse transcription-PCR analysis of endogenous Helios expression in thymocyte subsets sorted by FACS. Data shown are representative of 3 independent cDNA preparations (2 independent preparations for DN1) using mRNA from double-sorted cells. DN1 thymocytes were gated as CD4−CD8−CD25−B220−Mac-1−IL-7Rα −CD44+c-Kit+. cDNA from each subset was assayed in quadruplicate in each experiment. Error bars represent 1 SD from the mean. (C) CD4+ splenic T cells expressing either GFP or full-length Helios were stimulated in vitro with varying concentrations of anti-CD3 in the presence of irradiated APCs for 48 hours. Incorporation of tritiated thymidine into stimulated cells is shown. Error bars indicate ± SEM from quadruplicate wells from a representative experiment performed in triplicate.
Characterization of expression and function of full-length Helios. (A) Increased frequencies of DN thymocytes among cells that express full-length Helios. Data represent mice at 10 to 16 weeks after transplantation from 6 separate experiments. Asterisks indicate statistically significant differences between GFP control (n = 17) and Helios mice (n = 16) (DN1 and DN2, P < .001; DN3 and DN4, P < .05). DN1 thymocytes were analyzed as CD4−CD8−CD25−B220−Mac-1−CD44+ (B) Real-time reverse transcription-PCR analysis of endogenous Helios expression in thymocyte subsets sorted by FACS. Data shown are representative of 3 independent cDNA preparations (2 independent preparations for DN1) using mRNA from double-sorted cells. DN1 thymocytes were gated as CD4−CD8−CD25−B220−Mac-1−IL-7Rα −CD44+c-Kit+. cDNA from each subset was assayed in quadruplicate in each experiment. Error bars represent 1 SD from the mean. (C) CD4+ splenic T cells expressing either GFP or full-length Helios were stimulated in vitro with varying concentrations of anti-CD3 in the presence of irradiated APCs for 48 hours. Incorporation of tritiated thymidine into stimulated cells is shown. Error bars indicate ± SEM from quadruplicate wells from a representative experiment performed in triplicate.
Endogenous Helios is expressed throughout thymocyte development
To account for the reduction in absolute numbers of DN thymocytes and the overall reduction in thymopoiesis in the presence of constitutive Helios expression, we analyzed the expression pattern of endogenous Helios in FACS-sorted thymocyte subsets by real-time PCR using both β-actin and HPRT amplification to normalize Helios expression levels between samples. In 3 independent cDNA preparations (2 preparations for the DN1 subset) using RNA isolated from cells that were sorted once and then resorted prior to mRNA isolation, there was not a large variation in the relative level of Helios expression between thymocyte subsets (Figure 3B), with the exception that Helios seemed to be down-regulated in later DN stages (most notably, DN4) and again as DP thymocytes differentiated to the CD4 and CD8 SP lineages. These results establish a correlation between Helios down-regulation and thymocyte proliferation that normally occurs at the DN4 stage.31
Full-length Helios represses T-cell proliferation
Because Ikaros and Aiolos had previously been shown to function as tumor suppressor genes for T- and B-lineage cells, we tested whether full-length Helios might also function to suppress T-cell proliferation stimulated by anti-CD3 and irradiated APCs in vitro. Incorporation of tritiated thymidine into stimulated CD4+ thymocytes was inhibited 2- to 2.5-fold in the presence of full-length Helios compared with cells that expressed the control GFP vector at either concentration of anti-CD3 (Figure 3C). These results suggest that retroviral expression of full-length Helios in vivo may be inhibiting thymocyte development by partially restricting thymocyte proliferation within early DN subsets.
Full-length Helios expression leads to increased frequencies of γδ T cells and NK cells in lymphoid organs
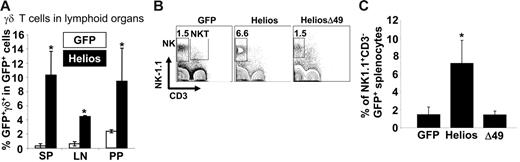
The observations that Ikaros null mice lack NK cells and certain subsets of γδ T cells2 and that Helios is quantitatively associated with Ikaros in thymocytes7 suggest that Helios may play an important role in development of both γδ T cells and NK cells. Analysis of γδ T-cell development within the GFP+ fraction of spleen, thymus, lymph node, and Peyer patches in animals reconstituted for at least 11 weeks showed significant increases in γδ T-cell frequencies in all lymphoid organs of full-length Helios reconstituted mice compared with GFP control mice (11-fold increase in spleen, P < .001; 7-fold increase in mesenteric lymph nodes, P < .001; 4.5-fold increase in Peyer patches, P < .01; Figure 4A). There was a 3-fold increase in γδ T-cell frequency among the GFP+ cells in the thymus of full-length Helios mice but this was not statistically significant (data not shown). There was no significant difference in γδ T-cell frequency in any lymphoid organ of animals reconstituted with cells expressing the Δ49-285 Helios isoform or the GFP control (data not shown).
Increased frequencies of NK and γδ T cells in full-length Helios reconstituted animals. (A) Frequency of γδ T cells among the total GFP+ population in spleen (SP), mesenteric lymph node (LN), and Peyer patches (PP). Data represent a total of 6 mice for each construct. Asterisks indicate a statistically significant difference between GFP control and Helios mice (spleen and mesenteric lymph node, P < .001; Peyer patches, P < .01). (B) NK-1.1 and CD3 expression in GFP+ spleen cells were analyzed in mice reconstituted with cells expressing the indicated retroviral vectors at 12 to 16 weeks after transplantation. (C) Frequency of NK cells among total GFP+ splenocytes (GFP, n = 13; Helios, n = 13; Helios Δ49-285, n = 6). Asterisk indicates a statistically significant difference between GFP control and Helios mice (P < .01). Error bars represent 1 D from the mean.
Increased frequencies of NK and γδ T cells in full-length Helios reconstituted animals. (A) Frequency of γδ T cells among the total GFP+ population in spleen (SP), mesenteric lymph node (LN), and Peyer patches (PP). Data represent a total of 6 mice for each construct. Asterisks indicate a statistically significant difference between GFP control and Helios mice (spleen and mesenteric lymph node, P < .001; Peyer patches, P < .01). (B) NK-1.1 and CD3 expression in GFP+ spleen cells were analyzed in mice reconstituted with cells expressing the indicated retroviral vectors at 12 to 16 weeks after transplantation. (C) Frequency of NK cells among total GFP+ splenocytes (GFP, n = 13; Helios, n = 13; Helios Δ49-285, n = 6). Asterisk indicates a statistically significant difference between GFP control and Helios mice (P < .01). Error bars represent 1 D from the mean.
Analysis of NK cell frequency in the spleens of reconstituted animals (n = 13 for full-length Helios and GFP control; n = 6 for Helios Δ49-285) indicated a 3.5-fold increase in the frequency of mature NK cells (NK1.1+CD3−) that expressed full-length Helios as compared with GFP control or Helios mutant animals (Figure 4B-C, P < .01). There was no difference in the mean frequencies of NK1.1+CD3+ NKT cells between full-length Helios, Helios Δ49-285, or GFP control reconstituted animals (Figure 4B).
Animals expressing the Δ49-285 Helios isoform develop T-cell lymphoma
As noted previously (Figures 2 and 4), there was little difference in the phenotypes of T-lineage subsets between control and Helios Δ49-285 animals at early times after transplantation (4-16 weeks). However, by 4 to 10 months after transplantation, 9 of 15 of the Δ49-285 Helios mice had become ill from apparent T-cell lymphoma with dramatically enlarged thymi. In contrast, none of 21 full-length Helios animals and one of over 150 GFP control animals developed T-cell lymphoma beyond 5 months after transplantation (data not shown). This indicates that random retroviral insertional mutagenesis contributes little to the phenotype observed in the Δ49-285 animals. The Δ49-285 animals with signs of lymphoma had high percentages of GFP+ thymocytes (Figure 5B), splenomegaly, and lymphadenopathy (data not shown). The normal architecture of the thymus and the spleen was completely disrupted in the sick animals by large numbers of blasts (Figure 5C and data not shown). Interestingly, all animals with lymphoma seemed to have selected for expansion of cells (or a clone) with very high levels of Δ49-285 Helios expression (compare GFP+ cells in the thymus of Helios Δ49-285 animals in Figures 5B and 2A).
Expression of the Helios Δ49-285 isoform results in T-cell lymphoma. (A) FACS analysis of hematolymphoid tissues in a nonsick Helios Δ49-285 animal at 5 months after transplantation showing an abnormal frequency of CD4 SP thymocytes among cells that are GFP+. The abnormal accumulation of CD4+ (or CD8+ in some cases) cells was restricted to the thymus at early stages of lymphoma. The extent of GFP chimerism in each tissue for the nonsick Δ49-285 Helios animal is shown in the left column. (B) FACS analysis of a moribund Helios Δ49-285 animal at 7 months after transplantation indicating high frequencies of GFP+ cells in the thymus and abnormal accumulation of DP and CD8 SP thymocytes in the spleen and bone marrow. Numbers in the quadrants of each plot represent percentages of the indicated populations among gated GFP+ cells. (C) Splenic architecture was severely disrupted in animals exhibiting lymphoma. FO indicates lymphoid follicle; RP, red pulp; original magnification, × 160. (D) Characterization of cell-surface marker profiles of lymphoma cells in the thymus of a representative moribund Δ49-285 Helios animal (n = 5) versus the GFP control. Analysis was done on gated GFP+ cells.
Expression of the Helios Δ49-285 isoform results in T-cell lymphoma. (A) FACS analysis of hematolymphoid tissues in a nonsick Helios Δ49-285 animal at 5 months after transplantation showing an abnormal frequency of CD4 SP thymocytes among cells that are GFP+. The abnormal accumulation of CD4+ (or CD8+ in some cases) cells was restricted to the thymus at early stages of lymphoma. The extent of GFP chimerism in each tissue for the nonsick Δ49-285 Helios animal is shown in the left column. (B) FACS analysis of a moribund Helios Δ49-285 animal at 7 months after transplantation indicating high frequencies of GFP+ cells in the thymus and abnormal accumulation of DP and CD8 SP thymocytes in the spleen and bone marrow. Numbers in the quadrants of each plot represent percentages of the indicated populations among gated GFP+ cells. (C) Splenic architecture was severely disrupted in animals exhibiting lymphoma. FO indicates lymphoid follicle; RP, red pulp; original magnification, × 160. (D) Characterization of cell-surface marker profiles of lymphoma cells in the thymus of a representative moribund Δ49-285 Helios animal (n = 5) versus the GFP control. Analysis was done on gated GFP+ cells.
The accumulation of abnormal T cells in the Δ49-285 animals originated in the thymus, which was suggested by the substantial increase in GFP+ thymocytes prior to GFP+ thymocyte expansion at other hematopoietic sites (Figure 5A). Among moribund Δ49-285 animals, abnormal DP thymocytes could be detected in both the bone marrow and spleen (Figure 5B).
Characterization of lymphoma cells in the thymus and spleen of Helios mutant animals
The Δ49-285 Helios mutation generates a Helios protein that closely resembles the Ikaros dominant-negative isoform created by the targeted deletion of the Ikaros DNA-binding domain.1 Expression of the dominant-negative Ikaros allele in heterozygous Ikaros knockout animals resulted in the rapid development (soon after 3 months of age) of a transplantable and clonal T-cell lymphoma.16 The abnormal cells in the Ikaros heterozygous animals expressed the CD3-TCR complex but exhibited variable expression of the CD4 and CD8 coreceptor molecules.16 In the moribund Helios Δ49-285 animals, we also observed variable expression of CD8 or CD4 SP cells in addition to DP thymocytes (Figure 5A-B). Animals also had abnormal accumulations of DP cells in the spleen and bone marrow (Figure 5B). In all cases of lymphoma, expanded GFP+ thymocytes expressed the Thy-1.1 T-cell marker, HSA (CD24), and low levels of TCRβ (Figure 5D and data not shown). Cells were negative for γδ T-cell receptor (Figure 5D). In the spleen, GFP+ cells were again positive for either CD4 or CD8 coreceptor expression and for CD43 and CD5, which are expressed on peripheral T-lineage cells (data not shown).
Helios Δ49-285 expression results in more rapid proliferation of SP splenic T cells
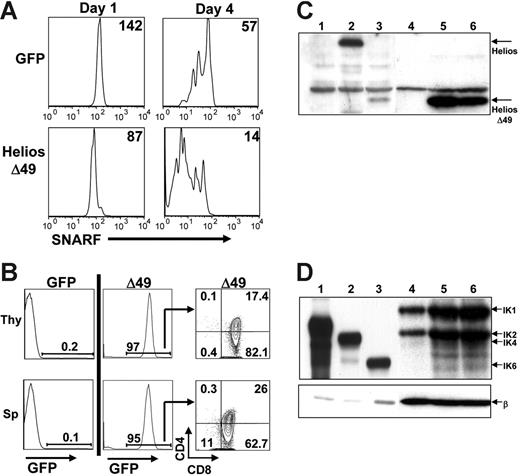
To test whether expression of Helios Δ49-285 would result in enhanced proliferation of SP thymocytes, we labeled splenocytes isolated from 3 Helios Δ49-285 animals that were not sick or from 3 transplant-age and sex-matched GFP control animals with the lipophilic cell-tracking dye, SNARF-1. Labeled cells were stimulated with anti-CD3 and anti-CD28 and were monitored for the percentage of responding CD4 and CD8 SP cells and the number of cell divisions that occurred over a 4-day in vitro culture period. Aliquots from each well were analyzed daily beginning at day 1 for GFP and SNARF-1 fluorescence, as well for CD4 and CD8 expression, using flow cytometry. The mean fluorescence intensity (MFI) of SNARF-1 for the GFP+-gated population in Helios Δ49-285 wells on day 1 was normalized to the MFI of SNARF-1 on GFP+ control cells at day 1 to control for differences in initial levels of SNARF labeling (Figure 6A; MFI is shown in upper right). Analysis of independent wells between day 1 and day 4 showed that CD8+ cells expressing Helios Δ49-285 went through an average of 1.8 more cell divisions compared with CD8+ cells expressing the GFP control vector. These results indicate that non–DNA-binding isoforms of Helios (like Ikaros) can accelerate the proliferation rate of in vitro-activated SP T cells.
Enhanced in vitro T-cell proliferation and aggressive T-cell expansion on secondary transfer of Δ49-285-expressing splenocytes. (A) Splenocytes from GFP control or Δ49-285–expressing animals were labeled with the lipophilic dye, SNARF-1, prior to costimulation with anti-CD3 and anti-CD28 treatment. The mean fluorescence intensity (MFI) of SNARF-1 is indicated following 1 and 4 days of culture after in vitro stimulation. All plots shown were first gated on GFP+CD8+ cells. Experiments were done using cells isolated from 3 independent GFP control and 3 nonsick Helios Δ49-285 animals at 4 months after transplantation. (B) Representative FACS analysis of the thymus (Thy) and spleen (Sp) at 4 to 5 weeks after transplantation of 2 × 106 splenocytes from primary GFP control (n = 2) or moribund Δ49-285 mice (n = 2) into 5 nonirradiated, congenic secondary recipient animals per primary animal. (C-D) Western blot analysis of Helios (C) and Ikaros (D) expression in 2 × 106 GFP+ thymocytes purified from 1 GFP control (lane 4) and 2 moribund Δ49-285 Helios animals (lanes 5 and 6). After probing with Helios or Ikaros, blots were stripped and reprobed with a monoclonal antibody to β-actin (β). Lanes 1 to 3 show extracts from BOSC23 cells transfected with retroviral vectors expressing the GFP parent (C: lane 1), full-length Helios (C: lane 2), Helios Δ49-285 (C: lane 3), IK2 (D: lane 1), IK4 (D: lane 2), and IK6 (D: lane 3) as controls.
Enhanced in vitro T-cell proliferation and aggressive T-cell expansion on secondary transfer of Δ49-285-expressing splenocytes. (A) Splenocytes from GFP control or Δ49-285–expressing animals were labeled with the lipophilic dye, SNARF-1, prior to costimulation with anti-CD3 and anti-CD28 treatment. The mean fluorescence intensity (MFI) of SNARF-1 is indicated following 1 and 4 days of culture after in vitro stimulation. All plots shown were first gated on GFP+CD8+ cells. Experiments were done using cells isolated from 3 independent GFP control and 3 nonsick Helios Δ49-285 animals at 4 months after transplantation. (B) Representative FACS analysis of the thymus (Thy) and spleen (Sp) at 4 to 5 weeks after transplantation of 2 × 106 splenocytes from primary GFP control (n = 2) or moribund Δ49-285 mice (n = 2) into 5 nonirradiated, congenic secondary recipient animals per primary animal. (C-D) Western blot analysis of Helios (C) and Ikaros (D) expression in 2 × 106 GFP+ thymocytes purified from 1 GFP control (lane 4) and 2 moribund Δ49-285 Helios animals (lanes 5 and 6). After probing with Helios or Ikaros, blots were stripped and reprobed with a monoclonal antibody to β-actin (β). Lanes 1 to 3 show extracts from BOSC23 cells transfected with retroviral vectors expressing the GFP parent (C: lane 1), full-length Helios (C: lane 2), Helios Δ49-285 (C: lane 3), IK2 (D: lane 1), IK4 (D: lane 2), and IK6 (D: lane 3) as controls.
Lymphoma is transplantable into secondary hosts
To determine whether thymocytes or splenocytes from moribund Δ49-285 animals would be malignant in secondary recipient animals, we transferred 2 × 106 cells (thymocytes or splenocytes) into 5 nonirradiated, congenic recipient mice. Four weeks after intravenous injection of Δ49-285–expressing cells from 2 independent primary animals, all mice were sick and displayed significant enlargement of lymphoid organs including the thymus, spleen, and lymph nodes (data not shown). There were no sick animals among the 5 recipients that received an equivalent number of splenocytes or thymocytes from transplant age-matched GFP control animals (low levels of GFP+ cell reconstitution in secondary recipients of GFP control cells was due to the transfer of splenocytes or thymocytes instead of bone marrow cells). FACS analysis of transplant recipients showed extremely high levels of GFP+ T cells in the thymus and spleen of all animals reconstituted with Δ49-285 cells (Figure 6B). Abnormal cells displayed the same heterogeneity of CD4 and CD8 expression that was observed in the primary animal used for the transplantation. These results indicate that splenocytes or thymocytes from Helios Δ49-285 animals with signs of lymphoma can aggressively expand in secondary recipient animals and can rapidly induce death with complete penetrance.
Loss of Ikaros is not required for lymphoma in moribund Δ49-285 animals
In the context of Ikaros heterozygous animals, loss of the wild-type Ikaros allele was associated with progression to lymphoma.16 To determine if loss of Ikaros was required for disease progression in the sick, Helios Δ49-285 animals, 2 × 106 GFP+ thymocytes were sorted from one GFP control and 2 moribund Helios Δ49-285 animals for Western analysis using polyclonal antibodies to detect Helios or Ikaros expression (Figure 6C and D, respectively). As shown in lanes 4 to 6 of Figure 6C, endogenous full-length Helios was almost nondetectable in both GFP control and Helios Δ49-285–expressing thymocytes. The very low level of Helios expression relative to Ikaros in thymocytes was previously observed by others.7 Based on densitometry, expression of the Helios Δ49-285 mutant was at least 50-fold higher than the endogenous full-length Helios protein. Importantly, expression of endogenous, DNA-binding isoforms of Ikaros (particularly IK1 and IK2) was not reduced in Helios Δ49-285–expressing thymocytes (lanes 5 and 6) compared with GFP control thymocytes (lane 4) after normalization with a β-actin control antibody (Figure 6D). These results indicate that non-DNA–binding isoforms of Helios are sufficient to promote T-cell lymphoma progression in the presence of wild-type (or even increased) levels of Ikaros.
Discussion
The contrasting phenotypes between Ikaros DN mutant animals,1 which displayed a complete absence of αβ T cells, and Ikaros null animals,2 which retain the ability to differentiate into mature αβ T cells, suggests that the dominant-negative allele of Ikaros may be interfering with the function of another factor that is essential for the earliest stages of αβ T-cell development. One possible candidate factor could be Helios, which is expressed at the earliest stages of thymocyte development (Figure 3B). If Helios were the factor being inhibited by the DN allele of Ikaros to block T-cell development, then we would have anticipated that expression of full-length Helios would promote αβ T-cell development and Helios Δ49-285 expression would inhibit thymocyte development. In contrast, full-length Helios suppressed thymocyte development by a mechanism that is perhaps best explained by the ability of Helios to inhibit thymocyte proliferation (Figures 2 and 3C). Loss-of-function mutations in other Ikaros family members, including Ikaros and Aiolos, have shown that both factors function as tumor suppressors for lymphocytes. Data showing that expression of the Δ49-285 Helios mutation in thymocytes results in T-cell lymphoma and enhanced SP T-cell proliferation (Figures 5–6) also support an antiproliferative function for the full-length DNA-binding isoforms of Helios.
Expression of a non–DNA-binding isoform of Helios resulted in no detectable abnormalities in development of T, B, or NK cells in the bone marrow, thymus, or peripheral lymphoid organs before the onset of T-cell lymphoma (Figures 2,Figure 3–4). This suggests that non–DNA-binding isoforms of Helios may not function to regulate developmental decisions but rather may act in association with DNA-binding isoforms of Helios or Ikaros to promote lymphocyte proliferation. In a similar manner, expression of a non–DNA-binding isoform of Ikaros contributed to enhanced thymocyte proliferation in response to stimulation through the TCR but did not result in skewing of hematopoietic development.16 Similar observations regarding development of αβ T cells were made in animals that expressed a non–DNA-binding isoform of Ikaros (IK-5) from the proximal lck promoter.32 This suggests that levels of Ikaros (or Helios) expressed from the wild-type alleles were sufficient to promote proper lymphocyte development in the context of constitutive expression of non–DNA-binding isoforms of either factor.
Animals that expressed the Δ49-285 Helios variant ultimately progressed to an aggressive and transplantable T-cell lymphoma by 4 to 10 months after transplantation. These results demonstrate that expression of non–DNA-binding isoforms of Helios can be oncogenic within the T-cell lineage. Rapid development of T-cell lymphoma (within 3 months) was also observed for expression of a non–DNA-binding isoform of Ikaros in the context of Ikaros heterozygous knockout animals.16 In the case of the Ikaros animals, lymphoma was accompanied by loss of the remaining wild-type Ikaros allele, thus resulting in the complete loss of Ikaros DNA-binding isoforms in leukemic thymocytes. In our experiments, the Δ49-285 Helios variant was introduced into wild-type C57BL/6 bone marrow cells, which retain 2 wild-type alleles of both Helios and Ikaros. The delay in the development of lymphoma in the context of the Δ49-285 Helios variant (4-10 months) could be due to the presence of the wild-type alleles, which would suppress lymphoma development. Notably, Western blot analysis of leukemic thymocytes showed that levels of endogenous DNA-binding isoforms of Ikaros are not down-regulated during disease progression. Rather, selection for expansion of cells that express extremely high levels of the non–DNA-binding isoform of Helios was correlated with lymphoma. This result is consistent with a number of studies showing enhanced expression of non–DNA-binding isoforms of either Ikaros or Helios in human cases of T-lymphocyte leukemia.12,19,20,24–26
The normal development of lymphoid cells and the control of lymphoproliferative responses is regulated by a complex interaction between multiple members of the Ikaros gene family and proteins that mediate changes in chromatin structure. The experiments described in this study establish Helios as an important regulator of thymocyte development and indicate that aberrant mRNA splicing or deletion of the DNA-binding domain of Helios results in significantly increased risk for development of T-cell lymphoma and lymphoproliferative disease.
Authorship
Contribution: Z.Z. performed research, designed experiments, and collected and analyzed data; C.S.S. performed research, designed experiments, and collected and analyzed data; J.T.B. performed research and analyzed data; R.K. performed research and analyzed data; C.V.C. performed research and analyzed data; and C.A.K. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Z.Z. and C.S.S. contributed equally to this manuscript.
Correspondence: Christopher A. Klug, Departments of Microbiology, Biochemistry, and Pathology, Division of Developmental and Clinical Immunology, University of Alabama at Birmingham, Birmingham, AL; e-mail: chrisk@uab.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by NIH grants RO1DK55650 and RO1AI055667. J.T.B. and C.S.S. were supported by the Immunologic Diseases and Basic Immunology training grant (T32AI07051).
We wish to thank Dr John F. Kearney for his support; Dr Stephen Smale and Dr Bradley Cobb for the Helios cDNA and insightful comments related to the work; and Drs Richard Lopez, Colleen Witt, and Sheetal Purohit for valuable discussions. Thanks to Weifan Jia for technical support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal