Abstract
Nasal NK/T-cell lymphoma is a rare disease entity with a poor outcome. Expression of antiapoptotic proteins has not been extensively investigated in this entity. Forty-eight patients with nasal T/NK-cell lymphoma who received first-line polychemotherapy (n = 44) or chemoradiotherapy (n = 4) were analyzed for expression of active caspase-3 (aC3), granzyme B protease inhibitor 9 (PI9), and Bcl-2 proteins. Lymphomas were CD3+/CD5−/granzyme B+ and EBV-associated. Median age was 46 years. Stage I/II disease was present in 75% of the cases and an International Prognostic Index (IPI) score less than 1 in 65%. With a median follow-up of 6.3 years, 5-year event-free survival (EFS) and overall survival (OS) rates were 39% and 49%, respectively. Apoptotic index was scored as high in 32% of cases and PI9 expression as positive in 68%, whereas 35% disclosed a high number of aC3+ tumor cells. Univariate analysis showed that absence of PI9 and low apoptotic index were associated with poor outcome, but not aC3 expression nor IPI score. By multivariate analysis, both parameters affected independently EFS (P = .02 and .08, respectively) and OS (P = .009 and .04). In view of its constitutive expression by normal NK cells, it is suggested that loss of PI9 expression in tumor cells may reflect some mechanism associated with progression.
Introduction
Nasal NK/T-cell lymphoma has been recently recognized as a distinct lymphoma subtype within the World Health Organization (WHO) classification.1 It shows a predilection for Asians, Mexicans, and South Americans contrasting with its rarity in Western countries. Clinically, it is characterized by a localized disease of the nasal cavity, the nasopharynx, paranasal sinuses, or palate. Nasal NK/T-cell lymphomas derive putatively from natural killer (NK) cells or more rarely from a subset of γδ or αβ cytotoxic T cells and show a striking association with Epstein-Barr virus (EBV).1,2 Treatment strategies have not been fully defined yet,3–11 and most series, mainly from Eastern countries, have pointed out their poor clinical outcome after treatment with radiotherapy given alone or in combination with chemotherapy.
It is generally accepted on the basis of in vitro studies that the resistance of neoplastic cells to cytotoxic agents may be due to inhibition of apoptosis,12,13 a programmed cell death activated by a cascade of proteases called caspases, leading to degradation of vital cytoplasmic and nuclear proteins. Three major pathways of caspase activation have been described14 : (1) a cell-surface death receptor-signaling pathway (Fas, tumor necrosis factor) leading to activation of caspase-8; (2) a stress-induced pathway defined by a pivotal event—mitochondrial outer membrane permeabilization—leading to cytochrome c release and activation of caspase-9; and (3) the cytotoxic granules pathway used by cytotoxic T lymphocytes (CTLs) and NK cells against target cells involving the release of cytolytic enzymes such as perforin and granzyme B (GrB). GrB has been shown to activate caspase-3 directly or indirectly via the stress-induced pathway.15 Because the final execution of apoptosis ultimately results in activation of caspase-3,16 it might be expected that high levels of activated caspase-3 (aC3) reflect proper functioning of at least one of the 3 apoptotic pathways and result in chemotherapy-sensitive tumor cells. In this regard, it has been reported that a high number of aC3+ Reed-Sternberg cells in pretreatment biopsies predicted a favorable clinical outcome in Hodgkin lymphoma.17
Execution of apoptosis also depends on the expression of apoptosis-regulating proteins, including Bcl-2 and the recently identified GrB-specific protease inhibitor 9 (PI9). Bcl-2 inhibits specifically the stress-induced pathway18 and its overexpression has been shown to be related to poor clinical outcome in diffuse large B-cell lymphomas.19,20 PI9 is a serine protease, also named serpin 9, which is present in cytotoxic effector cells to protect them against their own GrB.21 PI9 has also been shown to be expressed in the neoplastic cells of several lymphoma subtypes, including NK/T-cell lymphoma, suggesting a novel mechanism for tumor cells to escape their elimination by CTLs.22 In this respect, high numbers of PI9+ neoplastic cells have been reported to predict a poor outcome in anaplastic large cell lymphoma, whereas a high number of aC3+ tumor cells correlated with a more favorable prognosis.23
This prompted us to analyze a series of 48 patients with nasal Tl/NK-cell lymphoma who received chemotherapy as first-line treatment according to Groupe d'Etude des Lymphomes de l'Adulte (GELA) protocols and specially to investigate in pretreatment biopsies whether activation of caspase-3 and expression levels of the antiapoptotic Bcl-2 and PI9 proteins were related to clinical outcome.
Patients, materials, and methods
Patient selection
Forty-eight patients with proven nasal NK/T-cell lymphoma diagnosed between 1987 and 2003 were selected from the files of the LNH 87, 93, and 98 GELA protocols (45 patients) and from the files of the Department of Pathology, Hôpital Henri Mondor, Créteil, France (3 patients) on the basis of the following criteria: (1) diagnosis of NK/T-cell lymphoma in a primary biopsy of the nasopharyngeal region (excluding Waldeyer ring) and (2) clinical and follow-up data available. In this study, we required for a diagnosis of nasal NK/T-cell lymphoma that the malignant cells had a CD3ϵ+ CD20− phenotype, a cytotoxic profile, and disclosed association with EBV. According to French legislation at the time of the study, no informed consent was required to perform supplemental immunohistochemical studies on archival material. The LNH 87, LNH 93, and LNH 98 trials from the GELA are institutional review board–approved protocols, approved by the Hôpital Saint-Louis (Paris, France).
Clinical staging and treatment
Performance status was based on the Eastern Cooperative Oncology Group (ECOG) scale (0-4). Serum lactate dehydrogenase (LDH) level was expressed as the ratio over the maximum normal value. Patients were scored according to the International Prognostic Index (IPI).24
All patients but 3 were treated according to the consecutive LNH87, LNH93, and LNH98 trials of the GELA, as previously published.26–35 Consequently, 42 patients received an anthracycline-based chemotherapy, as follows: cyclophosphamide, hydroxydaunomycin, Oncovin, prednisone (CHOP) regimen alone in 5 cases25,26 or followed by involved field irradiation in 4 cases27 ; Adriamycin, cyclophosphamide, vindesine, bleomycin, prednisone (ACVBP) regimen, a dose-intensified CHOP-like combination, followed by consolidative sequential chemotherapy in 24 cases27–31 ; or high-dose therapy with autologous transplantation in 329,31 ; cyclophosphamide, Oncovin, prednisone, Adriamycin/Cytoxan, vincristine, etoposide (COPADM/CYVE) regimen in 6 patients.32 Six patients received non–anthracycline-based regimens as follows: cyclophosphamide, vincristine, prednisone (CVP) in 2 patients33 ; etoposide, Solu-Medrol, high-dose ara-C, Platinol (ESHAP) in 2 patients34 ; and dexamethasone-gemcitabine, oxaliplatin (Dexa-GEMOX; gemcitabine 1000 mg/m2 intravenously on day 1, oxaliplatin 100 mg/m2 intravenously on day 1, dexamethasone 40 mg daily, given orally on day 1-4) in the last 2 patients.
Response to treatment was considered as complete (CR), unconfirmed complete (CRu), or failure according to the International Workshop criteria.35
Histopathologic studies
At the time of enrollment in the LNH87, LNH93, and LNH98 protocols, tumor biopsies were reviewed and initially classified according to the updated Kiel classification and Working Formulation,36,37 based on morphologic examination of slides stained with hematoxylin and eosin or Giemsa and on immunohistochemistry comprising at least CD20 and CD3ϵ. For the purpose of the present study, 2 hematopathologists (P.G. and C.B.) reviewed all CD3+ lymphomas diagnosed on a nasopharyngeal biopsy and completed the phenotype to reclassify them according to the WHO classification1 and to evaluate the presence of the following parameters: angiocentrism/angioinvasion, epitheliotropism, and necrosis as well as to assess the predominant small/medium- or large-cell component. In addition, the apoptotic index was determined, based on counting the absolute number of apoptotic cells in 20 high-power fields (hpf's). We used the threshold of 10 apoptotic cells/hpf for statistical analysis. Detection of apoptotic cells was made at distance from necrotic areas, using the morphologic criteria as described by Walker et al.38
Immunohistochemical staining
Immunohistochemistry was performed on deparaffinized tissue sections using an indirect immunoperoxidase method to evaluate T-, NK- and B-cell differentiation antigens. After appropriate antigen retrieval, slides were stained for CD20, CD3ϵ (DakoCytomation, Glostrup, Denmark), CD5, GrB, CD56 (Novocastra, Newcastle, United Kingdom), and T-cell intracellular antigen 1 (TiA1; Immunotech, Marseille, France).
In addition, the expression of apoptotic-related proteins was investigated using antibodies against Bcl-2 (clone 124, DakoCytomation), the GrB inhibitor PI9,39 whereas for aC3, the monoclonal rabbit antibody against aC3 (PharMingen, San Diego, CA) was used. The 3 antibodies required antigen retrieval by microwave irradiation in a citrate buffer (pH 6), after which sections were incubated, respectively, overnight at 4°C (aC3) or for 1 hour at room temperature (PI9, Bcl-2). For aC3, a tyramide signal amplification system (CSAII kit, DakoCytomation) was applied.
EBV studies
LMP-1 (CS1-4, DakoCytomation) was detected by immunohistochemistry. The in situ hybridization (ISH) procedure for the detection of the EBV-encoded RNA (EBER) transcripts was performed using the fluorescein-conjugated EBV (EBERs 1 and 2) oligonucleotides probes (DakoCytomation), as previously reported.40
Evaluation of tumor cells expressing Bcl-2, PI9, and aC3
A semiquantitative evaluation of PI9 and Bcl-2 expression was performed by estimating the percentage of positive tumor cells and scored as follows: −, 0% to 10%; +, 10% to 25%; ++, 25% to 50%; +++, 50% to 100%. For the purpose of the study, a cut-off point of 10% stained tumor cells was chosen for statistical analysis. Therefore cases with more than 10% PI9+ cells were scored as positive for PI9 expression and cases with less than 10% PI9+ cells as negative.
For aC3, 20 hpf's in areas distant from necrosis were screened and the mean value of positive tumor cells per field was calculated. Only malignant cells with nuclear or nuclear/cytoplasmic staining were scored, thus excluding macrophages with apoptotic debris. A cut-off of 10 aC3+ tumor cells/hpf was chosen for statistical analysis.
Statistical analysis
Overall survival (OS) was measured from the date of diagnosis to the date of death from any cause or last follow-up. Event-free survival (EFS) was calculated from the date of diagnosis to disease progression, relapse, death, or last follow-up. Survival curves were estimated using the method of Kaplan-Meier41 and compared using the log-rank test.42 The prognostic value for EFS and OS of the following parameters was studied by univariate analysis: IPI score, histology, apoptotic index, and expression of PI9, aC3, and Bcl-2. Multivariate analysis was performed using the Cox proportional hazard model.43 Differences were considered significant when the 2-sided P < .05.
All statistical analyses were performed using SAS 9.13 software (SAS Institute, Cary, NC).
Results
Clinical characteristics of NK/T-cell lymphomas at presentation
The main clinical features are summarized in Table 1. There were 29 men and 19 women with a median age of 46 years. Most patients (79%) presented with a localized stage I/II nasopharyngeal disease. Ten patients had stage IV disease, as a result of involvement of distant lymph nodes (n = 3), central nervous system (n = 2), or bone marrow, lung, pleural cavity, liver, or skin (1 patient each). The serum LDH level at diagnosis was normal in 34 (71%) patients and the ECOG score was 0 to1 for 38 patients (79%). Thirty-one patients (65%) had an IPI score less than 2.
Clinical features and outcome of the 48 patients with nasal NK/T-cell lymphomas and of the subgroup of 39 patients evaluable for expression of PI9
| Clinical features . | Entire group . | Subgroup . |
|---|---|---|
| No. of patients | 48 | 39 |
| No. male/no. female | 29/19 | 27/12 |
| Median age, y (range) | 46 (22-79) | 46 (25-72) |
| Initial sites, no. (%) | ||
| Nasal cavity | 32 (67) | 27 (69) |
| Nasal cavity with adjacent structures | 16 (33) | 12 (31) |
| Ann Arbor stage, no. (%) | ||
| I | 36 (75) | 31 (80) |
| II | 2 (4) | 1 (2) |
| III | 0 (0) | 0 (0) |
| IV | 10 (21) | 7 (18) |
| ECOG score, no. (%) | ||
| 0 | 25 (52) | 23 (59) |
| 1 | 13 (27) | 8 (21) |
| 2 | 2 (4) | 2 (5) |
| 3 | 8 (17) | 6 (15) |
| LDH level, no. (%) | ||
| Normal | 34 (71) | 29 (74) |
| Elevated | 14 (29) | 10 (26) |
| IPI score, no. (%) | ||
| 0 | 22 (46) | 18 (47) |
| 1 | 9 (19) | 8 (20) |
| 2 | 9 (19) | 8 (20) |
| 3 | 5 (10) | 3 (8) |
| 4 | 3 (6) | 2 (5) |
| Survival, % | ||
| 5-y EFS rate | 39 | 41 |
| 5-y OS rate | 49 | 50 |
| Clinical features . | Entire group . | Subgroup . |
|---|---|---|
| No. of patients | 48 | 39 |
| No. male/no. female | 29/19 | 27/12 |
| Median age, y (range) | 46 (22-79) | 46 (25-72) |
| Initial sites, no. (%) | ||
| Nasal cavity | 32 (67) | 27 (69) |
| Nasal cavity with adjacent structures | 16 (33) | 12 (31) |
| Ann Arbor stage, no. (%) | ||
| I | 36 (75) | 31 (80) |
| II | 2 (4) | 1 (2) |
| III | 0 (0) | 0 (0) |
| IV | 10 (21) | 7 (18) |
| ECOG score, no. (%) | ||
| 0 | 25 (52) | 23 (59) |
| 1 | 13 (27) | 8 (21) |
| 2 | 2 (4) | 2 (5) |
| 3 | 8 (17) | 6 (15) |
| LDH level, no. (%) | ||
| Normal | 34 (71) | 29 (74) |
| Elevated | 14 (29) | 10 (26) |
| IPI score, no. (%) | ||
| 0 | 22 (46) | 18 (47) |
| 1 | 9 (19) | 8 (20) |
| 2 | 9 (19) | 8 (20) |
| 3 | 5 (10) | 3 (8) |
| 4 | 3 (6) | 2 (5) |
| Survival, % | ||
| 5-y EFS rate | 39 | 41 |
| 5-y OS rate | 49 | 50 |
Pathologic findings
The histopathologic findings are summarized in Table 2. Necrotic areas were present in most cases (42 of 48, 87%), associated with an angiocentric or angioinvasive growth pattern (or both) in 39 of 48 cases (81%). Variable degrees of epitheliotropism, defined by the presence of clusters of CD3ϵ+ tumor cells within the respiratory glands or surface epithelia, were observed in 37 of 48 cases (77%). NK/T-cell lymphomas were subclassified into 2 groups, according to their predominant small- to medium-sized (22 cases, 46%) and large-sized (26 cases, 54%) cell features (Figure 1A-B). Small/medium lymphoid cells disclosed a slightly irregular nucleus, sometimes responsible for a centrocyte-like appearance, whereas the large-cell category was characterized by a predominance of cells with a large irregular nucleus, showing in a few cases anaplastic features. In both groups, tumor cells frequently had an abundant clear cytoplasm. The apoptotic index was variable from case to case and was scored as high (> 10 apoptotic tumor cells/hpf) in 12 of 37 (32%) evaluable cases. Interestingly, it was higher in the large cell category than in the small/medium-cell category (32 and 4 apoptotic cells/hpf, respectively, P = .009). On immunohistochemistry, all lymphomas disclosed a characteristic CD3ϵ+/CD5− phenotype with expression of GrB or TiA1 cytotoxic molecules, whereas expression of CD56 was detected in 38 of 48 cases (79%). EBV association was demonstrated in all cases either by EBER ISH (n = 47 of 48) or LMP-1 immunostaining (n = 6 of 10).
Histopathologic and phenotypic characteristics of the 48 patients with nasal NK/T-cell lymphomas
| Characteristic . | No. patients/total (%) . |
|---|---|
| Angiocentric/angioinvasion pattern | 39/48 (81) |
| Epitheliotropism | 37/48 (77) |
| Necrosis | 42/48 (87) |
| Cytology | |
| Small/medium | 22/48 (46) |
| Medium/large | 26/48 (54) |
| CD3ε | 48/48 (100) |
| CD5 | 0/48 (0) |
| CD56 | 38/48 (79) |
| TiA1 | 32/32 (100) |
| GrB | 35/35 (100) |
| EBV* | 48/48 (100) |
| Characteristic . | No. patients/total (%) . |
|---|---|
| Angiocentric/angioinvasion pattern | 39/48 (81) |
| Epitheliotropism | 37/48 (77) |
| Necrosis | 42/48 (87) |
| Cytology | |
| Small/medium | 22/48 (46) |
| Medium/large | 26/48 (54) |
| CD3ε | 48/48 (100) |
| CD5 | 0/48 (0) |
| CD56 | 38/48 (79) |
| TiA1 | 32/32 (100) |
| GrB | 35/35 (100) |
| EBV* | 48/48 (100) |
EBV association was demonstrated by in situ hybridization in 47 cases and by LMP-1 immunostaining in 6 of 10 cases.
Histologic subtypes of nasal NK/T-cell lymphomas and detection of PI9 and aC3 in tumors cells of nasal NK/T-cell lymphomas. (A) Example of a lymphoma with a predominant small to medium-sized cell component with mild atypia. (B) Example of a lymphoma with pleomorphic large-cell features. (C) Example of a nasal NK/T-cell lymphoma scored as positive for PI9, with virtually all tumor cells surrounding glands positive. (D) Another nasal NK/T-cell lymphoma scored as negative for PI9 (< 10% tumor cells positive). A few scattered cells serve as internal positive control. (E) Example of a nasal NK/T-cell lymphoma with more than 10% aC3+ tumor cells (brown nuclear staining), whereas panel F illustrates a case with less than 10% aC3+ tumor cells. Only rare scattered cells with brown nuclear staining are present. Original magnification, × 400 for panels A-B, × 100 for panel C, and × 200 for panels D-F). Images were captured with a Zeiss Axioskop2 microscope (Oberkochen, Germany) and Neofluar 100×/0.1 NA optical lenses (Zeiss). Photographs were taken with a DP70 Olympus camera (Tokyo, Japan). Image acquisition was performed with Olympus DP Controller 2002, and images were processed with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Histologic subtypes of nasal NK/T-cell lymphomas and detection of PI9 and aC3 in tumors cells of nasal NK/T-cell lymphomas. (A) Example of a lymphoma with a predominant small to medium-sized cell component with mild atypia. (B) Example of a lymphoma with pleomorphic large-cell features. (C) Example of a nasal NK/T-cell lymphoma scored as positive for PI9, with virtually all tumor cells surrounding glands positive. (D) Another nasal NK/T-cell lymphoma scored as negative for PI9 (< 10% tumor cells positive). A few scattered cells serve as internal positive control. (E) Example of a nasal NK/T-cell lymphoma with more than 10% aC3+ tumor cells (brown nuclear staining), whereas panel F illustrates a case with less than 10% aC3+ tumor cells. Only rare scattered cells with brown nuclear staining are present. Original magnification, × 400 for panels A-B, × 100 for panel C, and × 200 for panels D-F). Images were captured with a Zeiss Axioskop2 microscope (Oberkochen, Germany) and Neofluar 100×/0.1 NA optical lenses (Zeiss). Photographs were taken with a DP70 Olympus camera (Tokyo, Japan). Image acquisition was performed with Olympus DP Controller 2002, and images were processed with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Expression of PI9, aC3, and Bcl-2 proteins in NK/T-cell lymphomas
Due to insufficient tissue or inadequate staining, a few cases could not be evaluated for expression of PI9, aC3, and Bcl-2 (Table 3). To note, as shown in Table 1, the subgroup of 39 patients evaluable for PI9 did not differ for the distribution of clinical characteristics. In agreement with the known constitutive expression of GrB and PI9 by normal NK cells,21 all evaluable NK/T-cell lymphomas disclosed positivity for both markers. However, the expression level of PI9 was heterogeneous from case to case and PI9 was scored as positive (eg, > 10% tumor cells) in 26 of 39 (67%) evaluable cases and as negative in 13 of 39 (33%) cases (Figure 1C-D). In PI9+ cases, the immunostaining was usually strong in virtually all tumor cells, but in a few cases it was heterogeneous, with positive clusters of neoplastic cells in a negative background.
Univariate analysis of prognostic factors in patients with nasal NK/T-cell lymphoma
| Prognostic factor . | No. of patients . | Estimated OS 5-y rate, % . | P . | Estimated EFS 5-y rate, % . | P . |
|---|---|---|---|---|---|
| Expression of P19* | .009 | .02 | |||
| Positive, more than 10% of tumor cells | 26 | 64 ± 9† | 61 ± 10† | ||
| Negative 10% or less of tumor cells | 13 | 23 ± 11† | 23 ± 12† | ||
| Apoptotic index* | .05 | .08 | |||
| Low, 10 or fewer cells/hpf | 25 | 36 ± 10† | 32 ± 10† | ||
| High, more than 10 cells/hpf | 12 | 67 ± 14† | 58 ± 14† | ||
| IPI score | .06 | .2 | |||
| Lower than 2 | 31 | 59 ± 9† | 47 | ||
| Higher than 2 | 17 | 29 ± 11† | 29 | ||
| Histology | .4 | .3 | |||
| Small/medium cells | 22 | 43 | 39 | ||
| Medium/large cells | 26 | 53 | 49 | ||
| Expression of aC3* | .8 | .7 | |||
| Low, fewer than 10 tumor cells | 23 | 43 | 39 | ||
| High, 10 or more tumor cells | 13 | 46 | 38 |
| Prognostic factor . | No. of patients . | Estimated OS 5-y rate, % . | P . | Estimated EFS 5-y rate, % . | P . |
|---|---|---|---|---|---|
| Expression of P19* | .009 | .02 | |||
| Positive, more than 10% of tumor cells | 26 | 64 ± 9† | 61 ± 10† | ||
| Negative 10% or less of tumor cells | 13 | 23 ± 11† | 23 ± 12† | ||
| Apoptotic index* | .05 | .08 | |||
| Low, 10 or fewer cells/hpf | 25 | 36 ± 10† | 32 ± 10† | ||
| High, more than 10 cells/hpf | 12 | 67 ± 14† | 58 ± 14† | ||
| IPI score | .06 | .2 | |||
| Lower than 2 | 31 | 59 ± 9† | 47 | ||
| Higher than 2 | 17 | 29 ± 11† | 29 | ||
| Histology | .4 | .3 | |||
| Small/medium cells | 22 | 43 | 39 | ||
| Medium/large cells | 26 | 53 | 49 | ||
| Expression of aC3* | .8 | .7 | |||
| Low, fewer than 10 tumor cells | 23 | 43 | 39 | ||
| High, 10 or more tumor cells | 13 | 46 | 38 |
In 11 cases, the small size of the sections or abundant areas of necrosis did not allow the assessment of the number of apoptotic cells on 20 hpf's. Due to insufficient tissue or inadequate staining, a few cases could not be evaluated for expression of PI9 (n = 9) and aC3 (n = 13).
95% CI.
Regarding aC3, patients were distributed into low (n = 23) and high (n = 13) expression groups (Figure 1E-F). Although most aC3+ tumor cells disclosed morphologic features of apoptosis, the number of aC3-labeled cells was overall smaller than that of morphologic apoptotic cells. Furthermore, no correlation was found between the level of PI9 and aC3. Finally, expression of Bcl-2 was scored as positive in at least 10% of tumor cells in only 6 of 25 (24%) tested cases.
Clinical outcome and prognostic parameters
CR/CRu to first-line treatment was achieved in 24 of 48 patients (50%), whereas 21 patients progressed and 3 died from disease during treatment. Five patients had a relapse, most often locally (4 cases). The median time to relapse was 20 months (range, 2-54 months), with 3 patients having the relapse in the first 2 months. Patients in failure or in relapse received a salvage treatment consisting of exclusive chemotherapy in 15 cases and chemotherapy followed by involved-field radiotherapy in 11 cases.
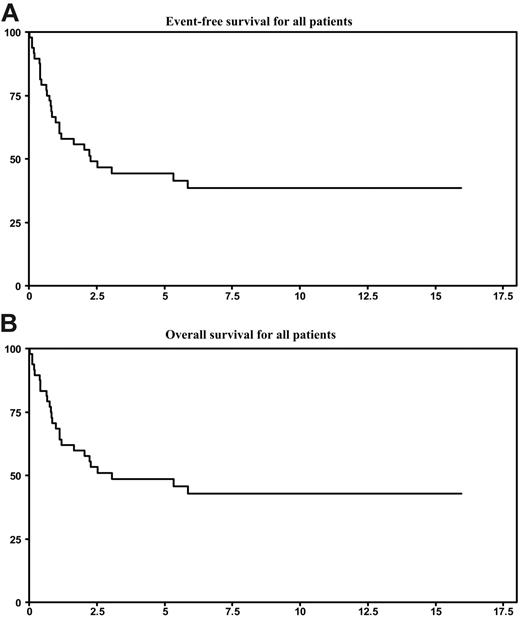
Among the 29 deaths registered, 27 resulted from lymphoma progression and 2 occurred in patients with persistent CR (myocardial infarction and radiotherapy-induced myelitis). With a median follow-up of 6.5 years, the 5-year estimates for EFS and OS were 39% and 49%, respectively (Figure 2). As shown in Table 1, the subgroup of 39 patients evaluable for PI9 did not differ for 5-year EFS and OS rates (41% and 50%, respectively).
Survival statistics. (A) EFS for all patients. (B) OS for all patients.
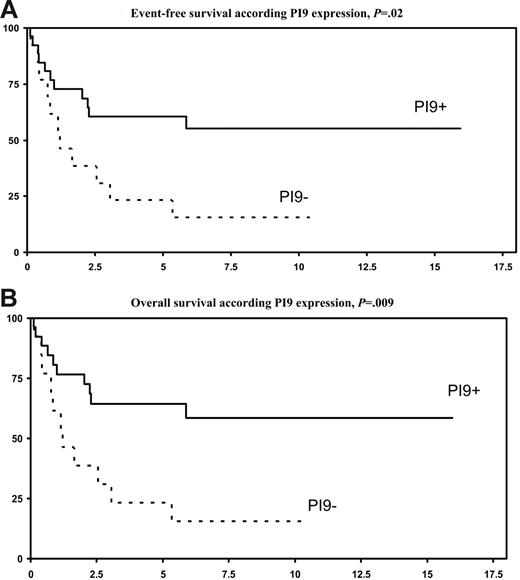
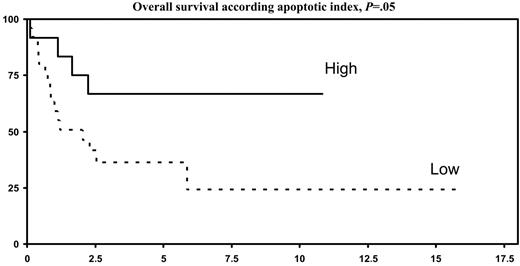
By univariate analysis (Table 3), expression of PI9 by tumor cells was associated with a highly significant better 5-year OS (P = .009) and 5-year EFS (P = .02), as compared with PI9− lymphomas (Figure 3A-B). A high apoptotic index (Figure 4), but not expression of aC3, was also associated with a significantly better 5-year OS (P = .05) and a trend for a better 5-year EFS (P = .08). A low IPI score (< 2) was associated with a trend for a better 5-year OS (P = .06). In a multivariate analysis (Table 4), OS and EFS were independently affected by PI9 expression (P = .009 and P = .02, respectively) and apoptotic index (P = .05 and P = .08, respectively).
Survival according to PI9 expression. (A) EFS, P = .02. (B) OS, P = .009.
Multivariate analysis of prognostic factors in patients with nasal NK/T-cell lymphoma
| Prognostic factor . | OS . | EFS . | ||||
|---|---|---|---|---|---|---|
| P . | Risk ratio . | 95% CI . | P . | Risk ratio . | 95% CI . | |
| Expression of PI9 | .009 | 3.55 | 2.19-5.88 | .02 | 2.98 | 1.86-4.76 |
| Apoptotic index | .04 | 3.75 | 2.87-4.9 | .03 | 4 | 2.09-7.62 |
| IPI score | .1 | 0.45 | .16 | 0.53 | ||
| Prognostic factor . | OS . | EFS . | ||||
|---|---|---|---|---|---|---|
| P . | Risk ratio . | 95% CI . | P . | Risk ratio . | 95% CI . | |
| Expression of PI9 | .009 | 3.55 | 2.19-5.88 | .02 | 2.98 | 1.86-4.76 |
| Apoptotic index | .04 | 3.75 | 2.87-4.9 | .03 | 4 | 2.09-7.62 |
| IPI score | .1 | 0.45 | .16 | 0.53 | ||
Discussion
We report here a series of 48 nasal NK/T-cell lymphomas, the largest so far reported Occidental series,5,44–47 in which diagnosis was based on strict histopathologic and phenotypic criteria including the demonstration of both a cytotoxic profile and EBV association. Following first-line chemotherapy with curative intent in most patients, we confirm the poor prognosis of the disease, as already reported with other treatment modalities.4,6,9 Interestingly, we show that the level of the GrB inhibitor PI9 and the apoptotic index are independent prognostic indicators.
In keeping with previous Asian and Western reports,2,6,8–10,12,43,44,46–51 we observed that the disease has a male predominance with a median age around 50 years and presents as a localized disease restricted to the nasal cavity and adjacent structures. As others,6,9,48,49 we found few adverse risk factors in the majority of the patients, resulting in a low (< 2) IPI score. We also confirm that the disease pursues a highly aggressive clinical course with only half the patients achieving a CR to treatment and surviving at 5 years. The optimal therapy of nasal NK/T-cell lymphoma remains debated because trials comparing prospectively radiotherapy with chemotherapy are lacking. However, several studies have suggested radiotherapy-based primary treatment to be superior to chemotherapy alone.3–5,7,9,11 In our series, all patients but 4 received chemotherapy alone as primary treatment. This approach resulted in a 49% 5-year survival rate, which compares to that reported in patients receiving radiotherapy-based primary treatment.3,4,6,7,11,48,49 Moreover, in our study, salvage with combined chemoradiotherapy appeared to be ineffective with only 1 of 11 cases in persistent second remission (data not shown).
Several attempts have been made to identify prognostic parameters in nasal NK/T-cell lymphomas. Advanced stage,4,9,11,50–52 high IPI score,7–9,51–53 and local tumor invasiveness10 have been reported to predict an unfavorable outcome. Recently, Lee et al48 proposed a prognostic model based on B symptoms, stage, LDH level, and regional lymph node involvement allowing a better prognostic discrimination as compared with IPI. There have been conflicting results regarding the relevance of cytologic grading in nasal NK/T-cell lymphomas53,54 ; our results in patients receiving primary chemotherapy do not support its prognostic impact.
Efforts are also ongoing in nasal NK/T-cell lymphoma to identify molecular abnormalities with clinical relevance that may help in defining mechanisms responsible for resistance to therapy-induced apoptosis and ultimately alternative therapies. For instance, P53 missense mutations have been associated with an aggressive course,54 whereas overexpression of CD94, an NK-cell antigen receptor, has been shown to predict a favorable outcome.55 In the present study, we show for the first time that high level of expression of the putative antiapoptotic protein PI9 by lymphoma cells is, unexpectedly, associated with a favorable outcome. This is in contrast with a previous report in anaplastic large-cell lymphoma23 showing that PI9 expression by neoplastic cells was associated with a poor outcome, an observation in keeping with the hypothesis that tumor cells use expression of PI9 to escape their elimination mediated by the release of GrB by cytotoxic cells.22 These discrepancies may be explained by the constitutive expression of both GrB and PI9 by NK cells,56 to protect themselves from a premature cell death. Indeed, GrB and PI9 form complexes into the cytoplasm of NK cells immediately after leakage of GrB from intracellular granules to rapidly inactivate extra granular GrB.57 On the other hand, the assumption that PI9 may confer resistance to apoptosis was not confirmed in a recent report.58 In this study, human lymphoma cell lines and primary lymphoma cells were sensitive, independently of their level of PI9 expression, to cytolysis by both CTLs and activated NK cells. Furthermore, recent data suggest that PI9 may become inactivated through its cleavage by granzyme M,59 another enzyme present in cytotoxic granules, which is specifically expressed by cells of the innate immune system and also by nasal NK/T-cell lymphomas.60 Finally, PI9 belongs to the serpin superfamily, which comprises several members constitutively expressed in epithelial cells.61,62 The expression of serpins has been shown to be down-regulated in several invasive carcinomas63,64 and the corresponding genes have been regarded as tumor suppressor genes, able to suppress tumor growth, invasion, and metastasis and to enhance tumor cell sensitivity to apoptosis. Interestingly, tumor cells expressing one of these serpins, named maspin, have a reduced level of bcl-2 protein,65 a finding that parallels our observation of a lack of Bcl-2 in most cases of nasal NK/T-cell lymphomas in the present series. In the light of these data in epithelial tumors, it is tempting to speculate that the loss of PI9 in nasal NK/T-cell lymphomas could reflect a dedifferentiation process associated with tumor progression, resistance to apoptosis, and ultimately poor outcome. Interestingly, we observed in some PI9+ cases an heterogeneous PI9 immunostaining with clusters of negative cells, which might suggest the emergence of PI9 down-regulated subclones possibly associated with aggressiveness and invasive potential. The mechanism by which NK/T tumor cells may down-regulate PI9 needs to be clarified. Cytogenetic studies have shown 6q deletion as a consistent aberration in NK/T-cell lymphomas,66 whereas PI9 is located at chromosome 6p.67 It is tempting to speculate that PI9 down-regulation might result from epigenetic inactivations by aberrant promoter hypermethylation, as described for tumor suppressor genes such as p73 in NK-cell disorders.68
In contrast to the association of a high apoptotic index with a favorable outcome, we observed a broad range of aC3 expression without correlation with prognosis. In most cases, the number of apoptotic cells appeared higher than the number of aC3+ cells, resulting in the absence of correlation between both parameters. Although the reasons for such discrepancies are unclear, it might reflect the involvement of a caspase-independent programmed cell death, as recently reported.69
We conclude that further studies at a large multicenter scale, with respect to the rarity of the disease, are warranted to confirm the prognostic relevance of PI9 expression in nasal NK/T-cell lymphomas. Such studies are facilitated by the fact that PI9 expression can be easily evaluated on routinely fixed material. They should evaluate whether PI9 expression, in addition to other clinical prognostic factors such as local tumor invasiveness and IPI, could help to stratify patients with nasal NK/T-cell lymphomas and possibly propose adapted innovative therapies.
Authorship
C.B., K.B., F.R. and P.G. wrote the paper; N.M.-G. and A.K. performed the experiments and provided the PI9 monoclonal antibody; C.B., P.G., F.B., J.B., A.C.-B., and J.B. participated in the pathologic review of patients entered in the GELA studies; K.B., P.G., N.M., and F.R. analyzed the data; F.R., V.R., C.G., and N.M. participated in designing the LNH87, LNH93, LNH98 studies and treating and documenting the patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Felix Reyes died on August 13, 2006.
A list of the participating members of the GELA is presented in Document S1 (available at the Blood website; see the Supplemental Document link at the top of the online version of the article).
Correspondence: Philippe Gaulard, Département de pathologie, Hôpital Henri Mondor, 51, Avenue du Maréchal de Lattre de Tassigny, 94010 Créteil Cedex, France; e-mail: philippe.gaulard@hmn.aphp.fr.
Presented in abstract form at the 46th annual meeting of the American Society of Hematology, San Diego, CA, December 6, 2004.70
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We indebted to Chafika Coppeaux and Karine Sardin for their technical help and to Nathalie Dordonne for her help in statistical analysis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal