Abstract
Suppression by T regulatory cells (Treg cells) is a major mechanism by which the immune system controls responses to self and nonharmful foreign proteins. Although there are many different types of Treg cells, the best characterized are those that constitutively express cell-surface IL-2Rα (CD25). We investigated whether altered T-cell–receptor (TCR)–mediated signaling in pure populations of ex vivo human CD4+CD25+ Treg cells might underlie their unique phenotype, including hyporesponsiveness to TCR–mediated activation and lack of cytokine production. CD4+CD25+ Treg cells displayed a consistent defect in phosphorylation of AKT at serine 473 and reduced phosphorylation of the AKT substrates FOXO and S6. Restoration of AKT activity via lentiviral-mediated expression of an inducibly active form of the kinase revealed that reduced activity of this pathway was necessary for the suppressive function of CD4+CD25+ Treg cells. These data represent the first demonstration of a causal association between altered signaling and the function of CD4+CD25+ Treg cells. Moreover, we have created the first system allowing inducible abrogation of suppression through manipulation of the suppressor cells. This system will be a powerful tool to further study the mechanism(s) of suppression by CD4+CD25+ Treg cells.
Introduction
Active suppression of immune responses by T regulatory cells (Treg cells) is a key mechanism for induction and maintenance of peripheral tolerance.1–4 The importance of Treg cells in vivo has been demonstrated in several mouse models: their absence results in systemic autoimmune disease, while their presence can inhibit antitumor, antiallergen, antiviral, and antiparasite immunity.2–5 Knowledge of exactly how Treg cells arise, the precise mechanisms that control their suppressive function, and how they differ from effector T cells at the molecular level remains largely unknown. A better understanding of the basic biologic characteristics of Treg cells will lead to novel therapies for diseases resulting from immune dysregulation.
Although there is evidence that T cells with a regulatory/suppressor function exist within all major subsets, most research has been focused on those that are CD4+ and constitutively express high levels of the IL-2Rα (CD25). These CD4+CD25+ Treg cells have more recently been further defined based on high expression of the FOXP3 transcription factor,6,7 and in humans relatively pure populations of CD4+CD25+FOXP3+ Treg cells can be isolated from peripheral blood by sorting 1% to 3% of the brightest CD25+ cells within the CD4+ T-cell subset.8–10 CD4+CD25+ Treg cells possess several characteristics that suggest their intracellular signaling following T-cell–receptor (TCR) activation may differ from that of effector T cells. For example, activated CD4+CD25+ Treg cells fail to produce most classic T-cell–derived cytokines, are hyporesponsive in the absence of exogenous growth factors, and suppress the functions of many different cell types.11,12 The molecular changes, however, that underlie this unique phenotype remain unknown.
TCR signaling is initiated by antigen (Ag) binding and results in activation of tyrosine kinases of the Src, Syk, and Tec families and assembly of scaffolds of adaptor molecules.13 Phosphorylation of these adaptors subsequently leads to activation of downstream effectors, including serine/threonine kinases, such as the mitogen-activated protein kinases (MAPKs) and protein kinase C (PKC), and phosphatidylinositide-3 kinase (PI3′K)–dependent serine/threonine kinases such as AKT. Activation of these cascades results in cytoskeletal rearrangements, cytokine production, cell-cycle progression, and engagement of T-cell effector functions. In contrast, T cells that are functionally hyporesponsive due to activation in the absence of costimulation have a block in activation of the Ras/MAPK pathway and/or in calcium mobilization.14
A detailed analysis of whether any of these pathways may be altered in human TCR-activated CD4+CD25+ Treg cells has been hampered by the scarcity of these cells, difficulties in isolating pure populations due to the lack of a reliable cell-surface maker, and their relatively poor proliferation in vitro. Nevertheless, there have been several attempts to characterize intracellular signaling events in mouse and human CD4+CD25+ Treg cells. Murine CD4+CD25+ Treg cells displayed reduced activation of AKT following stimulation with IL-2,15 and following stimulation with PMA/ionomycin they were reported to have a reduced capacity to activate JNK but not extracellular signal-related kinase (ERK) or p38 MAPK.16 In contrast, in vitro–expanded CD4+CD25+ Treg-cell lines derived from human cord blood showed normal activation of AKT but significantly reduced activation of Ras, MAP kinase kinase (MEK)1/2, and ERK1/2 upon TCR-mediated activation.17 Thus, there is currently no consistent evidence for a specific block in one or more signaling pathways in CD4+CD25+ Treg cells, and the potential biologic relevance of altered signal transduction to the unique function of CD4+CD25+ Treg cells has not been investigated.
To overcome the problem of limited numbers of CD4+CD25+ Treg cells that can be obtained ex vivo and avoid the caveats associated with studying molecular events in T-cell lines expanded in vitro upon superphysiologic activation, we took advantage of recent advances in flow cytometry–based methods18–20 to study TCR-mediated signaling in subpopulations of human CD4+ T cells. We report here that TCR-mediated activation of AKT is diminished in human ex vivo CD4+CD25+ Treg cells, as compared with CD4+CD25− T cells, and that their suppressive capacity is dependent upon this altered signal transduction.
Materials and methods
Cell purification
Peripheral blood was obtained from healthy volunteers following informed consent and approval of the protocol by the University of British Columbia (UBC) Clinical Research Ethics Board. Peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation over Ficoll (StemCell Technologies, Vancouver, BC, Canada), and CD4+ T cells were subsequently purified by negative selection with magnetic beads (Miltenyi Biotec [Auburn, CA] or StemCell Technologies). CD25+ cells were either purified by positive selection (Miltenyi Biotec) and passed over 2 MS columns to ensure 90% to 95% purity, or they were sorted by fluorescence-activated cell sorter (FACS). The flow-through from the CD25+ selection was then passed over an LD depletion column to remove any CD25lo cells and used as the CD25− fraction.
TCR-mediated activation of signaling
Following purification, CD4+ T cells or T-cell lines were rested overnight in RPMI (StemCell Technologies) with 1% human serum (NorthBio, Toronto, ON, Canada) and then starved in serum-free RPMI for 2 hours prior to stimulation. CD4+ T cells (2 × 106/mL to 4 × 106/mL) were activated by addition of αCD3 (OKT3, 1 μg/mL, Orthoclone; Ortho Biotech, Bridgewater, NJ), with or without αCD28 (1 μg/mL; BD Biosciences, San Jose, CA) monoclonal antibodies (mAbs), on ice for 15 minutes. Cells were washed once, and primary mAbs were crosslinked by addition of anti–mouse IgG F(ab′)2 (20 μg/mL; Jackson Immune Research, West Grove, PA) at 37°C for the indicated times. Activation was arrested by fixation in either 2% formaldehyde or the FOXP3-specific fix/perm buffer (eBioscience, San Diego, CA).
Flow cytometry
For signaling experiments, fixed samples were washed and permeabilized with ice-cold methanol and stained with anti-CD4 (conjugated to FITC, allophycocyanin, or allophycocyanin-Cy7 [eBioscience]), anti-CD25 (conjugated to PE [Miltenyi Biotec] or PE-Cy7 [BD PharMingen, San Diego, CA]), and the indicated Abs against signaling proteins. Anti–phospho-AKT (Ser473 or Thr308), anti-AKT, anti-PTEN, and anti–phospho-S6 ribosomal protein (Ser 235/236) were obtained from Cell Signaling Technology (Danvers, MA). For nonconjugated Abs, a secondary goat-antirabbit Alexa-488 (Molecular Probes, Eugene, OR) was used for detection. Anti–phospho-ERK Alexa-647 (T202/Y204), anti–phospho-p38 Alexa-488 (T180/Y182), anti–granzyme A (FITC), and anti–granzyme B (Alexa-647) were obtained from BD PharMingen. The PI3′K product, PI(3,4,5)P3, was detected by an anti-PIP3–FITC Ab obtained from Echelon Biosciences (Salt Lake City, UT). All anti-SHIP Abs were a generous gift from Dr Gerry Krystal. For detection of FOXP3, cells were fixed and permeabilized according to the manufacturer's instructions and incubated with anti-FOXP3–PE (eBioscience clone PCH101). Intracellular staining for CTLA-4 was performed as described.11
All samples were read on a BD FACS Canto and analyzed with FCS Express V3 (De Novo Software, Thornhill, ON, Canada).
Western blotting
Highly purified CD4+CD25+ Treg cells or CD4+CD25− T cells were activated as described and lysed using 1% NP-40 lysis buffer.21 Lysates from 1 × 106 to 3 × 106 cells were quantitated by bicinchoninic acid (BCA; Pierce, Rockford, IL), separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to PVDF membranes, and immunoblotted with anti–phospho-FOXO (S256) or anti–phospho-AKT S473 Abs (Cell Signaling Technology). Membranes were reprobed with anti-p38 Abs (Santa Cruz Biotechnology, Santa Cruz, CA) to determine equivalency of loading.
Lentiviral vectors
The bidirectional lentiviral vector (pCCL.sin.cPPT.SV40polyA.CTE.mCMV ΔNGFR.PGK)22 was modified by replacing the PGK promoter with the human EF1α promoter to improve transgene expression in human T cells. A cDNA cassette containing a truncated form of human AKT1 lacking its PH domain, fused to the src myristoylation signal at the amino terminus, and the steroid binding domain of the estrogen receptor (ER) along with an hemagglutinin (HA) epitope tag at its carboxyl terminus,23 was inserted downstream of the EF1α promoter. The resulting control (pCCL) and pCCL.AKT-ER (AKT-ER) vectors both drive constitutive expression of ΔNGFR as a marker gene. VSV-pseudotyped third-generation lentiviruses were produced by transient 4-plasmid cotransfection into HEK293T cells and concentrated by ultracentrifugation.22 Expression titers were determined on HEK293T cells by limiting dilution and ranged from 7 × 106 to 1.5 × 107 transducing units per milliliter.
Generation of T-cell lines and lentiviral transduction
CD4+ T cells were stained for CD4 and CD25 and sorted by FACS into CD25hi and CD25− fractions on a BD FACSAria as described.10 T cells (150 000 per well) were activated by stimulation with αCD3 (1 μg/mL) and antigen-presenting cells (APCs) (500 000 per well, irradiated at 50 Gy) or by coculture with CD32+CD58+CD80+ L cells (200 000 per well, irradiated at 75 Gy) and αCD3 (100 ng/mL)21 for 24 hours in T-cell medium (Xvivo-15, 5% human serum [NorthBio], 1× penicillin/streptomycin [Invitrogen, Carlsbad, CA], 1× Glutamax [Invitrogen]) in the presence of rhIL-2 (100 U/mL [Chiron], Emeryville, CA). Control pCLL or pCLL AKT-ER–encoding lentivirus was added to the activated cells at a multiplicity of infection of 2. The percentage of ΔNGFR+-transduced T cells was monitored after 6 days and was routinely 20% to 40%. ΔNGFR+ cells were purified with anti-NGFR magnetic beads (Miltenyi Biotec) following the manufacturer's instructions and allowed to expand. Equivalent expression of the AKT-ER protein in the various cell lines was confirmed by flow cytometric analysis using an anti-HA–FITC mAb (Roche Applied Science, Laval, QC, Canada) as described.24 Purified CD4+CD25+ Treg-cell and CD4+CD25− T-cell lines were restimulated every 14 days as previously described10 and monitored at the end of every cycle to ensure preservation of their suppressive capacity.
Proliferation and suppression of T cells
Transduced T cells were plated at 20 000 cells per well in 96-well plates and stimulated with soluble αCD3 mAbs (1 μg/mL) in the presence of APCs (CD3-depleted PBMCs, irradiated at 50 Gy, 50 000 cells per well). Proliferation was assessed after 72 hours by addition of [3H]thymidine (1 μCi [0.037 MBq] per well; Amersham Biosciences, Freiburg, Germany) for the final 16 hours of the assay. To test for suppressive capacity, CD4+ T cells (40 000 cells per well) were stimulated with αCD3/APCs in the absence or presence of transduced T cells (20 000 cells per well). Experiments were conducted in either vehicle alone (EtOH 0.15%) or 4-hydroxytamoxifen (4HT) at 150 nM (Sigma, St Louis, MO), a concentration that was found to stimulate maximum proliferation of transduced T cells. Suppression was assessed by determining [3H]thymidine incorporation after 72 hours. In all cases, untransduced CD4+CD25+ Treg-cell and CD4+CD25− T-cell lines were analyzed in parallel and found not to be different from control vector-transduced T-cell lines (data not shown).
Cell-surface marker expression and cytokine production
Transduced T cells (100 000 cells per well in 96-well plates) were stimulated with αCD3 (platebound, 1 μg/mL) and αCD28 (soluble, 1 μg/mL) mAbs. Experiments were conducted in either vehicle alone or 150 nM 4HT. Supernatants were collected at the indicated times, and the Th1/Th2 cytometric bead assays (BD Biosciences) were used to determine amounts of cytokine. After 48 hours, the cells were collected for flow cytometric analysis of cell-surface markers.
Statistical analyses
All analysis for statistically significant differences was performed with the Student paired t test. P values below .05 were considered significant. For cytokine analysis, log (base 10) values were used for statistical analysis to account for the variability between cell lines. All cultures were performed in triplicate, and error bars represent the SD.
Results
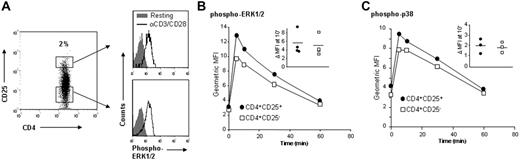
Single-cell analysis of intracellular signaling cascades reveals that ex vivo human CD4+CD25+ Treg cells and CD4+CD25− T cells have equivalent ERK1/2 and p38 MAPK phosphorylation following TCR activation
A major limitation in the molecular analysis of human CD4+CD25+ Treg cells is the inability to isolate homogeneous populations of live cells ex vivo. Although highly suppressive populations of CD4+CD25+ Treg cells can be isolated by sorting the brightest 2% to 3% of CD25+ cells, an in vitro expansion step is required to obtain sufficient cells to perform signal transduction assays using traditional Western blotting. In vitro expansion of CD4+CD25+ Treg cells is possible,11 but prolonged supraphysiologic TCR-mediated activation and the outgrowth of contaminating non-Treg cells represent confounding factors. To overcome these limitations, we have used flow cytometry to analyze intracellular signaling cascades in heterogeneous populations of CD4+ T cells.18–20,25–29 This method allows quantitative analysis of signaling within single cells, more accurately replicates the in vivo scenario, and decreases variables associated with cell isolation and handling. The specificity and sensitivity of Abs directed against TCR-activated proteins such as phospho-ERK, -p38, and -AKT have been validated by use of specific inhibitors and parallel Western blot analysis.25,28
Given the well-established role of MAPK pathways in TCR-stimulated cell division,14 we investigated the activation of these kinases in CD4+CD25+ Treg cells versus CD4+CD25− T cells upon TCR stimulation. CD4+ T cells were stimulated with αCD3/CD28 mAbs, fixed, and stained with anti-CD4, anti-CD25, and anti–phospho-ERK Abs. The relative amounts of ERK phosphorylation were compared in the 2 subsets by gating on the brightest 2% to 3% of CD25+ cells versus CD25− cells (Figure 1A). We consistently observed equivalent phosphorylation of ERK in CD4+CD25+ Treg cells and CD4+CD25− T cells following TCR activation (n = 4, P = NS). Analysis of these experiments at multiple time points showed that there was no difference between CD4+CD25+ Treg cells and CD4+CD25− T cells in the kinetics of ERK phosphorylation (Figure 1B). Similarly, no difference in the capacity of CD4+CD25+ Treg cells to activate p38 MAPK was observed (Figure 1C) (n = 4, P = NS). These data indicate that CD4+CD25+ Treg cells respond to TCR-mediated stimulation through the ERK and p38 MAPK pathways and demonstrate that these cells do not have a global defect in TCR-mediated signal transduction.
Single-cell analysis of MAPK activation in ex vivo human CD4+CD25+ Treg cells and CD4+CD25− T cells following TCR activation. (A) Ex vivo CD4+ T cells were left unstimulated or stimulated with crosslinked αCD3/CD28 mAbs for 10 minutes and stained for CD4, CD25, and phospho-ERK1/2. Histograms depicting levels of phospho-ERK were generated by gating on subsets of CD4+CD25high or CD4+CD25− T cells. (B) Geometric mean fluorescence intensities (MFIs) of cell populations stained with anti–phospho-ERK1/2 or (C) anti–phospho-p38 Abs were determined over a 60-minute time course following activation with αCD3/CD28 mAbs. The experiment was performed 3 times with similar results, and a representative analysis is shown. The inset is the fold change in MFI from resting to activation (at 10 minutes), with each point representing a separate experiment and horizontal bars representing the mean.
Single-cell analysis of MAPK activation in ex vivo human CD4+CD25+ Treg cells and CD4+CD25− T cells following TCR activation. (A) Ex vivo CD4+ T cells were left unstimulated or stimulated with crosslinked αCD3/CD28 mAbs for 10 minutes and stained for CD4, CD25, and phospho-ERK1/2. Histograms depicting levels of phospho-ERK were generated by gating on subsets of CD4+CD25high or CD4+CD25− T cells. (B) Geometric mean fluorescence intensities (MFIs) of cell populations stained with anti–phospho-ERK1/2 or (C) anti–phospho-p38 Abs were determined over a 60-minute time course following activation with αCD3/CD28 mAbs. The experiment was performed 3 times with similar results, and a representative analysis is shown. The inset is the fold change in MFI from resting to activation (at 10 minutes), with each point representing a separate experiment and horizontal bars representing the mean.
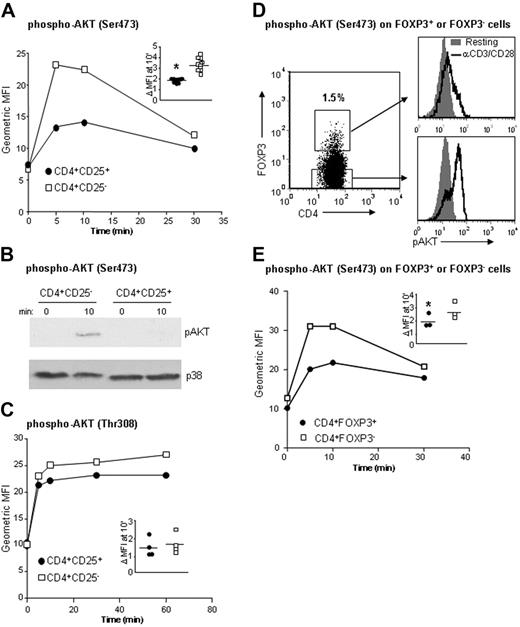
Human CD4+CD25+ Treg cells have a reduced capacity to phosphorylate AKT following TCR stimulation
Activation of the PI3′K-AKT pathway, which can be further enhanced by costimulation via CD28,30 promotes T-cell survival and is required for production of both Th1- and Th2-associated cytokines.31,32 We observed a profound defect in the capacity of CD4+CD25+ Treg cells to activate AKT upon stimulation via CD3 and CD28 and, after 10 minutes, the increase in levels of phospho-AKT (Ser473) was only 1.8-fold ± 0.17-fold in CD4+CD25+ Treg cells compared with 3.2-fold ± 0.58-fold in CD4+CD25− T cells (P < .001, n = 11; Figure 2A). Analysis more than 30 minutes following stimulation revealed that the reduction in phosphorylation of Ser473 was not due to altered kinetics of activation. Defective AKT phosphorylation in CD4+CD25+ Treg cells was also observed using traditional Western blotting (Figure 2B). A similar defect was found in the absence of CD28 costimulation (Figure S1A, available on the Blood website; see the Supplemental Figures link at the top of the online article). The amount of total AKT protein was equivalent in the 2 cell types, indicating that differential expression of AKT was not responsible for this phenotype (Figure S1B). For AKT to be fully activated, both Ser473 and Thr308 must be phosphorylated.33,34 Examination of the phosphorylation state of AKT at Thr308 following TCR activation showed that CD4+CD25+ Treg cells were no different from CD4+CD25− T cells in this respect (Figure 2C).
Human CD4+CD25+ Treg cells have a reduced capacity to phosphorylate AKT following TCR stimulation. (A) Ex vivo CD4+ T cells were stimulated with crosslinked αCD3/CD28 mAbs for the indicated times and stained for CD4 and CD25, and geometric MFIs of cells stained with anti–phospho-AKT (Ser473) were determined in CD4+CD25high or CD4+CD25− T cells. (B) More than 90% pure CD4+CD25high and CD4+CD25− T cells were left unstimulated or stimulated with αCD3/CD28 mAbs for 10 minutes, lysed, and analyzed by Western blotting for amounts of AKT (Ser473) phosphorylation. Blots were reprobed with anti-p38 Abs to ensure equivalency of loading. (C) Ex vivo CD4+ T cells were stimulated and analyzed as in panel A, and MFIs following staining with anti–phospho-AKT (Thr308) were determined. (D-E) Ex vivo CD4+ T cells were stimulated as in panel A and then stained for CD4, FOXP3, and phospho-AKT (Ser473). (D) Histograms depicting levels of phospho-AKT were generated by gating on subsets of CD4+FOXP3+ or CD4+FOXP3− T cells, and (E) MFIs of cells stained with anti–phospho-AKT (Ser473) were determined in CD4+FOXP3+ or CD4+FOXP3− T cells. Panels A, C, and E represent a single experiment with the inset depicting the fold change in MFI from resting to activation (10 minutes) for all experiments, and with horizontal bars indicating means and asterisks indicating significance. For panels B and D, a single representative example of 3 experiments performed is depicted.
Human CD4+CD25+ Treg cells have a reduced capacity to phosphorylate AKT following TCR stimulation. (A) Ex vivo CD4+ T cells were stimulated with crosslinked αCD3/CD28 mAbs for the indicated times and stained for CD4 and CD25, and geometric MFIs of cells stained with anti–phospho-AKT (Ser473) were determined in CD4+CD25high or CD4+CD25− T cells. (B) More than 90% pure CD4+CD25high and CD4+CD25− T cells were left unstimulated or stimulated with αCD3/CD28 mAbs for 10 minutes, lysed, and analyzed by Western blotting for amounts of AKT (Ser473) phosphorylation. Blots were reprobed with anti-p38 Abs to ensure equivalency of loading. (C) Ex vivo CD4+ T cells were stimulated and analyzed as in panel A, and MFIs following staining with anti–phospho-AKT (Thr308) were determined. (D-E) Ex vivo CD4+ T cells were stimulated as in panel A and then stained for CD4, FOXP3, and phospho-AKT (Ser473). (D) Histograms depicting levels of phospho-AKT were generated by gating on subsets of CD4+FOXP3+ or CD4+FOXP3− T cells, and (E) MFIs of cells stained with anti–phospho-AKT (Ser473) were determined in CD4+FOXP3+ or CD4+FOXP3− T cells. Panels A, C, and E represent a single experiment with the inset depicting the fold change in MFI from resting to activation (10 minutes) for all experiments, and with horizontal bars indicating means and asterisks indicating significance. For panels B and D, a single representative example of 3 experiments performed is depicted.
CD25 is a not a true lineage marker for Treg cells, because it can also be expressed by activated effector cells. Recent data indicate that FOXP3 may be a more specific marker,35 although it may also represent an activation marker in a subset of cells in humans.8,24,36 We therefore examined the capacity of CD4+FOXP3+ Treg cells to activate AKT. Similar to CD25+ cells, FOXP3+ cells displayed a consistent defect in their capacity to phosphorylate AKT: After 10 minutes of stimulation, phospho-Ser473 in CD4+FOXP3+ Treg cells was only 1.86-fold ± 0.57-fold compared with 2.65-fold ± 0.7-fold in CD4+FOXP3− T cells (P = .009, n = 3) (Figure 2D). This reduced activity was not due to altered kinetics, because levels of phospho-AKT in CD4+FOXP3+ Treg cells remained low throughout a 30-minute course (Figure 2E). These data indicate that the altered signaling in CD4+CD25+ putative Treg cells was not due to contaminating T effector cells.
To determine if reduced AKT phosphorylation was the result of an upstream defect in the activity of PI3′K, we investigated the levels of phosphatidylinositol-3,4,5-triphosphate (PIP3), the biologically active product of PI3′K, in stimulated CD4+CD25+ Treg cells. As shown in Figure S2A, no difference was observed between CD4+CD25+ Treg cells and CD4+CD25− T cells in PIP3 levels following TCR activation, indicating that PI3′K function in these cells is equivalent. This finding is consistent with observations made in the murine system downstream of the IL-2R.15
We then investigated whether CD4+CD25+ Treg cells have high basal expression and/or increased activity of src homology 2 (SH2)–containing inositol phosphatase-1 (SHIP1) or phosphatases and tensin homolog deleted on chromosome 10 (PTEN), the lipid phosphatases that counteract the activity of PI3′K.37 As shown in Figure S2B-C, CD4+CD25+ Treg cells and CD4+CD25− T cells did not differ in their expression of, or capacity to phosphorylate, SHIP1. Additionally, no differences in the basal expression levels of PTEN were detected (Figure S2D).
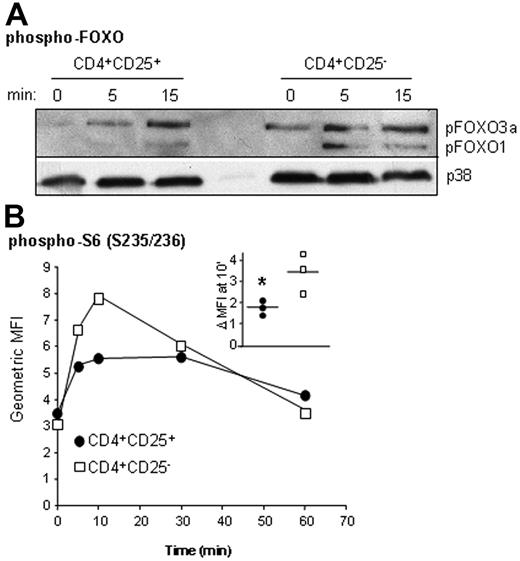
Diminished AKT phosphorylation in CD4+CD25+ Treg cells results in decreased activation of downstream effectors
To evaluate if decreased Ser473 phosphorylation was indicative of a drop in AKT kinase activity, we examined the activation state of FOXO1 (FRKHL) and FOXO3a (FRKHL-1), proteins known to be directly phosphorylated by AKT.38 Because Abs validated for flow cytometry were not available, we purified CD4+CD25+ Treg cells and CD4+CD25− T cells, activated them with αCD3/CD28 mAbs, and performed Western blotting of cell lysates (Figure 3A). CD4+CD25+ Treg cells had consistently lower levels of FOXO phosphorylation as compared with CD4+CD25− T effectors, suggesting that the reduced AKT phosphorylation resulted in reduced kinase activity (densitometric analysis: 1.82-fold ± 1.3-fold in CD4+CD25+ Treg cells versus 3.3-fold ± 1.3-fold in T effectors at 5 minutes, n = 3, P = .006). In addition, we examined the capacity of CD4+CD25+ Treg cells to phosphorylate the S6 ribosomal protein. S6 is a direct target of mammalian target of rapamycin (mTOR), a Ser/Thr kinase that is also a known target of AKT.39 Ex vivo CD4+CD25+ Treg cells displayed significantly lower amounts of phospho-S6 after activation than CD4+CD25− T cells (1.9-fold ± 0.28-fold versus 3.6-fold ± 0.96-fold at 10 minutes, n = 3, P = .03) (Figure 3B). Thus, the defect in AKT phosphorylation observed in ex vivo CD4+CD25+ Treg cells results in reduced kinase activity and appears to cause a general blockade of activation of downstream effector molecules.
Diminished AKT phosphorylation in CD4+CD25+ Treg cells results in decreased activation of downstream effectors. (A) More than 90% pure CD4+CD25high and CD4+CD25− T cells were left unstimulated or stimulated with αCD3/CD28 mAbs for the indicated times, lysed, and analyzed by Western blotting for amounts of phosphorylated FOXO1 and FOXO3a. Blots were reprobed with anti-p38 Abs to ensure equivalency of loading. Shown is a representative experiment of 3 performed. (B) Ex vivo CD4+ T cells were stimulated with αCD3/CD28 Abs for the indicated times, and MFIs following staining with anti–phospho-S6 (Ser235/236) were determined in CD4+CD25high or CD4+CD25− T cells. A single representative experiment is depicted, and the inset represents the fold change in MFI from resting to activation (10 minutes) for each separate experiment, with horizontal bars indicating means and the asterisk indicating significance.
Diminished AKT phosphorylation in CD4+CD25+ Treg cells results in decreased activation of downstream effectors. (A) More than 90% pure CD4+CD25high and CD4+CD25− T cells were left unstimulated or stimulated with αCD3/CD28 mAbs for the indicated times, lysed, and analyzed by Western blotting for amounts of phosphorylated FOXO1 and FOXO3a. Blots were reprobed with anti-p38 Abs to ensure equivalency of loading. Shown is a representative experiment of 3 performed. (B) Ex vivo CD4+ T cells were stimulated with αCD3/CD28 Abs for the indicated times, and MFIs following staining with anti–phospho-S6 (Ser235/236) were determined in CD4+CD25high or CD4+CD25− T cells. A single representative experiment is depicted, and the inset represents the fold change in MFI from resting to activation (10 minutes) for each separate experiment, with horizontal bars indicating means and the asterisk indicating significance.
Enforced activation of AKT reverses the suppressive capacity of CD4+CD25+ Treg cells
Given the well established role of the PI3′K-AKT pathway in cell-cycle progression,39 we hypothesized that diminished activation of this pathway in CD4+CD25+ Treg cells might be the direct cause of their hyporesponsive and suppressive phenotype. To test this possibility we first generated a series of T-cell lines and confirmed that expanded CD4+CD25+ Treg-cell lines displayed diminished phosphorylation of AKT and S6 (Figure S3). We then used lentiviral-mediated gene transfer to overexpress a conditionally active form of AKT consisting of the AKT kinase domain fused to an amino-terminal myristoylation sequence and the hormone binding domain of the ER at the carboxy-terminus (AKT-ER).23 The resulting protein remains in an inactive state in the cytoplasm until 4HT is added, whereupon a 17- to 50-fold increase in kinase activity is induced.23 CD4+CD25+ Treg cells and CD4+CD25− T cells were sorted and infected with control- (pCCL) or AKT-ER–encoding (AKT-ER) lentiviruses (Figure S4A). The activity of the AKT-ER protein was confirmed by analysis of phosphorylation of a downstream target, S6, upon addition of 4HT (Figure S4B). Following isolation and expansion of transduced cells, their proliferative capacity was tested in the absence or presence of 4HT. Surprisingly, although the presence of the AKT-ER resulted in an increase in the proliferative capacity of the CD4+CD25+ Treg cells upon TCR stimulation in the presence of 4HT (5.5-fold ± 2.25-fold compared with control, n = 4, P = .014), these cells remained hyporesponsive in comparison with CD4+CD25− T effector cells (Figure 4A). As expected, activation of AKT-ER by addition of 4HT in the AKT-ER+ CD4+CD25− T cells also resulted in increased proliferation (1.64-fold ± 0.35-fold compared with control, n = 4, P = .017). 4HT had no effect on control pCCL-transduced T-cell lines (Figure 4A).
Enforced activation of AKT reverses the suppressive capacity of CD4+CD25+ Treg cells. CD4+CD25high or CD4+CD25− T cells were transduced with lentivirus encoding an inducible AKT-ER or control (pCCL) vector. (A) Cells were stimulated with αCD3/APCs in the presence of vehicle alone or 4HT (150 nM). The inset is the fold increase in proliferation induced by 4HT in AKT-ER+ CD4+CD25+ Treg-cell or CD4+CD25− T-cell lines, with each point representing a single experiment. (B) CD4+ T cells were stimulated with αCD3 (soluble 1 μg/mL) and irradiated APCs in the absence or presence of a 1:2 ratio (T-cell lines–target) of control-transduced or of AKT-ER+ CD4+CD25+ Treg-cell or CD4+CD25− T-cell lines in the presence of either vehicle alone or 4HT (150 nM). Data are depicted as percent suppression ([1 − (Te + Treg/Te alone] × 100); negative values are plotted as 0. Proliferation and suppression were assessed by 3H-thymidine incorporation. A single representative experiment of 4 is depicted, and error bars represent SD from an individual experiment.
Enforced activation of AKT reverses the suppressive capacity of CD4+CD25+ Treg cells. CD4+CD25high or CD4+CD25− T cells were transduced with lentivirus encoding an inducible AKT-ER or control (pCCL) vector. (A) Cells were stimulated with αCD3/APCs in the presence of vehicle alone or 4HT (150 nM). The inset is the fold increase in proliferation induced by 4HT in AKT-ER+ CD4+CD25+ Treg-cell or CD4+CD25− T-cell lines, with each point representing a single experiment. (B) CD4+ T cells were stimulated with αCD3 (soluble 1 μg/mL) and irradiated APCs in the absence or presence of a 1:2 ratio (T-cell lines–target) of control-transduced or of AKT-ER+ CD4+CD25+ Treg-cell or CD4+CD25− T-cell lines in the presence of either vehicle alone or 4HT (150 nM). Data are depicted as percent suppression ([1 − (Te + Treg/Te alone] × 100); negative values are plotted as 0. Proliferation and suppression were assessed by 3H-thymidine incorporation. A single representative experiment of 4 is depicted, and error bars represent SD from an individual experiment.
We next determined whether restoration of AKT activity altered the suppressive capacity of CD4+CD25+ Treg cells. Responder CD4+ T cells were stimulated with αCD3/APC in the absence or presence of CD4+CD25+ Treg-cell or CD4+CD25− effector T-cell lines and in the absence or presence of 4HT. As expected, in the absence of 4HT, pCCL-transduced or AKT-ER–expressing CD4+CD25+ Treg-cell lines suppressed proliferation by 70% to 80% (ratio, 1:2 Treg/Teffector) (Figure 4B). In contrast, in the presence of 4HT, the capacity of CD4+CD25+ Treg cells to suppress effector T-cell proliferation was almost completely abrogated (percent suppression in the presence of 4HT for AKT-ER Treg-cell lines was 12.5% ± 17% compared with 76% ± 16% for pCCL controls, n = 5, P = .001) (Figure 4B). The degree of increase in proliferation upon activation of AKT in CD4+CD25+ Treg-cell lines (Figure 4A) is clearly insufficient to account for the lack of suppression observed in these assays. CD4+CD25− effector T-cell lines did not exhibit suppressive capacity in either control or 4HT conditions; nor did the presence of 4HT itself cause T-effector cells to proliferate (data not shown).
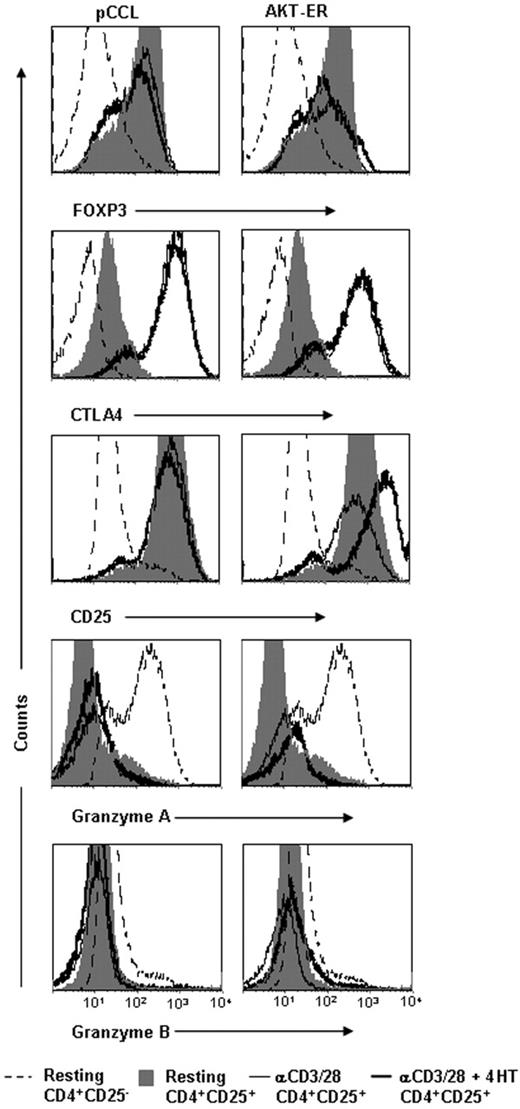
Enhanced AKT activity in CD4+CD25+ Treg cells does not suppress expression of FOXP3, CTLA-4, CD25, or granzyme A or B
Although the mechanism of suppressive action of CD4+CD25+ Treg cells remains to be identified, it has been speculated that FOXP3,6 CTLA4,2 granzymes A or B,40,41 and possibly also CD25 itself42 may play a role. We therefore determined whether expression of any of these 5 proteins was altered upon addition of 4HT to AKT-ER+ CD4+CD25+ Treg-cell lines. Because the control pCCL CD4+CD25+ Treg cells did not survive upon stimulation with 4HT alone for the time required for these assays (data not shown), the T-cell lines were activated with αCD3/CD28 for 48 hours in the absence or presence of 4HT and analyzed by flow cytometry. As expected, both control pCCL and AKT-ER+ CD4+CD25+ Treg-cell lines expressed high levels of FOXP3, CTLA4, and CD25 compared with CD4+CD25− T-cell lines in the resting state (Figure 5). Upon stimulation with 4HT, expression of FOXP3 and CTLA4 was unchanged (or slightly increased), and CD25 was increased by 2.84-fold ± 1.0-fold (n = 3, P = .046) (Figure 5). Although previous reports have suggested a role for granzymes in CD4+CD25+ Treg-cell function,40,41 we did not observe high levels of granzyme A or B expression on resting or activated CD4+CD25+ Treg cells compared with CD4+CD25− T cells. Addition of 4HT to AKT-ER+ CD4+CD25+ Treg cells either did not change, or slightly increased, the minimal expression of granzyme A and B. Therefore, the loss of suppressive activity upon AKT activation is not due to decreased expression of any of these Treg-cell–associated proteins.
Enhanced AKT activity in CD4+CD25+ Treg cells does not reduce expression of FOXP3, CTLA-4, CD25, or granzymes A or B. AKT-ER– (or control pCCL-) transduced CD4+CD25+ Treg cells or CD4+CD25− T cells were activated with αCD3/CD28 mAbs in the presence of either vehicle alone (EtOH) or 4HT (150 nM). After 48 hours, cells were stained for CD25, CTLA-4, FOXP3, and granzyme A and B. Unstimulated CD4+CD25+ and CD4+CD25− T-cell lines are included for comparison. A single representative experiment of 4 is depicted.
Enhanced AKT activity in CD4+CD25+ Treg cells does not reduce expression of FOXP3, CTLA-4, CD25, or granzymes A or B. AKT-ER– (or control pCCL-) transduced CD4+CD25+ Treg cells or CD4+CD25− T cells were activated with αCD3/CD28 mAbs in the presence of either vehicle alone (EtOH) or 4HT (150 nM). After 48 hours, cells were stained for CD25, CTLA-4, FOXP3, and granzyme A and B. Unstimulated CD4+CD25+ and CD4+CD25− T-cell lines are included for comparison. A single representative experiment of 4 is depicted.
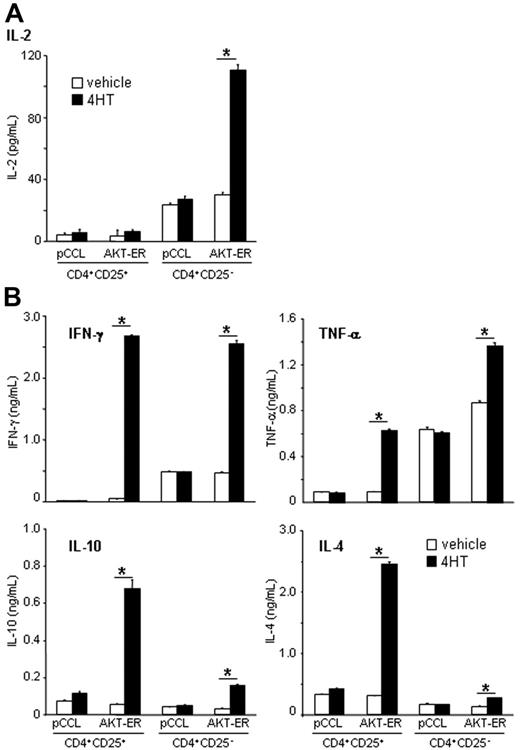
Enhanced AKT activity in CD4+CD25+ Treg cells restores their capacity to produce IFN-γ, TNF-α, IL-4, and IL-10, but not IL-2
One of the defining characteristics of CD4+CD25+ Treg cells is their inability to produce significant amounts of cytokines, particularly IL-2 and IFN-γ.10 Given the capacity of exogenous IL-2 to reverse suppression, and evidence that constitutive AKT activation in T cells can induce production of IL-2 and IFN-γ,31 it was important to determine whether the lack of suppression in the AKT-ER CD4+CD25+ Treg cells in the presence of 4HT was simply due to increased IL-2 production. Control pCCL- and AKT-ER–expressing CD4+CD25+ Treg-cell and CD4+CD25− T-cell lines were stimulated with αCD3/CD28 mAbs in the absence or presence of 4HT, and supernatants were collected after 24 hours (Figure 6A). As expected, control pCCL and, in the absence of 4HT, AKT-ER+ CD4+CD25+ Treg cells produced significantly lower levels of IL-2 than the CD4+CD25− T-cell lines. Surprisingly, the capacity of the AKT-ER CD4+CD25+ Treg-cell lines to produce IL-2 was not altered upon addition of 4HT. In contrast, addition of 4HT to the AKT-ER+ CD4+CD25− T-cell lines resulted in a 3.46-fold ± 0.59-fold increase in IL-2 production (n = 3, P = .009).
Enhanced AKT activity in CD4+CD25+ Treg cells restores their capacity to produce IFN-γ, TNF-α, IL-4, and IL-10, but not IL-2. AKT-ER– (or control pCCL-) transduced CD4+CD25+ Treg-cell or CD4+CD25− T-cell lines were activated with αCD3/CD28 mAbs in the presence of either vehicle alone or 4HT (150 nM). Supernatants were collected after 24 hours (A) or 48 hours (B) and assayed for amounts of cytokines by cytometric bead array (CBA). A representative experiment of 3 performed is depicted, and error bars represent SD from an individual experiment.
Enhanced AKT activity in CD4+CD25+ Treg cells restores their capacity to produce IFN-γ, TNF-α, IL-4, and IL-10, but not IL-2. AKT-ER– (or control pCCL-) transduced CD4+CD25+ Treg-cell or CD4+CD25− T-cell lines were activated with αCD3/CD28 mAbs in the presence of either vehicle alone or 4HT (150 nM). Supernatants were collected after 24 hours (A) or 48 hours (B) and assayed for amounts of cytokines by cytometric bead array (CBA). A representative experiment of 3 performed is depicted, and error bars represent SD from an individual experiment.
We next examined whether activation of AKT in the CD4+CD25+ Treg-cell lines affected production of other Th1- or Th2-associated cytokines. In contrast to IL-2, activation of AKT by addition of 4HT in AKT-ER+ CD4+CD25+ Treg cells restored their capacity to produce IFN-γ (7.8- to 58-fold compared to without 4HT control, n = 4, P = .003), TNF-α (2.5- to 21.3-fold, n = 4, P = .01), IL-4 (3.2- to 7.9-fold, n = 4, P = .005), and IL-10 (3.1- to 12.1-fold, n = 4, P = .004) (Figure 6B) to levels equivalent to, or in the case of IL-4 and IL-10, higher than those produced by CD4+CD25− T cells.
Discussion
This report represents the first characterization of intracellular signaling cascades in ex vivo human CD4+CD25+ Treg cells. Using flow cytometry–based assays, we found that CD4+CD25+ Treg cells have a capacity equivalent to that of non–Treg cells to activate ERK and p38 MAPKs but have a significant defect in the phosphorylation of AKT upon TCR-mediated activation. This abnormal AKT activation resulted in decreased activity of downstream effectors. Moreover, TCR-independent conditional activation of exogenous AKT in Treg cells reversed their suppressive capacity, indicating that the defect in the capacity of CD4+CD25+ Treg cells to fully activate AKT contributes to their unique suppressive function. Interestingly, enforced activation of AKT in CD4+CD25+ Treg cells neither reversed their inability to produce IL-2 following TCR stimulation nor decreased expression of FOXP3, CTLA4, or CD25, indicating that additional factors are involved in the reversal of suppression. Together, these data represent the first causal association between an altered molecular signal and the biologic function of CD4+CD25+ Treg cells.
Phosphorylation of AKT at both Thr308 and Ser473 is required for maximal kinase activity.33 Activated CD4+CD25+ Treg cells showed decreased phosphorylation of AKT at Ser473 but no decrease in phosphorylation at Thr308. AKT activity in CD4+CD25+ Treg cells, however, was clearly impaired because the phosphorylation of both direct (FOXO1 and FOXO3A) and indirect (S6) downstream targets was also reduced. This observation is consistent with the finding that murine CD4+CD25+ Treg cells had lower AKT activation upon IL-2 receptor stimulation15 and suggests these cells may have a global defect in their capacity to activate this kinase. In contrast, Li et al recently reported that in vitro–expanded CD4+CD25+ Treg cells derived from cord blood were found to have normal activation of AKT at longer (15 minutes to 16 hours) time points after activation.17 Our finding that CD4+CD25+ Treg-cell lines from adult peripheral blood have reduced phosphorylation of AKT and S6 indicates that this difference is not simply related to in vitro expansion. Rather, the apparent discrepancy between our observations and those of Li et al could be due to contamination by effector T cells and/or the time points chosen by these authors for examination of AKT activation.
Our data indicate that the reduced phosphorylation of AKT in CD4+CD25+ Treg cells does not result from changes in the activity of PI3′K itself or of the phosphatases SHIP or PTEN. Thus, the upstream defect must lie either in their capacity to activate or recruit PDK2, the putative Ser473 kinase, or in increased function of a Ser473-specific phosphatase. The identity of PDK2 has long been the subject of intensive research, and kinases such as integrin-linked kinase (ILK),43–45 DNA-PK,46 and even AKT itself47 can phosphorylate this residue. More recently, it has been suggested that when complexed with rictor, mTOR is the dominant Ser473 kinase.48 In terms of Ser473-specific phosphatases, the recently identified PH domain leucine-rich repeat protein phosphatase (PHLPP)49 or the more broadly acting protein phosphatase 2A (PP2A)50 may be involved. Currently, the relative contribution of these molecules to the state of Ser473 phosphorylation in primary human T cells has not been investigated, and further studies are required before the upstream defect in CD4+CD25+ Treg cells can be explored.
Activation of the PI3′K-AKT pathway is a fundamental requirement for T-cell survival and cell-cycle progression,39 and transgenic mice expressing a constitutively active form of AKT displayed enhanced T-cell activation and cytokine production, lost the requirement for CD28 costimulation to fully activate T-cells, and developed autoimmune-like syndromes.51,52 We therefore hypothesized that restoration of AKT signaling in CD4+CD25+ Treg cells would reverse their hyporesponsive state. Upon lentiviral-mediated expression of an inducibly active form of AKT, however, their capacity to proliferate was only slightly (about 5-fold) enhanced and remained significantly lower than that of CD4+CD25− T cells. CD4+CD25+ Treg cells may therefore have undergone irreversible epigenetic changes and/or unidentified blocks in other signaling pathways that underlie this phenotype. This finding may also be related to the continued high expression of FOXP3 in the AKT-ER CD4+CD25+ Treg cells, because ectopic expression of FOXP3 alone is sufficient to induce anergy in human CD4+ T cells.24
Unexpectedly, activation of AKT did not restore the capacity of CD4+CD25+ Treg cells to produce IL-2, although it significantly enhanced production of all other cytokines tested. In contrast, activation of AKT in CD4+CD25− T cells resulted in significantly enhanced IL-2 production. These latter data are consistent with findings from T cells from AKT transgenic mice, which produced 10-fold higher levels of both IL-2 and IFN-γ (but not IL-4)31 than wild-type T cells, and with the known role of AKT in activation of NFAT.39 The lack of IL-2 production by CD4+CD25+ Treg cells with restored AKT activity suggests that these cells have a permanent block in production of this cytokine, which may be related to their inability to undergo chromatin remodeling at the IL-2 locus after activation.16
The mechanism of CD4+CD25+ Treg-cell suppression has yet to be fully defined. We investigated whether restoration of AKT activity might alter expression of molecules previously associated with suppressive activity. Our data, however, indicate that the loss of suppressive capacity is not due to decreased expression of FOXP3, CD25, CTLA4, and/or granzymes A and B. We also considered the possibility that expression of AKT-ER may enhance the capacity of CD4+CD25+ Treg cells to respond to IL-2. Restoration of AKT activity, however, neither caused the cells to respond to lower amounts of IL-2 nor significantly increased the magnitude of their response (data not shown). Thus, the basis for the reversal of suppression by expression of active AKT in CD4+CD25+ Treg cells remains unclear. It is possible that enhanced production of cytokines other than IL-2 may be involved. It seems unlikely that IL-10, IFN-γ, or TNF-α would be implicated, because these cytokines generally have antiproliferative, prosuppressive effects.53–55 High levels of IL-4 might be involved, because exogenous IL-4 has been shown to reverse suppression in murine cells,56 although we have found this not to be the case for human cells (M.K.L., unpublished data, June 2001). Future experiments involving neutralization of these cytokines will be required to investigate their possible role.
In conclusion, we have demonstrated for the first time a causal connection between the biologic characteristics of CD4+CD25+ Treg cells and an altered signaling pathway. Our data provide further evidence that the in vitro hyporesponsive state of CD4+CD25+ Treg cells does not correlate with suppressive capacity. In addition, the observation that CD4+CD25+ Treg cells have a profound inability to produce IL-2, even when their capacity to secrete other cytokines is restored, suggests that these cells have undergone irreversible epigenetic changes. Finally, the conditionally suppressive AKT-ER CD4+CD25+ Treg cells represent the first system allowing inducible abrogation of suppression at the level of the Treg cell rather than by changing the susceptibility to suppression of the target T cells. Induction of AKT activity in CD4+CD25+ Treg cells represents a powerful tool for the study of the mechanism(s) of suppression and a major advance toward therapeutic modulation of peripheral tolerance.
Authorship
Contribution: N.K.C. designed and performed research, analyzed data, and wrote the paper; R.V.G. performed research and analyzed data; and M.K.L. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Megan K. Levings, Department of Surgery, UBC, 2660 Oak St, Vancouver, BC, Canada V6H 3Z6; e-mail: mlevings@interchange.ubc.ca.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by the Canadian Institutes for Health Research (grant MOP57834). M.K.L. holds a Canada Research Chair in Transplantation and is a Michael Smith Foundation for Health Research (MSFHR) Scholar. N.K.C. holds an MSFHR Senior Graduate Studentship award.
We thank Richard Roth for the gift of the myrAKT-ER-HA cDNA, Mario Amendola and Luigi Naldini for the parent bidirectional lentivector, and Gerry Krystal and Laura Sly for the anti-SHIP Abs. We also thank Rupinder Dhesi and the MSFHR-funded Immunity and Infection Research Centre's core virus facility for production of lentivirus. We gratefully acknowledge the support of Barrett Benny, Michael Barnett, Raewyn Broady, and the Cell Separator Unit at Vancouver General Hospital for providing PBMCs. We thank Paul Orban, Sarah Allan, and Natasha Locke for critical reading of the manuscript.

![Figure 4. Enforced activation of AKT reverses the suppressive capacity of CD4+CD25+ Treg cells. CD4+CD25high or CD4+CD25− T cells were transduced with lentivirus encoding an inducible AKT-ER or control (pCCL) vector. (A) Cells were stimulated with αCD3/APCs in the presence of vehicle alone or 4HT (150 nM). The inset is the fold increase in proliferation induced by 4HT in AKT-ER+ CD4+CD25+ Treg-cell or CD4+CD25− T-cell lines, with each point representing a single experiment. (B) CD4+ T cells were stimulated with αCD3 (soluble 1 μg/mL) and irradiated APCs in the absence or presence of a 1:2 ratio (T-cell lines–target) of control-transduced or of AKT-ER+ CD4+CD25+ Treg-cell or CD4+CD25− T-cell lines in the presence of either vehicle alone or 4HT (150 nM). Data are depicted as percent suppression ([1 − (Te + Treg/Te alone] × 100); negative values are plotted as 0. Proliferation and suppression were assessed by 3H-thymidine incorporation. A single representative experiment of 4 is depicted, and error bars represent SD from an individual experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/5/10.1182_blood-2006-07-035279/4/m_zh80050709170004.jpeg?Expires=1767769710&Signature=x8I4Z9gpIJHuQQphfilhM5y0-4Ls~9njPsSorCV~3TtMNRWQANu9x-dWlWXJ3EzDBh9JVSeCpJDMMjOsAc0U3Ojy8EebQ8Btyc8U~7e6DoRSJytIsfhzoIUcrz3ncNbbE4xWcFE~xOHJZL5DSEyyLZ4PgGM7HsSPKJGFevqQZBvht~97AXcEzib-CRFJNwq4V-fAHZ5-FFE9y-dHfQ1pzkB5hCQEYuauPq3fKunmcghYK4bhpNiDw6Qffrd5w-Jahk1EAWJGKdXNtGtWW53jgB1PSQ5WioAvVRkqO-bzJS4k2rkoHB7w93uIvk2cFjTDTvjSdKpDhM4tdfBDwyp1VA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal