Abstract
An important mechanism of host defense to Cryptococcus neoformans involves the direct microbicidal activity of lymphocytes. The importance of CD4+ T cells is illustrated by the incidence of this infection in the acquired immunodeficiency syndrome (AIDS) patients; however, the relative activity of microbicidal CD4+ T cells compared with CD8+ T cells and natural killer (NK) cells has not been established. Further, although NK cells and CD8+ T cells use perforin or granulysin, respectively, to kill C neoformans, the effector molecule used by CD4+ T cells is not known. Experiments demonstrated that IL-2–activated peripheral blood lymphocytes from healthy adults acquire anticryptococcal activity, and surprisingly, that CD4+ T cells had the most profound effect on this activity. Using SrCl2induced degranulation and siRNA knockdown, granulysin was shown to be the effector molecule. Although activation by anti–CD3 + IL-2 resulted in the additional expression of perforin, this did not improve the anticryptococcal activity. Cryptococcal killing by CD4+ T cells was defective in human immunodeficiency virus (HIV)–infected patients due to dysregulated granulysin and perforin production in response to IL-2 or anti–CD3 + IL-2. In conclusion, CD4+ T cells are the major subset of cells responsible for killing C neoformans in peripheral blood. These cells use granulysin as the effector molecule, and priming is dysregulated in HIV-infected patients, which results in defective microbicidal activity.
Introduction
CD4+ T cells function as both regulators and effectors in the immune response.1 CD4+ T cells produce cytokines, providing help in the immune response to pathogens, and have often been referred to as Th cells.2 However, over the past 2 decades, the ability of CD4+ T cells to become cytotoxic has been reported in both mice and humans against alloantigens, microbe-infected cells, and cancer.1,3–8 Previous studies in mice demonstrate that CD4+ cytotoxic T lymphocytes (CTLs) cause target cell lysis primarily via Fas/FasL interactions.9–12 Later, studies on human CD4+ T cells demonstrated that CD4+ CTLs also use perforin and granzyme-based granule exocytosis.13–15 This pathway involves disruption of the target cell membrane through extracellular Ca2+-dependent polymerization of perforin, accompanied by uptake of granzymes, which are responsible for downstream activation of caspases and subsequent DNA fragmentation, resulting in target cell apoptosis.
Although the importance of CTL-mediated killing of tumor or microbe-infected cells is well established, direct CTL-mediated microbial killing has been underinvestigated. Evidence has suggested that CTLs have very potent direct antimicrobial activity. This activity requires cell contact and involves regulated exocytosis of granules from the cytoplasm of the effector cells.16 Perforin and granzymes are well-known cytolytic molecules17 ; however, granulysin is less well recognized.16,18 The human granulysin cDNA was originally isolated by subtractive hybridization in a search for T-cell–specific molecules expressed “late” (3-5 days) after activation.19 Granulysin, a member of the saposin-like protein family of lipid binding proteins, colocalizes with perforin and granzymes in the cytolytic granules of human CTLs and natural killer (NK) cells.20–22 Two major protein products of granulysin, 15 and 9 kDa, are detected in CTL and NK cells.22 It is apparent that the 9-kDa form is processed from the larger, precursor 15-kDa form,22 and recombinant 9-kDa granulysin disrupts artificial liposomes and cell membranes, damages mitochondria, and activates caspase 9 to induce apoptosis.23,24 Additionally, granulysin exhibits potent cytotoxic activity against a broad panel of microbial pathogens and tumor cells.18,20 Our previous observation, using Cryptococcus neoformans, one of the most important microbial pathogens in patients with compromised CD4+ T-cell–dependent immunity, such as the acquired immunodeficiency syndrome (AIDS),25–27 indicated that granulysin is required for the CD8+ T-cell–mediated direct antifungal activity.28 By contrast, NK cells use perforin, rather than granulysin, as the effector molecule to kill C neoformans.29
Although CD4+ and CD8+ T cells and NK cells are important in cryptococcal host defense,30–32 defective CD4+ T cells are clearly the major risk factor for disease in human immunodeficiency virus (HIV)–infected patients.33 Previous studies have demonstrated that IL-2–activated CD4+ T cells, like CD8+ and NK cells, form conjugates with, and directly inhibit growth of, C neoformans in vitro.34,35 Despite the importance of CD4+ T cells in cryptococcal host defense, the relative importance of CD4+ T cells and the mechanism by which CD4+ T cells facilitate the elimination of cryptococci is not known. C neoformans have previously been shown to be susceptible to recombinant granulysin added to the culture,18 and recent studies have demonstrated that CD4+ T cells express granulysin after activation, which in turn correlates with cytotoxicity to Epstein-Barr virus–infected cells, Mycobacterium tuberculosis–infected cells, and Mycobacterium leprae antigen–pulsed macrophages,36–38 thus suggesting that granulysin-expressing CD4+ T cells could be directly microbicidal to C neoformans.
Despite the importance of granulysin, the role of perforin is better known. Perforin is a 70-kDa protein found in lytic granules of CTL and NK cells,39 and an important component of granule exocytosis and cell-mediated cytotoxicity.40 Perforin-mediated cytotoxicity is well documented in CTL-mediated defense against host cells infected with viruses, bacteria, tumor, and parasites,41 although expression of perforin by CD4+ CTLs is controversial. Some studies demonstrated that CD4+ CTLs express perforin,12,42–46 while others did not.36 Nevertheless, the role of granulysin or perforin in CD4+ T-cell–mediated direct antimicrobial activity, in which CD4+ T cells are directly in contact with an extracellular pathogen, is unclear.
The relative importance of CD4+ T cells in cryptococcal killing was examined by depletion of each of the major subsets (CD4+ T cells, CD8+ T cells, and NK cells) from IL-2–activated peripheral blood lymphocytes (PBLs). To determine whether CD4+ T cells use granulysin like CD8+ T cells or perforin like NK cells to kill C neoformans,28,29 cells were stimulated with IL-2 or anti–CD3 + IL-2, and the expression of granulysin and perforin was detected by Western blot. To determine whether granulysin is necessary for the anticryptococcal activity of IL-2–activated or anti–CD3 + IL-2–activated CD4+ T cells, granulysin was depleted with strontium chloride (SrCl2), or silenced with granulysin-specific small interference RNA (siRNA) using RNA interference (RNAi). To examine whether perforin is required for the anticryptococcal effect of granulysin, CD4+ T cells were treated with concanamycin A, an inhibitor of the perforin-mediated cytotoxicity pathway, or silenced with perforin-specific siRNA. Finally, the ability of IL-2–activated or anti–CD3 + IL-2–activated CD4+ T cells isolated from antiretroviral therapy (ART)–naive patients to increase granulysin and/or perforin expression and the ability of activated CD4+ T cells to kill C neoformans were investigated.
Patients, materials, and methods
Preparation of C neoformans
C neoformans CAP67 (ATCC 52817) was obtained from the American Type Culture Collection (Manassas, VA). The organisms were maintained on Sabouraud dextrose slants (Difco, Detroit, MI) and passaged to fresh slants every month as previously described.47
PBL and CD4+ T-cell isolation, activation, and subset depletion
Human peripheral heparinized blood samples were collected by venipuncture from healthy young adults or ART-naive HIV-infected young adults. These patients came from a cohort of HIV-infected individuals with CD4+ T-cell counts from 400 to 1000/μL, so that enough cells could be recovered to perform experiments. No other criteria were used to exclude participants. These HIV patients had no other comorbidities; none of these patients had documented cryptococcal infection before, or within 6 months subsequent to, testing; and the viral load was low (undetectable: 58 × 103/mm3). Peripheral blood mononuclear cells (PBMCs) were prepared immediately after collection by separating over a Ficoll-Hypaque density gradient (Sigma-Aldrich, Oakville, ON, Canada) as described previously.48 PBMCs were harvested and washed 3 times in HBSS (Life Technologies, Burlington, ON, Canada) followed by subset isolation.
PBLs were isolated by CD14+ cell depletion and CD4+ T cells were purified by CD4+ T-cell negative isolation using an autoMACS separator following the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). The purified PBLs or CD4+ T cells were resuspended in medium containing RPMI 1640 (Life Technologies), 5% human AB serum (BioWhittaker, Walkersville, MD), 100 U penicillin/mL, 100 μg streptomycin/mL, 0.25 μg amphotericin B/mL, 2 mM l-glutamine, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids (all from Life Technologies). The purity of CD4+ T cells from healthy donors and HIV-infected patients was routinely more than 95% and 90%, respectively, and the contamination of CD8+ T cells or NK cells was routinely lower than 1% as determined by flow cytometry (Guava EasyCyte; Guava Technologies, Hayward, CA). Previous studies have shown that this number of CD8+ T cells or NK cells has no detectable killing of C neoformans.28,29 All of the study subjects participated voluntarily and gave informed consent, and human research was conducted in accordance with the approval of the conjoint Board of Medical Ethics at the University of Calgary, in accordance with the Declaration of Helsinki.
PBLs or CD4+ T cells were stimulated with IL-2 (100 U/mL; R&D Systems, Hornby, ON, Canada) or with anti–human CD3 monoclonal antibody (Ab, 1 μg/mL; eBioscience, San Diego, CA) for 7 days unless otherwise specified. For some experiments, IL-2–stimulated CD4+ T cells were treated with 25 mM SrCl2 (Sigma-Aldrich) for 18 hours49,50 or 10 nM concanamycin A (CMA; Sigma-Aldrich) for 2 hours.51 The cells were washed 3 times in medium and placed in culture for the experiment. The viability of cells was not altered by these treatments as assessed by trypan blue exclusion.
Western blot analysis
Cell pellets were lysed with lysing buffer (50 mM Tris [pH 6.8], 1% SDS, 0.025% bromophenol blue, 10% glycerol, 20 mM DTT) supplemented with a cocktail of protease inhibitors (Calbiochem, San Diego, CA). Protein from the lysates of 1 × 105 cells was loaded in each lane of a 4% to 12% gradient Tris-glycine gel (Invitrogen, San Diego, CA), separated by electrophoresis, transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA), and blotted with polyclonal antigranulysin Ab 519/GST rabbit serum (1:1000 dilution),18 a mouse monoclonal anti–human β actin Ab (Chemicon International, Temecula, CA), and/or a monoclonal anti–human perforin Ab (eBioscience). The reactive bands were visualized with an Odyssey Infrared Imaging System (LI-COR Biotechnology, Lincoln, NE) using IRDye 800CW purified goat anti–mouse IgG (1:20 000 dilution) or with IRDye 700DX purified goat anti–rabbit IgG (1/10 000 dilution; Rockland, Gilbertsville, PA).
siRNA preparation and gene silencing assays
The siRNA sequences targeting perforin (GenBank accession number M28393) and 2 separate sequences within granulysin (GenBank accession number NM_012483) were synthesized by Dharmacon Research (Lafayette, CO). The siRNA for perforin was 5′-AAGCCCACCCAGAGAAGTGTT-3′, and the siRNAs for granulysin were 5′-AAGACCCACCAGGACCAGTAC-3′ (G1) and 5′-GGAGGTATCAGTCTAGAGTTT-3′ (G2). The sequences of CD20 and a nonsilencing siRNA are 5′-AACCACTCTTCAGGAGGATGT-3′ (Dharmacon Research) and 5′-AATTCTCCGAACGTGTCACGT-3′ (Ambion, Austin, TX), which served as negative controls. It has been shown that CD20 siRNA could decrease the constitutive expression of CD20 on Ramso B cells (a B-cell line that constitutively expresses CD20) by approximately 50% (Dr J. P. Deans, University of Calgary, oral communication, 2006). CD4+ T cells were mixed with each siRNA (5 nM) and electroporated in 0.4-cm cuvette using the Gene Pulser apparatus (Bio-Rad) with a pulse of 200 V and capacitance of 800 μF. The treated cells were stimulated with IL-2 as described under “PBL and CD4+ T-cell isolation, activation, and subset depletion.”
Semiquantitative reverse-transcription–polymerase chain reaction (RT-PCR)
Total RNA was extracted from individual cell samples using an RNA extraction kit (Qiagen, Chatsworth, CA). The extracted total RNA was quantified by optical density (Thermo Spectronic spectrophotometer; Thermo Electron, Shelton, CT). Total RNA (1 μg from each sample) was reversely transcribed using the ImProm-II Reverse Transcription System (Promega, Madison, WI) following the manufacturer's instructions. The following granulysin-, perforin-, or GAPDH-specific primers were used: 5′ GGCCGTGACTACAGGACCTGTC 3′ and 5′ CCTGAGGTCCTCACAGATCTG 3′52 ; 5′ ATGTAACCAGGGCCAAAGTCA 3′ and 5′ AGTGATGGCATGGACTGTGG 3′53 ; and 5′ TCACCATCTTCCAGGAGCGA 3′ and 5′-AGTGATGGCATGGACTGTGG-3′. The PCR profile for granulysin, perforin, and GAPDH was as follows: denaturing at 94°C for 1 minute, followed by 25 cycles of denaturing at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 30 seconds. A final extension at 72°C for 5 minutes was used. The number of cycles was adjusted so that amplification occurred over the linear range. The PCR products were separated by electrophoresis on a 1.5% agarose gel and visualized by ethidium bromide staining.
Anticryptococcal activity of CD4+ T cells
A CFU assay was performed as previously described.34,54,55 C neoformans (2 × 103/well [200 μL]) were incubated with or without 5 × 105 lymphocytes (effector-target ratio [E/T] =250:1, unless otherwise specified). The number of CFUs of C neoformans per well was determined at 0, 24, or 48 hours by lysing the effector cells with dH2O followed by diluting and spreading onto Sabouraud dextrose agar plates. Preliminary experiments established that dH2O lysed effector cells without affecting fungal viability. Results are expressed as CFU/mL. A decrease in the CFU of C neoformans compared with the growth of C neoformans alone indicates growth inhibition. Values lower than the inoculum indicate killing.
Statistical analysis
Data were expressed as mean ± SEM. Each experiment was performed with different donors on different days. Statistical analysis was performed by using the ANOVA. For this purpose, the Fisher least-squares difference was used when allowed by the F test. Student t test was used further to do the pairwise comparison using the Bonferroni correction. For these tests, a P value of less than .05 was considered significant.
Results
CD4+ T cells are responsible for the anticryptococcal activity of IL-2–stimulated PBLs
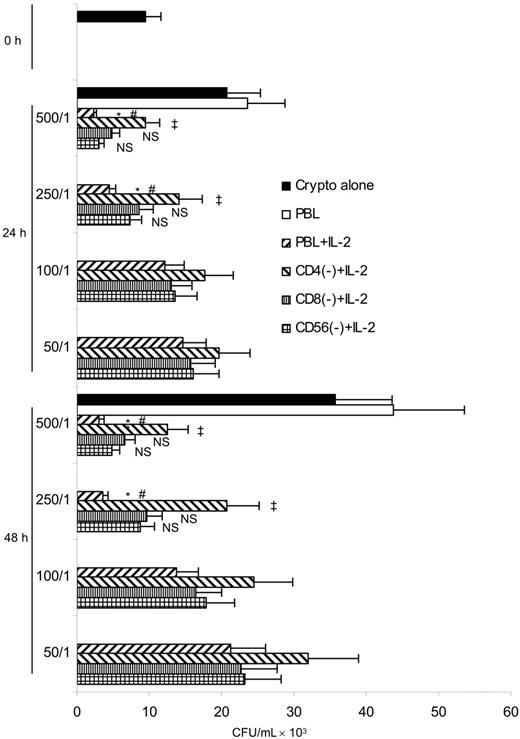
Previous studies have shown that activated CD4+ T cells, CD8+ T cells, or NK cells can directly inhibit the growth of C neoformans in vitro.34,35,54,56 However, the relative contribution of these cell subpopulations in PBLs is unknown. Experiments were performed to deplete each cell subset from IL-2–activated PBLs and assess the reduction in anticryptococcal activity. These experiments showed that IL-2–activated PBLs killed C neoformans at higher E/T ratios (500:1 and 250:1) (P < .05) (Figure 1), but resting PBLs did not. We were surprised to find that CD4+ T-cell depletion significantly inhibited killing at higher E/T ratios (500:1 and 250:1) (P < .05), while CD8+ T-cell or NK-cell depletion did not. The data demonstrate that CD4+ T cells have a greater contribution to cryptococcal killing than CD8+ T cells or NK cells in PBLs.
Anticryptococcal activity of IL-2–activated PBLs was due to CD4+ T cells. PBLs were cultured with or without IL-2 for 7 days and activated PBLs were depleted of CD4+ T cells, CD8+ T cells, or NK cells by magnetic separation. C neoformans was incubated with PBLs at different E/T ratios or PBLs depleted of CD4+ T cells (CD4(−)), CD8+ T cells (CD8(−)), or NK cells (NK(−)) for 24 or 48 hours. The number of C neoformans (CFU) was determined in each group. Results are expressed as mean ± SEM. *P < .05 compared with the inoculum; #P < .01 compared with the number of C neoformans alone at 24 or 48 hours; ‡P < .05 compared with the number of C neoformans in the presence of IL-2–stimulated PBLs; NS, no significant difference, compared with the number of C neoformans in the presence of IL-2–stimulated PBLs. Data are representative of 2 independent experiments.
Anticryptococcal activity of IL-2–activated PBLs was due to CD4+ T cells. PBLs were cultured with or without IL-2 for 7 days and activated PBLs were depleted of CD4+ T cells, CD8+ T cells, or NK cells by magnetic separation. C neoformans was incubated with PBLs at different E/T ratios or PBLs depleted of CD4+ T cells (CD4(−)), CD8+ T cells (CD8(−)), or NK cells (NK(−)) for 24 or 48 hours. The number of C neoformans (CFU) was determined in each group. Results are expressed as mean ± SEM. *P < .05 compared with the inoculum; #P < .01 compared with the number of C neoformans alone at 24 or 48 hours; ‡P < .05 compared with the number of C neoformans in the presence of IL-2–stimulated PBLs; NS, no significant difference, compared with the number of C neoformans in the presence of IL-2–stimulated PBLs. Data are representative of 2 independent experiments.
Granulysin expression correlates with the anticryptococcal activity of IL-2–activated CD4+ T cells from healthy adults
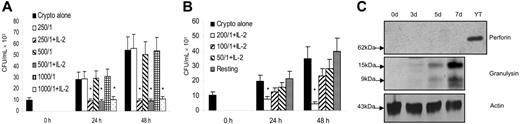
It has been shown that IL-2–activated CD4+ T cells from peripheral blood have a direct effect on C neoformans.34,35 To establish the anticryptococcal effect and determine the effector molecules that were induced by IL-2, purified CD4+ T cells were stimulated with IL-2. The ability of stimulated CD4+ T cells to kill or inhibit the growth of C neoformans was compared with that of resting CD4+ T cells. When C neoformans were cultured in medium without CD4+ cells, there was a 4- to 6-fold increase in the number of organisms after 48 hours of incubation (Figure 2A). There was no anticryptococcal effect when the untreated CD4+ T cells were added to the culture, even at an E/T ratio of 1:1000 (Figure 2A). By contrast, when C neoformans were incubated with IL-2–stimulated CD4+ T cells, the CFU at 24 and 48 hours was significantly lower than in the group containing C neoformans alone (P < .01) (Figure 2A), indicating that IL-2–stimulated CD4+ T cells had acquired anticryptococcal activity and killed C neoformans. The anticryptococcal effect at 250:1, 500:1, and 1000:1 was similar, and therefore experiments were also performed at lower ratios. There was a dose-dependent decrease in the anticryptococcal activity such that the effect was no longer statistically significant at E/T ratios of 100:1 and 50:1 (Figure 2B).
IL-2–activated peripheral blood CD4+ T cells possessed anticryptococcal activity, and this activity correlated with the expression of granulysin but not perforin. (A) CD4+ T cells were treated with IL-2 and incubated with C neoformans at 3 higher E/T ratios (E/T =250:1, 500:1, 1000:1). The number of C neoformans (CFU) was determined in each group as indicated. Results are expressed as mean ± SEM. Data are representative of 3 independent experiments. (B) CD4+ T cells were treated with IL-2 and incubated with C neoformans at 4 lower E/T ratios (E/T =200:1, 100:1, 50:1). The number of C neoformans was determined in each group as indicated. Results are expressed as mean ± SEM. Data are representative of 2 independent experiments. (A-B) *P < .01 compared with the number of C neoformans alone or resting CD4+ T cells incubated with C neoformans. (C) The time course of granulysin or perforin expression in IL-2–activated CD4+ T cells was detected by Western blot using an Ab (519/GST) that detects both the 15- and 9-kDa forms of granulysin or affinity-purified anti–human perforin, respectively. Data are representative of 3 independent experiments.
IL-2–activated peripheral blood CD4+ T cells possessed anticryptococcal activity, and this activity correlated with the expression of granulysin but not perforin. (A) CD4+ T cells were treated with IL-2 and incubated with C neoformans at 3 higher E/T ratios (E/T =250:1, 500:1, 1000:1). The number of C neoformans (CFU) was determined in each group as indicated. Results are expressed as mean ± SEM. Data are representative of 3 independent experiments. (B) CD4+ T cells were treated with IL-2 and incubated with C neoformans at 4 lower E/T ratios (E/T =200:1, 100:1, 50:1). The number of C neoformans was determined in each group as indicated. Results are expressed as mean ± SEM. Data are representative of 2 independent experiments. (A-B) *P < .01 compared with the number of C neoformans alone or resting CD4+ T cells incubated with C neoformans. (C) The time course of granulysin or perforin expression in IL-2–activated CD4+ T cells was detected by Western blot using an Ab (519/GST) that detects both the 15- and 9-kDa forms of granulysin or affinity-purified anti–human perforin, respectively. Data are representative of 3 independent experiments.
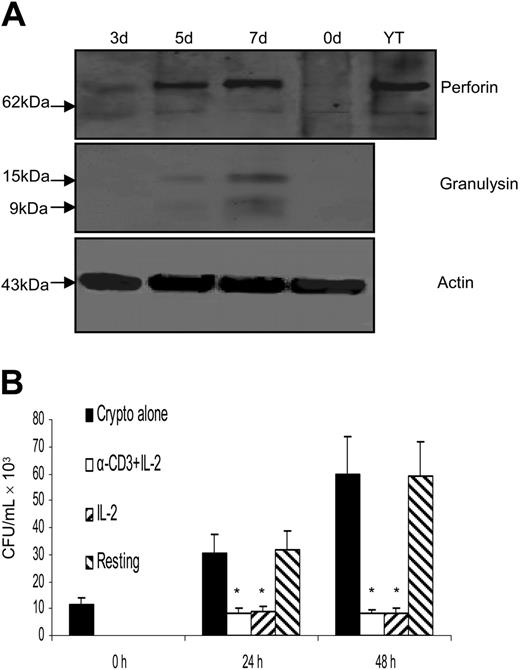
It has been shown that purified granulysin protein kills C neoformans in vitro,18 and the granulysin gene (mRNA) was first found to be expressed late (3-5 days) after activation in Th helper and CTL cell lines.19 However, perforin can also kill C neoformans in vitro.57 Further, CD8+ T cells use granulysin as the effector molecule, while NK cells use perforin.28,29 Therefore, the time course of granulysin and perforin expression in purified peripheral blood CD4+ T cells stimulated with IL-2 was examined. Neither perforin, nor granulysin, could be detected in resting CD4+ T cells. Although little granulysin expression was detected after 3 days of stimulation, both the 15- and 9-kDa forms of granulysin were expressed after 5 days of stimulation and increased further at 7 days (Figure 2C). By contrast, perforin was not detected at any time after stimulation with IL-2, but was easily detected in YT cells (an NK cell line). Hence, the acquired anticryptococcal activity correlated with induction of granulysin expression, but not perforin in CD4+ T cells. We also found that IL-2 provided optimal induction of granulysin expression (data not shown), which was in contrast to CD8+ T cells, in which IL-15 was superior to IL-2 in its ability to induce granulysin expression and activate cells for anticryptococcal activity (data not shown).
Granulysin is necessary for CD4+ T-cell–mediated anticryptococcal activity
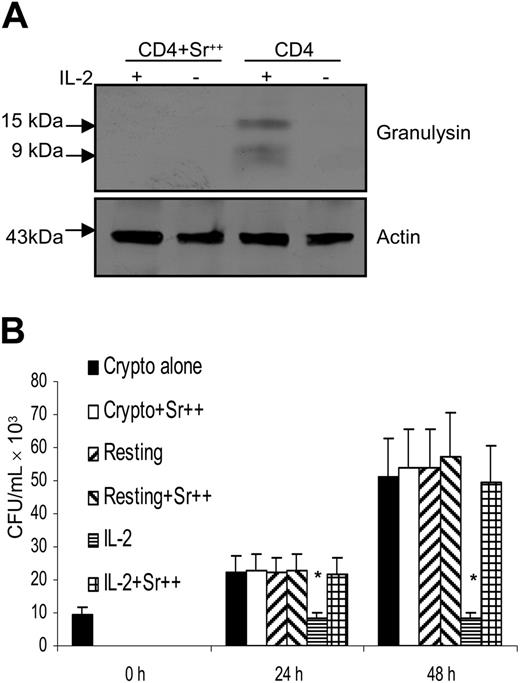
After demonstrating a correlation between granulysin expression and acquisition of anticryptococcal activity, experiments were performed to determine whether depletion of granules containing granulysin abrogated the antifungal activity in IL-2–activated CD4+ T cells. Many investigators have used SrCl2, which depletes the granule components of the cells without causing cellular toxicity.18,58 Strontium stimulates degranulation in several cell types and it has been suggested that it acts by mimicking the receptor-linked rise in calcium, which triggers degranulation.59 Treatment with SrCl2 abrogated the expression of granulysin (Figure 3A). In parallel, the IL-2–stimulated CD4+ T cells that had been SrCl2 treated were challenged with C neoformans. Treatment of IL-2–stimulated CD4+ T cells with SrCl2 abrogated the anticryptococcal activity (Figure 3B). Strontium treatment alone did not affect the growth of C neoformans or the viability of CD4+ T cells (data not shown).
Depletion of granules containing granulysin abrogated the anticryptococcal activity of IL-2–activated CD4+ T cells from healthy adults. (A) CD4+ T cells were activated with IL-2 (+) and treated with SrCl2 (Sr++). (B) IL-2–activated CD4+ T cells were treated with SrCl2, and the number of C neoformans was determined as indicated. Results are expressed as mean ± SEM. *P < .01 compared with the number of C neoformans alone. Data are representative of 3 independent experiments.
Depletion of granules containing granulysin abrogated the anticryptococcal activity of IL-2–activated CD4+ T cells from healthy adults. (A) CD4+ T cells were activated with IL-2 (+) and treated with SrCl2 (Sr++). (B) IL-2–activated CD4+ T cells were treated with SrCl2, and the number of C neoformans was determined as indicated. Results are expressed as mean ± SEM. *P < .01 compared with the number of C neoformans alone. Data are representative of 3 independent experiments.
Perforin induction did not improve the anticryptococcal activity
Perforin and granulysin can work cooperatively, and both are necessary for the lymphocyte-mediated microbicidal activity to Mycobacteria.18 Consequently, after demonstrating that granulysin is necessary for CD4+ T-cell–mediated anticryptococcal activity, experiments were performed to determine whether perforin contributes to this cytotoxic activity.
Since it has been shown that anti-CD3 Ab and IL-2 can induce perforin expression in CD4+ T cells,60 CD4+ T cells were stimulated with anti–CD3 + IL-2. Western blot analysis demonstrated that granulysin and perforin were both induced (Figure 4A), and granulysin expression was similar to that in CD4+ T cells stimulated with IL-2 alone (Figure 2A). Meanwhile, the anticryptococcal activity of CD4+ T cells stimulated with IL-2 alone was compared with that of CD4+ T cells stimulated with anti–CD3 + IL-2. Of interest, neither anti–CD3 + IL-2 nor the associated increase in perforin expression enhanced the anticryptococcal activity compared with IL-2 alone (Figure 4B).
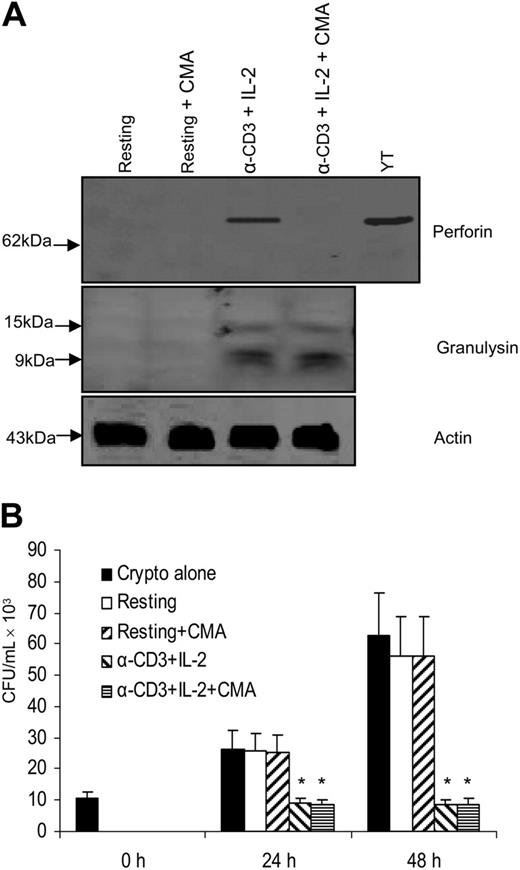
Activation with anti–CD3 + IL-2 and up-regulation of perforin did not enhance the anticryptococcal activity of CD4+ T cells from healthy adults.(A) CD4+ T cells were activated with anti–CD3 + IL-2; Western blot was performed for granulysin and perforin. (B) CD4+ T cells were activated with anti–CD3 + IL-2 or IL-2, and the number of C neoformans was determined as indicated. Results are expressed as mean ± SEM. *P < .01 compared with the number of C neoformans alone. Data are representative of 3 independent experiments.
Activation with anti–CD3 + IL-2 and up-regulation of perforin did not enhance the anticryptococcal activity of CD4+ T cells from healthy adults.(A) CD4+ T cells were activated with anti–CD3 + IL-2; Western blot was performed for granulysin and perforin. (B) CD4+ T cells were activated with anti–CD3 + IL-2 or IL-2, and the number of C neoformans was determined as indicated. Results are expressed as mean ± SEM. *P < .01 compared with the number of C neoformans alone. Data are representative of 3 independent experiments.
Granulysin-mediated anticryptococcal activity was not dependent on perforin
We also considered the possibility that perforin might not augment the activity of granulysin, but may contribute to its activity. Thus, experiments were performed to examine whether perforin is required for the anticryptococcal activity of granulysin. Two methods were used to block perforin expression. First, perforin was inhibited by CMA, an inhibitor of the vacuolar ATPase that is required to maintain perforin in lytic granules and inhibits perforin-mediated T-lymphocyte cytotoxicity for alloantigen-specific targets.51 Western blot analysis revealed that perforin expression was profoundly reduced by CMA treatment, but granulysin was not affected in anti–CD3 + IL-2–stimulated CD4+ T cells (Figure 5A). In addition, experiments were performed to investigate the effect of CMA treatment on the anticryptococcal activity of CD4+ T cells. Anti–CD3 + IL-2–stimulated CD4+ T cells incubated with CMA had similar levels of antifungal activity to those of CMA-untreated anti–CD3 + IL-2–stimulated CD4+ T cells (Figure 5B).
Granulysin-mediated anticryptococcal activity did not require perforin to function. (A) CD4+ T cells were activated with anti–CD3 + IL-2 and treated with CMA to deplete perforin. Expression of perforin and granulysin was assessed by Western blot. (B) CD4+ T cells were activated with anti–CD3 + IL-2 and treated with CMA. The number of C neoformans was determined as indicated. Results are expressed as mean ± SEM. *P < .01 compared with the number of C neoformans alone at 24 or 48 hours. Data are representative of 3 independent experiments.
Granulysin-mediated anticryptococcal activity did not require perforin to function. (A) CD4+ T cells were activated with anti–CD3 + IL-2 and treated with CMA to deplete perforin. Expression of perforin and granulysin was assessed by Western blot. (B) CD4+ T cells were activated with anti–CD3 + IL-2 and treated with CMA. The number of C neoformans was determined as indicated. Results are expressed as mean ± SEM. *P < .01 compared with the number of C neoformans alone at 24 or 48 hours. Data are representative of 3 independent experiments.
Experiments were also performed to specifically inhibit perforin production at the level of translation by using RNAi. CD4+ T cells were transfected by electroporation with perforin-specific siRNA. As controls, CD4+ T cells were transfected with siRNA targeted to CD20, nonsilencing (NS) siRNA, or sham transfection. siRNA to CD20 served as a control since it is expressed by B cells, but not by CD4+ T cells. Perforin and granulysin mRNA expression was determined by semiquantitative RT-PCR. In CD4+ T cells transfected with perforin-specific siRNA, perforin mRNA was dramatically decreased compared with control siRNAs (CD20 siRNA and NS siRNA) or sham-transfected cells (Figure 6A). By contrast, granulysin mRNA was not affected by transfection with perforin siRNA (Figure 6A).
Granulysin-mediated anticryptococcal activity did not require perforin to function. (A) Perforin or granulysin steady-state mRNA was assessed by RT-PCR from resting CD4+ T cells, activated cells sham electroporated without siRNA (−), and activated cells electroporated with perforin siRNA (P), CD20 siRNA (CD20), or nonspecific siRNA (NS). (B) Granulysin and perforin were detected by Western blot. (C) Activated and treated CD4+ T cells were incubated with C neoformans, and the anticryptococcal activity was assessed. Results are expressed as mean ± SEM. *P < .01 compared with the number of C neoformans alone at 24 or 48 hours. Data are representative of 3 independent experiments.
Granulysin-mediated anticryptococcal activity did not require perforin to function. (A) Perforin or granulysin steady-state mRNA was assessed by RT-PCR from resting CD4+ T cells, activated cells sham electroporated without siRNA (−), and activated cells electroporated with perforin siRNA (P), CD20 siRNA (CD20), or nonspecific siRNA (NS). (B) Granulysin and perforin were detected by Western blot. (C) Activated and treated CD4+ T cells were incubated with C neoformans, and the anticryptococcal activity was assessed. Results are expressed as mean ± SEM. *P < .01 compared with the number of C neoformans alone at 24 or 48 hours. Data are representative of 3 independent experiments.
To determine whether perforin protein expression was also reduced, CD4+ T cells were transfected with perforin-specific siRNA and perforin and granulysin were detected by Western blot. Perforin protein expression was abrogated in the group transfected with perforin siRNA, but not affected in the control groups. By contrast, granulysin was not affected by perforin siRNA, control, or NS siRNA (Figure 6B).
CD4+ T cells in which perforin was knocked down were used to examine the effect of perforin on the anticryptococcal activity of CD4+ T cells. Anti–CD3 + IL-2–stimulated CD4+ T cells transfected with perforin siRNA had no reduction in their anticryptococcal activity compared with the various control groups (Figure 6C). These results indicate that specific silencing of perforin did not affect the anticryptococcal activity of anti–CD3 + IL-2–activated CD4+ T cells. This is in marked contrast to human NK cell–mediated anticryptococcal activity that is dependent on the perforin.29
Granulysin is required for the anticryptococcal activity of anti–CD3 + IL-2–activated CD4+ T cells
The previous experiments showed that treatment with SrCl2 caused degranulation, resulting in depletion of granulysin and abrogation of anticryptococcal activity. However, the contents of granules are complex and contain other effector molecules that would be depleted by SrCl2 treatment. Consequently, it was necessary to show that specifically targeting granulysin would affect the anti-cryptococcal activity. For this purpose, anti–CD3 + IL-2–activated CD4+ T cells, which displayed both increased granulysin and perforin expression, were targeted with granulysin-specific siRNA. As a control, CD4+ T cells were also transfected with siRNA to CD20, NS siRNA, or sham transfection. Granulysin mRNA was assessed by semiquantitative RT-PCR. The response of treated cells was compared with that of sham-transfected cells. In CD4+ T cells transfected with siRNA G1 specific for granulysin, granulysin mRNA could not be detected (Figure 7A). By contrast, there was no change in the granulysin expression of stimulated CD4+ T cells transfected with control siRNAs (CD20 siRNA and NS siRNA) or sham-transfected cells (Figure 7A). Moreover, perforin and actin mRNA were not affected by granulysin siRNA G1 (Figure 7A).
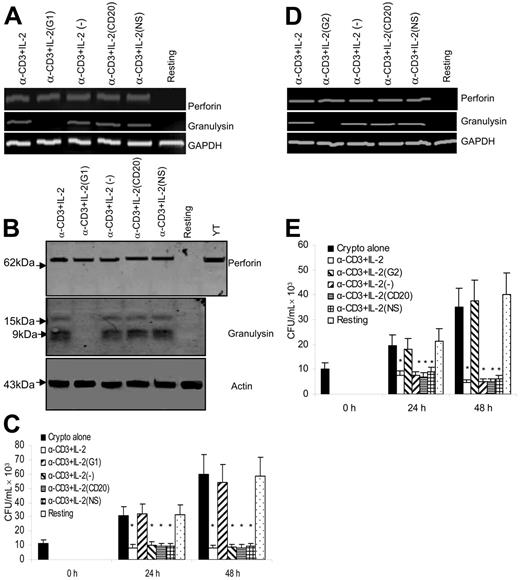
Granulysin is responsible for the anticryptococcal activity of anti–CD3 + IL-2–activated CD4+ T cells from healthy donors. (A) Granulysin or perforin steady-state mRNA was assessed by RT-PCR from CD4+ T cells silenced with granulysin siRNA (G1) or control siRNA as described in Figure 5A. (B) Corresponding granulysin and perforin were detected by Western blot. (C) The anticryptococcal activity of activated CD4+ T cells from healthy donors was determined as indicated. *P < .01 compared with the number of C neoformans alone at 24 or 48 hours. Data are representative of 3 independent experiments. (D) Granulysin or perforin steady-state mRNA was assessed by RT-PCR from CD4+ T cells treated with a different siRNA to granulysin (G2) or the control siRNA. Data are representative of 2 independent experiments. (E) The corresponding anticryptococcal activity of activated CD4+ T cells from healthy donors was determined. Results in panels C and E are expressed as mean ± SEM. *P < .01 compared with the number of C neoformans alone at 24 or 48 hours. Data are representative of 2 independent experiments.
Granulysin is responsible for the anticryptococcal activity of anti–CD3 + IL-2–activated CD4+ T cells from healthy donors. (A) Granulysin or perforin steady-state mRNA was assessed by RT-PCR from CD4+ T cells silenced with granulysin siRNA (G1) or control siRNA as described in Figure 5A. (B) Corresponding granulysin and perforin were detected by Western blot. (C) The anticryptococcal activity of activated CD4+ T cells from healthy donors was determined as indicated. *P < .01 compared with the number of C neoformans alone at 24 or 48 hours. Data are representative of 3 independent experiments. (D) Granulysin or perforin steady-state mRNA was assessed by RT-PCR from CD4+ T cells treated with a different siRNA to granulysin (G2) or the control siRNA. Data are representative of 2 independent experiments. (E) The corresponding anticryptococcal activity of activated CD4+ T cells from healthy donors was determined. Results in panels C and E are expressed as mean ± SEM. *P < .01 compared with the number of C neoformans alone at 24 or 48 hours. Data are representative of 2 independent experiments.
To determine whether granulysin protein expression was abrogated, CD4+ T cells were transfected with granulysin-specific siRNA or control siRNAs, and granulysin was detected by Western blot. Granulysin expression was not detected in the group treated with siRNA to granulysin, but unperturbed in the groups treated with control siRNA. By contrast, protein expression of perforin was not affected by granulysin or control siRNA (Figure 7B).
After demonstrating that expression of granulysin was blocked at the level of both mRNA and protein expression, experiments were performed to examine the effect of silencing granulysin on the antifungal activity. CD4+ T cells were transfected with granulysin siRNA and control siRNAs, and challenged with live C neoformans. The growth of C neoformans was inhibited in the presence of anti–CD3 + IL-2–activated CD4+ T cells. By contrast, anticryptococcal activity was abrogated by treatment of anti–CD3 + IL-2–activated CD4+ T cells with granulysin siRNA (Figure 7C). Taken together, these data indicate that granulysin, but not perforin, is required for the activated CD4+ T-cell–mediated anticryptococcal activity.
Although RNA interference results in robust silencing of the desired target, off-target gene regulation can occur as a result of degradation of mRNA transcripts with partial identity to the siRNA sequence.61 To examine potential off-target effects, a second siRNA duplex G2 to granulysin was used. The second siRNA also inhibited mRNA expression of granulysin (Figure 7D) and blocked the anticryptococcal effect of anti–CD3 + IL-2–stimulated CD4+ T cells (Figure 7E). Since the sequence of G2 was completely different from G1, off-target effects were unlikely to be responsible for the anticryptococcal activity.
The anticryptococcal activity of PBLs and CD4+ T cells from HIV patients is defective
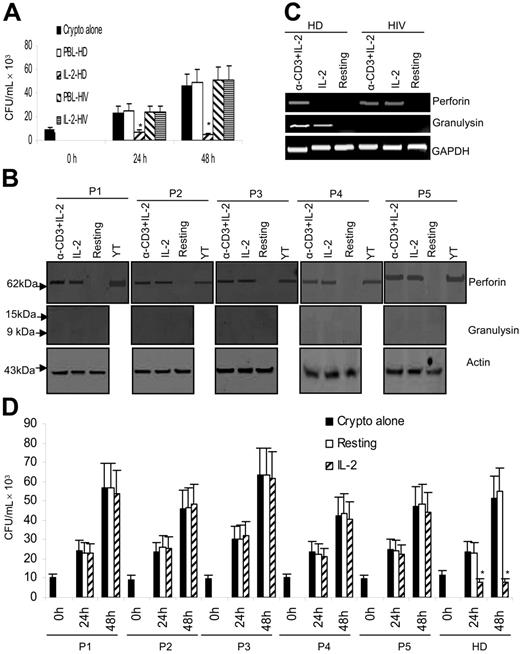
Since HIV-infected patients, who have defective CD4 T-cell immunity, are at risk of cryptococcosis, and our results show that CD4+ T cells are important mediators of the anticryptococcal activity in PBLs, experiments were performed to examine whether PBLs from HIV-infected patients have defective anticryptococcal activity. For this purpose, PBLs from HIV-infected patients were activated with IL-2 and the anticryptococcal activity was assessed. PBLs from HIV patients were defective in cryptococcal killing compared with PBLs from healthy donors (Figure 8A). Surprisingly, while healthy donors expressed perforin in response to only anti–CD3 + IL-2, IL-2 alone induced perforin expression in CD4+ T cells from HIV-infected patients (Figure 8B-C). Moreover, in HIV-infected patients, neither IL-2 nor anti–CD3 + IL-2 induced granulysin expression (Figure 8B-C), in contrast to healthy adults, in whom both IL-2 and anti-CD3 + IL-2 induced granulysin expression (Figures 2C and Figures 4A).
Activation of PBLs and CD4+ T cells from HIV-infected patients is dysregulated and the anticryptococcal activity is defective. (A) PBLs from a healthy donor (HD) and HIV-infected patient (HIV) were activated with IL-2, and the inhibition of number of C neoformans was determined as indicated. *P < .01 compared with the number of C neoformans alone at 24 or 48 hours. Data are representative of 2 independent experiments. (B) CD4+ T cells from HIV-infected patients were activated with IL-2 or anti–CD3 + IL-2. Expression of perforin and granulysin was assessed by Western blot. P1 to P5 stand for patients 1 to 5. Data are shown from 5 patients in 5 independent experiments. (C) CD4+ T cells from HD or HIV-infected patients were activated with IL-2 or anti–CD3 + IL-2. Granulysin or perforin steady-state mRNA was assessed by RT-PCR. Result from 1 of the 2 representative patients was shown. (D) CD4+ T cells were activated with IL-2, and the inhibition of number of C neoformans was determined at 0, 24, or 48 hours. HD indicates healthy donors. Results in panels A and D are expressed as mean ± SEM. *P < .01 compared with the number of C neoformans alone. Data are shown from 5 patients in 5 independent experiments.
Activation of PBLs and CD4+ T cells from HIV-infected patients is dysregulated and the anticryptococcal activity is defective. (A) PBLs from a healthy donor (HD) and HIV-infected patient (HIV) were activated with IL-2, and the inhibition of number of C neoformans was determined as indicated. *P < .01 compared with the number of C neoformans alone at 24 or 48 hours. Data are representative of 2 independent experiments. (B) CD4+ T cells from HIV-infected patients were activated with IL-2 or anti–CD3 + IL-2. Expression of perforin and granulysin was assessed by Western blot. P1 to P5 stand for patients 1 to 5. Data are shown from 5 patients in 5 independent experiments. (C) CD4+ T cells from HD or HIV-infected patients were activated with IL-2 or anti–CD3 + IL-2. Granulysin or perforin steady-state mRNA was assessed by RT-PCR. Result from 1 of the 2 representative patients was shown. (D) CD4+ T cells were activated with IL-2, and the inhibition of number of C neoformans was determined at 0, 24, or 48 hours. HD indicates healthy donors. Results in panels A and D are expressed as mean ± SEM. *P < .01 compared with the number of C neoformans alone. Data are shown from 5 patients in 5 independent experiments.
In addition, the anticryptococcal activity of IL-2–stimulated CD4+ T cells isolated from HIV-infected patients was assessed. IL-2–stimulated CD4+ T cells from HIV-infected patients, which failed to up-regulate granulysin, also failed to exert any anticryptococcal effect (Figure 8D). Thus, consistent with the observations in healthy donors, the increased expression of perforin in activated CD4+ T cells was not sufficient to inhibit the growth of C neoformans (Figure 8D). This further verifies that perforin is not functional in activated CD4+ T-cell–mediated killing of C neoformans. Of more importance, these data indicate that CD4+ T cells isolated from HIV-infected patients have defective regulation of granulysin expression and impaired anticryptococcal activity after stimulation.
Discussion
In this study, we have demonstrated that (1) CD4+ T cells are the major contributor to the anticryptococcal activity of IL-2–stimulated PBLs; (2) stimulation with IL-2 alone is sufficient to induce granulysin expression in human primary CD4+ T cells, while anti–CD3 + IL-2 cells are required for the expression of perforin; (3) the anticryptococcal activity of CD4+ T cells is dependent on granulysin, but independent of perforin; and (4) CD4+ T cells from HIV-infected patients have dysregulated production of perforin and granulysin in response to IL-2 and are defective in killing of C neoformans.
We were somewhat surprised to find that depletion of CD4+ T cells resulted in a profound reduction in cryptococcal killing by PBLs, while depletion of CD8+ T cells and NK cells did not. There are a number of possible reasons for this observation. First, CD4+ T cells are responsive to IL-2, while CD8+ T cells are more responsive to IL-15. Consequently, stimulation with IL-2 would favor CD4+ T-cell microbicidal activity. Additionally, CD4+ T cells are the major subset in PBLs, and therefore depletion of this subset reduces the number of effector cells to a greater degree than depletion of other subsets. In this regard, previous publications using different conditions have shown that the ability of CD4+ T cells to inhibit C neoformans was similar to CD8+ T cells or NK cells at the same E/T ratio.34,56 Nevertheless, the current studies demonstrate that CD4+ T cells are the most important subset in PBLs and provide the motivation to examine the mechanism of this microbicidal activity.
Although perforin was originally observed in the cytoplasmic granules of CTLs and NK cells and plays a central role in lymphocyte-mediated cytolysis,40,41 this study and our previous observations clearly demonstrate that granulysin, but not perforin, is an effector molecule of T cells responsible for the anticryptococcal activity.28 Previous studies demonstrated that activated CD4+ T cells have higher cytolytic potential than resting, but perforin-positive, CD8+ T cells and predicted that this cytolytic activity was perforin independent.60 Consistent with previous studies, our data show that perforin is nonessential in CD4+ CTL-mediated lysis of a fungus and that granulysin is an effector granule molecule involved in lymphocyte-mediated cytolysis.
Granulysin has been implicated in a variety of cytotoxic roles. Recombinant granulysin lyses a broad range of microbes, including bacteria, fungi, and parasites,18,62,63 and kills a variety of tumor cell lines by inducing apoptosis.24 Accordingly, granulysin has the potential to play a critical role in the defense against tumor growth and microbial infection. An extension of this observation reveals that increased expression of granulysin by CD4+ CTLs correlated with their cytotoxicity to viral and M tuberculosis–infected cells or M leprae–Ag–pulsed macrophages36–38,64–66 and that strontium ions abrogated the antimicrobial activity.38,67,68 However, SrCl2 depletes all of the granule components without molecular specificity, and consequently none of these studies has demonstrated a direct role of granulysin in cellular cytotoxicity. Additionally, since the microbial synapse is not amenable to blocking antibodies, alternate methods to prove molecular involvement in cytotoxicity were required. With this in mind, the RNAi technique was applied to specifically inhibit granulysin or perforin without affecting the other contents of the cytotoxic granules. The data indicate that induced granulysin or perforin mRNA and protein expression are profoundly reduced by siRNA. This is in contrast to the usual levels (70%-80%) of inhibition achieved with siRNA directed to constitutively expressed proteins. Granulysin or perforin mRNA and protein expression are not affected by control siRNA, CD20 siRNA, and nonsilencing siRNA, indicating that the inhibitory effects of siRNA for either granulysin or perforin were specific. Although there are off-target effects of RNAi,61 we showed that siRNA to 2 independent sequences of granulysin had a similar effect on anticryptococcal activity. It would be highly unlikely that both sequences would inhibit expression of the same critical unidentified gene. Therefore, the data is most consistent with siRNA knock-down of granulysin, rather than an off-target effect, being responsible for the anticryptococcal activity.
Previous studies showed that CD4+ T cells cultured with IL-2 acquire the ability to profoundly inhibit the growth of C neoformans.34,56,69 Those studies predicted that granule exocytosis was involved in killing the organisms, but the molecular mechanism was not identified.56,69 Of particular interest, the optimal conditions for growth inhibition of C neoformans included a minimum of 5 days of incubation with IL-2.34 This is consistent with our findings and previous observations that cDNA encoding granulysin is expressed “late” (3-5 days) after activation in T cells. This suggests that the microbicidal activity of lymphocytes occurs in an organized temporal sequence. NK cells do not require prior activation and therefore are available for early microbicidal activity.29 CD8+ T cells are activated by IL-15, which is rapidly expressed from preformed stores by translocating to the cell surface to exert its effect,28 followed by rapid expression of granulysin, while CD4+ T cells are activated by IL-2 and take 5 to 7 days to be active, a time course that is consistent with the development of cell-mediated immunity.19
Previous studies have demonstrated that granulysin was dependent on perforin to mediate killing of intracellular Mycobacterium.18 However, in the current experiments, CMA treatment, which selectively blocks the perforin and granzyme cytotoxicity pathway,51,70 had no effect on the anticryptococcal activity of CD4+ T cells. Moreover, when perforin was specifically silenced by its siRNA, there was no effect, further suggesting that perforin was not involved in the granulysin-mediated anticryptococcal activity. An obvious difference between perforin-independent killing of C neoformans and perforin-dependent killing of mycobacteria18 is that cryptococci were extracellular while mycobacteria are intracellular. Although the mechanism by which perforin contributes to granulysin-mediated cytotoxicity is not clear, a possible mechanism is that perforin acts as a gateway for granulysin, as it does for granzymes,41 to access the compartment containing intracellular microbes, such as mycobacteria. In this regard, the CD4+ T cells in tuberculoid leprosy contain granulysin, but not perforin, and therefore would be incapable of exerting a cytotoxic effect on intracellular M leprae without the additional contribution of perforin-expressing cells.38 By contrast, granulysin is directly active on extracellular C neoformans at the conjugation synapse of effector and target cells as suggested in previous studies using electron microscopy.34,35,56 Furthermore, supernatants from the activated CD4+ T cells fail to inhibit the growth of C neoformans (data not shown); this further supports the case for direct killing of C neoformans, and suggests that direct contact of CD4+ T cells and C neoformans is required for granulysin-mediated anticryptococcal activity.
In vitro activation with IL-2 alone could not induce the expression of perforin in CD4+ T cells,71–73 however, anti–CD3 + IL-2 induced the expression of perforin in CD4+ T cells.60 Our results demonstrating induction of perforin expression are consistent with these previous reports. Moreover, IL-2 alone could induce perforin expression in CD4+ T cells from HIV-infected patients, which differed from results obtained with CD4+ T cells from healthy donors and previous reports.60,72,73 One critical difference between these 2 groups is that CD4+ T cells from HIV-infected patients might be preactivated in vivo by specific cytokines or other mediators. These potential differences remain to be determined.
Finally, since HIV-infected patients are at high risk of cryptococcosis, the ability of CD4+ T cells isolated from ART-naive patients to kill C neoformans was assessed. Surprisingly, CD4+ T cells from HIV patients failed to induce granulysin expression and resulted in defective killing or growth inhibition of C neoformans. We speculate that defective CD4+ T-cell–mediated microbicidal activity is necessary but not sufficient for the acquisition of cryptococcosis since the microbicidal activity of CD4+ T cells occurred at a stage of disease (400-1000 CD4+ T cells/mm3) prior to the stage of HIV disease when patients are predisposed to cryptococcosis.
In summary, we investigated granulysin function and regulation in primary CD4+ T cells. We found that granulysin, but not perforin, was necessary for CD4+ T-cell–mediated antifungal activity. Moreover, priming of CD4+ T cells from HIV-infected patients is defective and results in failure to acquire anticryptococcal activity.
Authorship
Contribution: C.F.Z. designed the research, did experiments, analyzed data, and wrote the paper; L.M. had the conception of the research, designed the research, analyzed data, and wrote the paper; G.J.J. designed the research, analyzed data, and wrote the paper; M.J.G. recruited and managed patients, and wrote the paper; A.M.K. analyzed data, contributed vital new reagents or analytical tools, and wrote the paper; P.K. designed the research and wrote the paper; C.H.M. had the conception of the research, designed the research, analyzed data, and wrote the paper. C.F.Z. and L.L.M. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christopher H. Mody, Rm 273, Heritage Medical Research Building, University of Calgary, Calgary, Alberta, Canada, T2N 4N1; e-mail: cmody@ucalgary.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Canadian Institute of Health Research (C.H.M.), the Canadian Foundation for AIDS Research (C.H.M.), the Jessie Bowden Lloyd Professorship in Immunology (C.H.M.), and the Canadian Institute of Health Research (CIHR) Training Program in Immunology (C.F.Z.).
We would like to thank Dr J. P. Deans for the generous gift of CD20 siRNA, Brenda Beckthold for the assistance in recruiting and enrolling HIV patients to this study, and Danuta Stack and Tineka Asma-Schollaardt for their technical assistance. We also thank the participants and patients from the Southern Alberta Clinic for their cooperation.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal