Abstract
The anticoagulant, activated protein C (aPC), possesses antithrombotic, profibrinolytic, anti-inflammatory, and antiapoptotic properties, and the level of this protein is an important marker of acute inflammatory responses. Although infusion of aPC improves survival in a subset of patients with severe sepsis, evidence as to how aPC decreases mortality in these cases is limited. Because a total deficiency of PC shows complete neonatal lethality, no animal model currently exists to address the mechanistic relationships between very low endogenous aPC levels and inflammatory diseases. Here, we show for the first time that novel genetic dosing of PC strongly correlates with survival outcomes following endotoxin (LPS) challenge in mice. The data provide evidence that very low endogenous levels of PC predispose mice to early-onset disseminated intravascular coagulation, thrombocytopenia, hypotension, organ damage, and reduced survival after LPS challenge. Furthermore, evidence of an exacerbated inflammatory response is observed in very low PC mice but is greatly reduced in wild-type cohorts. Reconstitution of low-PC mice with recombinant human aPC improves hypotension and extends survival after LPS challenge. This study directly links host endogenous levels of PC with various coagulation, inflammation, and hemodynamic end points following a severe acute inflammatory challenge.
Introduction
Sepsis, with accompanying systemic inflammatory response syndrome, is a serious health problem affecting critically ill patients. It is estimated that 751 000 cases occur annually in the United States, with a mortality rate of 28.6%.1 This low therapeutic success rate is mainly due to a paucity of effective drugs for treating late-stage disease.
Sepsis and endotoxemia are characterized by multiple disturbances in the inflammatory, coagulation, anticoagulation, and fibrinolytic systems. Following an immune challenge by pathogenic bacteria or Gram-negative bacterial cell wall endotoxin (lipopolysaccharide [LPS]), proinflammatory and anti-inflammatory proteins are released from cells to control further infection. However, such an overwhelming acute inflammatory response is poorly regulated by the host, thereby leading to up-regulation of potent inflammatory cell-stimulated coagulation. Coagulation proteases, for example, thrombin, further stimulate production of inflammatory cytokines via cell signaling, which then propagate the procoagulant state,2 resulting in severe hypotension, disseminated intravascular coagulation (DIC), multiple organ distress, and death.3
The protein C (PC) system is an important regulator of hemostasis and also plays a crucial role during the systemic response to acute inflammatory challenge. The anticoagulant, activated PC (aPC), arises from activation of its zymogen, PC, through catalysis with thrombin. The reaction is optimal when PC is bound to its endothelial cell receptor (EPCR) and thrombin is present in complex with another cellular receptor, thrombomodulin (TM).4 The resulting aPC, along with Ca2+, phospholipids, and a cofactor, protein S, catalyzes inactivation of 2 potent procoagulant cofactors, FVa5 and FVIIIa.6 This leads to aPC-mediated attenuation of thrombin generation and consequently an inhibition of coagulation and inflammation.7 aPC also stimulates fibrinolysis by directly inactivating plasminogen activator inhibitor-1 (PAI-1).8 Additionally, by limiting thrombin generation, aPC indirectly suppresses both the thrombin-induced inactivation of PAI-19 and the thrombin-mediated activation of another fibrinolysis inhibitor, TAFI.10 By suppressing the inflammatory response to a relevant challenge, aPC also inhibits the induction of tissue factor (TF) on cells and the thrombus formation that is a result of the inflammatory insult.11 These anticoagulant and profibrinolytic functions of aPC render it an effective protein for the maintenance of blood fluidity.
During acute inflammation, plasma aPC levels are diminished. Cytokines such as IL-1β and TNF-α, as well as endotoxin, can attenuate TM and EPCR expression.12 This further reduces the ability of endothelial cells (ECs) to generate aPC. Studies have shown that patients with severe sepsis also have a rapid decrease in aPC production13 and aPC levels are strongly correlated with sepsis prognosis.14 The anti-inflammatory mechanisms of aPC are unclear. Reduction of cytokine production, protection of the endothelium against injury by inflammatory agents, and inhibition of neutrophil influx into tissues are among the proposed mechanisms.2,15 It is notable that many of the anti-inflammatory effects of aPC have been largely shown in vitro or with supra-pharmacologic concentrations of aPC. Whether such findings are physiologically relevant to human biology requires cautious interpretation. Thus, the question of how aPC controls inflammation in vivo remains to be answered.
Administration of recombinant human aPC (rhaPC) led to reduced mortality in a subset of patients with severe sepsis and a deficiency of endogenous PC exacerbated the endotoxic16 and septic17 responses in mice. Despite its clinical use, the precise mechanism of aPC in rescue of patients with severe sepsis is not well understood. A variety of coagulation, inflammation, cell migratory, and hemodynamic factors18–24 have been offered as predictors of outcome and targets of therapy in these patients. However, the side effects of such strong interventional approaches, such as bleeding in aPC-treated patients, remain a major challenge. Defining more precise mechanisms of aPC activity in modulating sepsis will lead to a more refined use of aPC.
We have previously described the generation of a novel group of mice possessing stable very low levels of endogenous PC, along with characterization of their unchallenged phenotypes.25 In the present investigation, selected lines of these mice, expressing 1% to 3% of wild-type (WT) PC, were used to examine the importance of the endogenous host level of PC in the presentation of various LPS-induced acute inflammatory end points. Because a PC deficiency contributes to sepsis/endotoxemia pathogenesis,20 the reduction of endogenous aPC would be expected to promote severe phenotypic responses following LPS administration, if PC is, indeed, an important member of the numerous mechanistic pathways associated with the disease. The present report summarizes the findings of this study.
Materials and methods
Mice
Transgenic mice expressing different levels of endogenous plasma PC were generated as described.25 Male mice, 7 to 8 weeks of age, were used. An experimentally optimized dosage of LPS (Escherichia coli endotoxin serotype 0111:B4) at 2 μg/g body weight was administered as an intraperitoneal bolus injection. This dosage allowed nearly complete lethality in very low PC mice (1%-3% of wild-type [WT] levels), but progressively lower lethality at higher endogenous PC levels (5% of WT). For survival analysis, mice were checked every hour following LPS administration until death occurred.
All experimental protocols were approved by the University of Notre Dame Institutional Animal Care and Use Committee.
Plasma and organ collection
Mice were killed at 3, 6, and 12 hours after LPS administration. Blood samples were withdrawn from the inferior vena cava into syringes containing citrate, heparin, or EDTA-containing buffer, depending on the assay requirements. Mice were then subjected to cardiac perfusion and livers, kidneys, lungs, and hearts were harvested for histologic examination (5 mice at each time point).
Histology and immunohistochemistry
Sections (4 μm) were prepared from periodate-lysine-paraformaldehyde (PLP)–fixed, paraffin-embedded tissues. Slides were stained with hematoxylin and eosin (H&E) or specific immunohistochemical stains for fibrin (ogen), neutrophils, and CD45. Cells undergoing apoptosis were detected by the TUNEL assay (Calbiochem, San Diego, CA). The specifics of our histologic procedures have been published.26
All histological images were visualized using a Nikon Eclipse E600 light microscope (Nikon, Melville, NY) equipped with a 20× or a 40× objective lens (0.10 numerical aperture). Micrograph images were captured using a SPOT RT-SE Slider-6 9.4 camera, and were acquired using SPOT 4.0.9 software (both from Diagnostic Instruments, Sterling Heights, MI). Images were then transferred to Adobe Photoshop version 7.0 (Adobe Systems, San Jose, CA) for final reproduction.
Radiotelemetric measurements of central blood pressures and heart rate
Male mice (20-25 g) were anesthetized with a cocktail of 0.075 mg ketamine/0.015 mg xylazine/0.0025 mg acepromazine/g body weight via intraperitoneal injection. Radiotelemetric catheters (model TA-F20 Mouse Transmitter; Data Sciences International, Arden Hills, MN) were implanted in the thoracic aortas of mice as described earlier.26 After the implantation surgeries, mice were kept warm on a heating pad and monitored for 1 week to allow full recovery. At this time, the cages were placed on telemetric receivers and the radiotelemeters were magnetically activated. Pulse rate, systolic and diastolic blood pressures (BPs), mean arterial pressure (MAP), heart rate (HR), and physical activity were recorded every 5 minutes for the duration of the experiment. All parameters were recorded 1 day prior to LPS administration for baseline assessments and continued for an additional 72 hours after LPS administration. Control low-PC and WT mice received saline injections in place of LPS.
Administration of rhaPC by continuous infusion
Male mice (7-8 weeks of age) were anesthetized with isoflurane (1.4%) introduced via inhalation. For rhaPC (Eli Lilly, Indianapolis, IN) infusion, a central venous catheter was inserted and rhaPC administered via a regulated pump at 1.8 μg/g/h. The optimal dose of rhaPC was determined from experiments in WT mice infused with rhaPC at 1.8 or 4 μg/g/h. No adverse side effects were associated with aPC treatment at 1.8 μg/g/h and the mice appeared well. The steady-state aPC concentration in low-PC mice after 15 hours was approximately 100 ng/mL, which is comparable to the level achieved in patients with severe sepsis receiving 24 to 30 μg/kg/h aPC.27 For survival analyses, mice were either infused with rhaPC or saline for 24 hours. LPS (2 μg/g) was injected intraperitoneally concurrent with the start of aPC infusion. Mice were checked hourly until death.
Enzyme-linked immunosorbent assay for measuring human aPC activity
Mice (n = 5) were continuously infused with rhaPC at a rate of 1.8 μg/g body weight/h. Blood was collected from mice at various times into citrate buffer (1:9) containing 20 mM benzamidine and assayed for circulating levels of rhaPC. The concentrations of rhaPC were determined in 96-well microtiter plates as published,13 using a monoclonal antibody against human aPC (HAPC1555; a gift from Dr C. T. Esmon, Oklahoma Medical Research Foundation, Oklahoma City) as the capture antibody for aPC. Nonspecific binding was blocked with 1% BSA. Because binding of aPC to HAPC1555 requires Ca2+, the citrated mouse plasma was recalcified with CaCl2 (5 mM) and supplemented with heparin (2 U/mL). Test plasma (100 μL) was added to the wells, followed by rinsing. The aPC substrate (200 μL), Spectrozyme PCa (American Diagnostica, Stamford, CT), was added to each well and incubated at 37°C for 30 minutes. Hydrolysis of p-nitroanilide from the substrate was determined at 405 nm using a SPECTRAmax Plus 384 spectrophotometer (Molecular Devices, Sunnyvale, CA).
Assays of plasma proteins
Plasma levels of IL-6 and TNF-α were measured by enzyme-linked immunosorbent assay (ELISA) using commercial antibodies specific for each antigen (R&D Systems, Minneapolis, MN). The D-dimer ELISA kit and reagents for activated partial thromboplastin time (aPTT) and prothrombin time (PT) assays were purchased from Diagnostica Stago (Parsippany, NJ). Antibodies for the thrombin-antithrombin (TAT) complex ELISA were obtained from ERL (South Bend, IN). Plasma fibrinogen levels were determined using Fibritest clotting time assays (Diagnostica Stago). Platelet counts were determined by VetScan analysis (Abaxis, Union City, CA).
Markers for assessment of organ distress
Organ damage in LPS-challenged mice was assessed by measuring various enzymes in plasma using the Vet test chemistry analyzer (Idexx Laboratories, Westbrook, ME). Blood was collected in heparin and plasma levels of alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatine kinase (CK), and lactate dehydrogenase (LDH) were analyzed using the relevant chemistry analysis strips.
Statistical analysis
Data are expressed as mean ± SEM, unless indicated otherwise. Comparisons between 2 group means were conducted using the paired Student t test. One-way ANOVA (Newman-Keuls test) was used for a multiple group comparison over time. Survival curves (Kaplan-Meier) were compared by the log-rank and Gehan-Wilcoxon test. P values less than .05 were considered statistically significant.
Results
Plasma PC levels dictate survival outcome in mice following LPS challenge
Administration of LPS resulted in 80% to 90% lethality within the first 24 hours in transgenic mice expressing 3% (PC−/−(PCTg785)) or 1% (PC−/−(PCTg4)) of WT plasma PC (Figure 1A). In contrast, 40% mortality was observed in mice that expressed 5% of WT plasma PC (PC−/−(PCTg12)). The average time of death for PC−/−(PCTg12) mice was significantly longer compared to those with lower endogenous PC levels. These data were in sharp contrast to mice expressing 18% (PC−/−(PCTg527)), 50% (PC+/−), and 100% (WT) PC. These latter mice had 100% survival rates under this treatment regimen. Furthermore, a higher mortality rate was observed in all PC genotypes under a higher dose of LPS, but the PC dependency on relative survival remained consistent (Figure 1A inset).
Low levels of PC predispose mice to LPS-induced lethality. Survival of transgenic mice stably expressing various low levels of endogenous PC following LPS challenge at 2 μg/g (A) or 10 μg/g (inset). Endogenous PC plasma concentrations are represented as percent of WT levels. Induction of TAT (B) and D-dimer (C) levels at various times after LPS (2 μg/g) administration in WT (▪) and PC−/−(PCTg785) (□) mice. Effect of LPS (2 μg/g) on the aPTT (D), PT (E), platelet count (F), and plasma fibrinogen levels (G) of WT (▪) and PC−/−(PCTg785) (□) mice. Data are shown as mean ± SEM (n = 5). Pairwise comparisons between low-PC mice and WT mice were analyzed by the Student t test. One-way ANOVA with the Newman-Keuls test was used for multiple group comparison over time. *Statistical significance with P < .05. Fibrinogen/fibrin stains in various organs from WT (H,J,L,N,P) and low-PC (I,K,M,O,Q) mice at 3 hours after LPS exposure. Heart (H, I), kidney (J, K), liver (L, M), lung (N, O) and spleen (P, Q). (H-Q) Original magnification, 200×.
Low levels of PC predispose mice to LPS-induced lethality. Survival of transgenic mice stably expressing various low levels of endogenous PC following LPS challenge at 2 μg/g (A) or 10 μg/g (inset). Endogenous PC plasma concentrations are represented as percent of WT levels. Induction of TAT (B) and D-dimer (C) levels at various times after LPS (2 μg/g) administration in WT (▪) and PC−/−(PCTg785) (□) mice. Effect of LPS (2 μg/g) on the aPTT (D), PT (E), platelet count (F), and plasma fibrinogen levels (G) of WT (▪) and PC−/−(PCTg785) (□) mice. Data are shown as mean ± SEM (n = 5). Pairwise comparisons between low-PC mice and WT mice were analyzed by the Student t test. One-way ANOVA with the Newman-Keuls test was used for multiple group comparison over time. *Statistical significance with P < .05. Fibrinogen/fibrin stains in various organs from WT (H,J,L,N,P) and low-PC (I,K,M,O,Q) mice at 3 hours after LPS exposure. Heart (H, I), kidney (J, K), liver (L, M), lung (N, O) and spleen (P, Q). (H-Q) Original magnification, 200×.
A PC insufficiency predisposes mice to the development of DIC
Since preliminary experiments with LPS indicated that PC−/−(PCTg785) mice showed dramatic responses compared to WT mice, this line, as well as the next lowest PC-expressing line, PC−/−(PCTg4), were used in the current study. In the resting state, these 2 strains of mice have higher levels of TAT complex compared to WT mice.25 At 3 hours after LPS administration, the plasma levels of TAT doubled from the resting values, both in PC−/−(PCTg785) and WT mice (Figure 1B). The plasma concentrations of TAT decreased in WT mice after 3 hours and by 12 hours the TAT concentration was not significantly different from the baseline levels. Meanwhile, TAT levels in PC−/−(PCTg785) mice remained elevated at 6 hours and 12 hours after LPS. These higher TAT levels account for the observed prothrombotic phenotype in this very low-PC mouse strain.
No significant changes in plasma D-dimer levels were observed in WT mice following LPS challenge (Figure 1C), suggesting that DIC does not occur in WT mice with this dose of LPS. In contrast, PC−/−(PCTg785) mice have increased D-dimer concentrations during disease progression. The D-dimer levels were significantly higher in PC−/−(PCTg785) mice compared to WT mice at all times examined after LPS administration.
These results suggest that mice severely lacking in PC were more susceptible to the development of DIC in response to LPS administration, consistent with observations in previous studies using PC+/− mice.16,17 Determination of PTs and aPTTs showed that there were statistical differences between WT and low-PC mice. Prolonged aPTTs were observed in resting PC−/−(PCTg785) mice and were further increased at 3 hours and 6 hours after LPS (Figure 1D). However, aPTTs were only slightly elevated in similarly treated WT mice. The PTs were significantly different between WT and PC−/−(PCTg785) mice at 3 hours and 6 hours after LPS challenge (Figure 1E).
Platelet counts decreased with time in WT mice following LPS challenge but were significantly lower in PC−/−(PCTg785) mice. By 6 hours, platelets were almost completely depleted in the low-PC group (Figure 1F). The plasma fibrinogen concentrations were higher in resting PC−/−(PCTg785) mice compared to WT mice and, at 3 hours after LPS exposure, fibrinogen decreased rapidly in low-PC mice. In contrast, WT mice showed an increase in plasma fibrinogen following LPS administration after 3 hours (Figure 1G). Consistent with rapid consumption of plasma fibrinogen and the emergence of DIC, more thrombus deposits were observed in multiple organs of low-PC mice as compared to WT mice following LPS exposure (Figure 1H-Q). These data support our findings that early onset of DIC occurs in very low PC-expressing mice, after LPS administration.
Organ damage is more prevalent in low-PC mice following LPS challenge
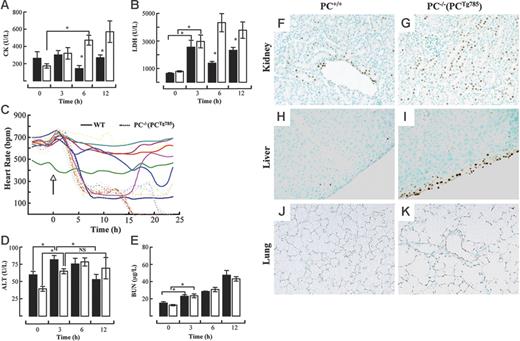
Various enzyme markers in plasma were measured to assess the degree of organ damage in PC−/−(PCTg785) mice and WT mice after LPS challenge. CK levels increased following LPS administration in a time-dependent manner in PC−/−(PCTg785) mice (Figure 2A). At 6 hours and 12 hours after LPS, plasma CK levels were statistically different between WT and the low-PC mice, suggestive of cardiac dysfunction. This was consistent with the up-regulation of plasma LDH at 12 hours in PC−/− (PCTg785) mice (Figure 2B). Further evidence of LPS-induced cardiac failure was indicated by a profound decline in HR at 6 hours after LPS challenge in low-PC mice compared to WT mice (Figure 2C). These data together support the presence of cardiac distress in low-PC mice. Similarly, a marker for hepatic damage, ALT, increased at 3 hours in the WT and PC−/− (PCTg785) groups (Figure 2D). These values were significantly different compared to the resting state. At 12 hours, ALT values in WT mice significantly decreased, whereas in PC−/−(PCTg785) mice, ALT was sustained at higher levels after 6 hours. Statistically, there was no significant difference between the 6-hour and 12-hour values for ALT in PC−/−(PCTg785) mice. BUN levels increased in a time-dependent manner following LPS exposure, but there were no significant differences between the WT and PC−/−(PCTg785) groups (Figure 2E). Organ damage as indicated by increased apoptosis was noted in the kidneys, livers, and lungs of low-PC mice compared to WT mice (Figure 2F-K).
Organ distress in low-PC mice after LPS administration. Plasma levels of CK (A) and LDH (B) at various times after LPS (2 μg/g) in WT (▪) and PC−/−(PCTg785) (□) mice. Heart rate measurements (C) in individual WT (solid lines) and PC−/−(PCTg785) (dashed lines) mice after LPS (2 μg/g, administered at the time marked by the arrow). Plasma levels of ALT (D) and BUN (E) at various times after LPS (2 μg/g) in WT (▪) and PC−/−(PCTg785) (□) mice. For panels A-B and D-E, the data represent the means of n = 3-5 mice from each group. *Statistical significance with P < .05. TUNEL assays showing apoptosis (brown staining) in various organs from WT (F,H,J) and low-PC (G,I,K) mice at 12 hours after LPS (2 μg/g) exposure. Kidney (F-G), liver (H-I), and lung (J-K). Original magnification, × 200 for all panels. (A-B, D-E) Data are represented as mean ± SEM (n = 5).
Organ distress in low-PC mice after LPS administration. Plasma levels of CK (A) and LDH (B) at various times after LPS (2 μg/g) in WT (▪) and PC−/−(PCTg785) (□) mice. Heart rate measurements (C) in individual WT (solid lines) and PC−/−(PCTg785) (dashed lines) mice after LPS (2 μg/g, administered at the time marked by the arrow). Plasma levels of ALT (D) and BUN (E) at various times after LPS (2 μg/g) in WT (▪) and PC−/−(PCTg785) (□) mice. For panels A-B and D-E, the data represent the means of n = 3-5 mice from each group. *Statistical significance with P < .05. TUNEL assays showing apoptosis (brown staining) in various organs from WT (F,H,J) and low-PC (G,I,K) mice at 12 hours after LPS (2 μg/g) exposure. Kidney (F-G), liver (H-I), and lung (J-K). Original magnification, × 200 for all panels. (A-B, D-E) Data are represented as mean ± SEM (n = 5).
Enhanced tissue infiltration of inflammatory cells in the absence of PC
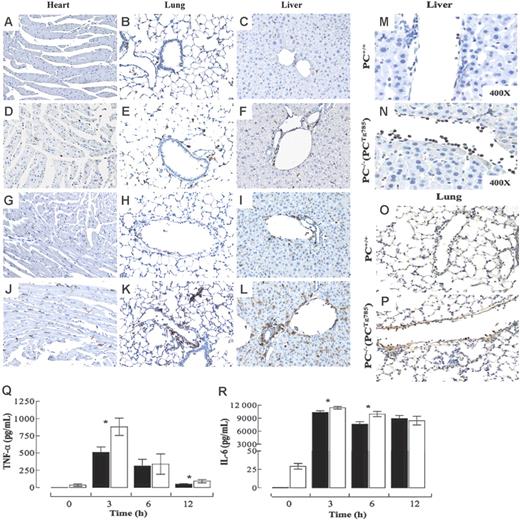
Tissue sections were analyzed immunohistochemically for various inflammatory markers. The resting levels of CD45+ leukocytes in the heart, lungs, and liver were more elevated in low-PC mice than in the WT group (Figure 3A-F). At 3 hours, increased CD45+ cells were noted in both groups (data not shown). By 6 hours, tissue CD45+ cells were considerably elevated in PC−/−(PCTg785) mice than in the WT group (Figure 3G-L). The majority of these leukocytes were neutrophils. Evidence of neutrophil margination and migration into the surrounding tissue was more profound in the low-PC mice than in the WT mice, consistent with increased neutrophil influx into the tissue (Figure 3M-P).
Inflammatory state in low-PC mice after LPS challenge. CD45+ immunostains (brown) in various organs from WT (A-C,G-I) or PC−/−(PCTg785) (D-F,J-L) mice at resting (A-F) and at 6 hours (G-L) after LPS (2 μg/g) administration. Neutrophils in the livers and lungs of WT (M-O) and PC−/−(PCTg785) (N-P) mice, respectively at 6 hours after LPS (2 μg/g) challenge. Original magnification, × 200 for all panels, unless indicated otherwise. Circulating levels of TNF-α (Q) and IL-6 (R) in WT (▪) and low-PC mice (□) at various times after LPS challenge. The values are means of 5 to 12 mice ± SEM.
Inflammatory state in low-PC mice after LPS challenge. CD45+ immunostains (brown) in various organs from WT (A-C,G-I) or PC−/−(PCTg785) (D-F,J-L) mice at resting (A-F) and at 6 hours (G-L) after LPS (2 μg/g) administration. Neutrophils in the livers and lungs of WT (M-O) and PC−/−(PCTg785) (N-P) mice, respectively at 6 hours after LPS (2 μg/g) challenge. Original magnification, × 200 for all panels, unless indicated otherwise. Circulating levels of TNF-α (Q) and IL-6 (R) in WT (▪) and low-PC mice (□) at various times after LPS challenge. The values are means of 5 to 12 mice ± SEM.
Inflammation state in low-PC mice following LPS exposure
Plasma levels of TNF-α and IL-6 were measured as markers of inflammation in PC−/−(PCTg785) mice following LPS-induced endotoxemia. Both proteins peaked at 3 hours followed by a time-dependent decrease. TNF-α (Figure 3Q) levels were significantly higher in low-PC mice at 3 hours compared to WT mice. This cytokine persisted in the plasma of PC−/−(PCTg785) mice and remained significantly higher than in WT mice at 12 hours after LPS. In the case of IL-6 (Figure 3R) small but significant differences were observed at 3 and 6 hours after LPS.
LPS-induced severe hypotension in low-PC mice
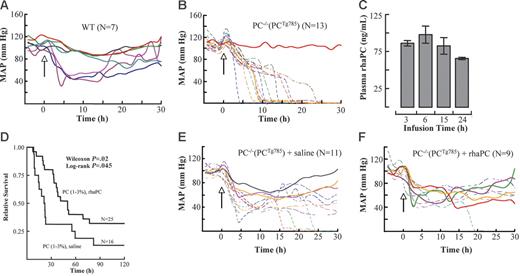
Central BPs were continuously monitored in PC−/−(PCTg785) mice and WT mice following LPS administration. LPS induced BP decreases in WT and low-PC mice at approximately 2 hours following challenge. By 5 hours, 40% of WT mice displayed BPs of approximately 50 mm Hg. Importantly, these BPs reached a plateau at these levels and began to recover at 15 hours (Figure 4A). In contrast, in low-PC mice, BP decreases lacked the plateau phase. In these cases, the declines in BP were more rapid and continued to decrease with time. The gradient of BP drop was steeper in mice that died early while others showed a steady decline. By 15 hours, 70% mortality was noted in low-PC mice. No change in BP was observed in mice that survived (Figure 4B).
aPC suppressed hypotension and prolonged survival in low-PC mice. The mean arterial blood pressures (MAPs) in LPS-challenged WT (A) and low-PC mice (B). The onset of LPS injection (2 μg/g) is indicated by the arrow (time 0). Continuous infusion of rhaPC to PC−/−(PCTg785) mice resulted in a steady-state aPC concentration of 100 ng/mL at 15 hours. By 24 hours, the rhaPC concentration was 60 ng/mL (C). The data represent means of 5 mice per group ± SEM. Survival analysis of PC−/−(PCTg785) mice challenged with LPS and treated with rhaPC (solid line) or saline (dotted line) (D). The data represent 16 to 25 mice/treatment group. Mice were challenged with LPS and infused with rhaPC or saline for 24 hours. Mice treated with rhaPC showed improved MAPs and overall longer survival (F) compared to saline-treated mice (E). In all graphs, solid lines represent mice that survived and dashed lines represent nonsurvivors.
aPC suppressed hypotension and prolonged survival in low-PC mice. The mean arterial blood pressures (MAPs) in LPS-challenged WT (A) and low-PC mice (B). The onset of LPS injection (2 μg/g) is indicated by the arrow (time 0). Continuous infusion of rhaPC to PC−/−(PCTg785) mice resulted in a steady-state aPC concentration of 100 ng/mL at 15 hours. By 24 hours, the rhaPC concentration was 60 ng/mL (C). The data represent means of 5 mice per group ± SEM. Survival analysis of PC−/−(PCTg785) mice challenged with LPS and treated with rhaPC (solid line) or saline (dotted line) (D). The data represent 16 to 25 mice/treatment group. Mice were challenged with LPS and infused with rhaPC or saline for 24 hours. Mice treated with rhaPC showed improved MAPs and overall longer survival (F) compared to saline-treated mice (E). In all graphs, solid lines represent mice that survived and dashed lines represent nonsurvivors.
Administration of rhaPC improves LPS-induced hypotension and reduces mortality
To further examine the importance of aPC in this model of LPS-induced acute inflammation, the survival outcomes of PC−/−(PCTg785) mice were studied after administration of rhaPC and compared to a control group treated with saline. PC−/−(PCTg785) mice infused with rhaPC at a dose of 1.8 μg/g/h, resulted in a plasma aPC concentration of approximately 60 ng/mL after 24 hours (Figure 4C). Mice treated with aPC showed a partial but significant improvement in 5-day survival (low-PC 32.5% versus WT 12.5%; Figure 4D). In addition, the onset of death was significantly delayed. At 22 hours, only 20% of aPC-treated mice died, compared to 56% mortality in saline-treated mice. Wilcoxon analysis revealed a P value of .02. Having demonstrated that reconstitution of rhaPC significantly reduced mortality in PC-deficient mice, we next investigated whether the reduction in mortality was related to an improved BP response. At 2 hours after LPS challenge, BP decreases were observed in low-PC mice, whether they received rhaPC or saline. At approximately 9 hours, 64% of the saline-treated mice had BPs of 40 mm Hg (Figure 4E), compared to 44% in rhaPC-treated mice (Figure 4F). In the saline treated group, no mice with BP of 40 mm Hg survived, whereas 25% of the rhaPC-treated mice that had BP of 40 mm Hg recovered and survived.
Discussion
In this investigation, our findings strongly support the concept that PC levels are relevant predictors for survival outcomes in a mouse model of endotoxemia. During the course of a related disease, sepsis, the levels of PC and aPC are severely depleted. Restoring plasma aPC with rhaPC has been shown to benefit a subset of patients with severe sepsis.28–32 Data from the PROWESS (Recombinant Human Protein C Worldwide Evaluation in Severe Sepsis) and ENHANCE (Extended Evaluation of Recombinant Human Protein C) clinical studies demonstrated that baseline plasma PC levels were an independent predictor of sepsis outcome. Patients with severe PC deficiencies (≤ 40% activity) correlated with a significantly higher mortality than those with a lesser PC deficiency (baseline PC level 41%-80% and > 80%).33,34
A previous study showed that mice homozygous for a total deficiency of PC died following birth.35 Consequently, mice heterozygous for the PC deficiency have been used as models of endotoxemia16 and polymicrobial sepsis.17 In the former case because 50% levels of PC could sufficiently protect them against LPS challenge, a high dose of LPS (ie, 50 mg/kg) was used to induce endotoxemia. Thus, cytotoxicity due to a high concentration of LPS is an issue that requires consideration. The availability of novel mouse lines stably producing a variety of very low endogenous PC levels,25 provided the capability of investigating the relevance of PC in LPS-induced endotoxemia. In the current investigation, we studied coagulation, inflammation, and hemodynamic outcomes after LPS challenge in these mice. Our findings support clinical observations showing that plasma PC levels were directly related to sepsis survival.
The dose-dependent effect of LPS on lethality was tested using mice expressing 1% to 3% of WT PC. LPS given at 10 μg/g caused very early onsets of thrombotic events. Clotting in these mice was so severe that blood samples could not be withdrawn. A much lesser response was observed with 2 μg/g LPS. Based on this observation, the latter dose was used in this investigation. In 3 groups of mice with different endogenous levels of PC, it was demonstrated that PC levels strongly correlated with survival. This PC dependency effect was also noted when high doses (10 μg/g) of LPS were used. At higher doses of LPS, higher mortality rates were observed for all PC genotypes but the relative rates remained inversely proportional to PC levels. These results serve as valuable guides for evaluating the extent to which PC must be maintained to benefit survival after an acute inflammatory challenge.
Coagulation parameters (eg, prolonged PTs and aPTTs), together with elevated plasma D-dimer and decreased fibrinogen levels, support the existence of DIC in low-PC mice compared to WT mice. This observation was further confirmed by the finding that low-PC mice had a severe depletion of platelets, thereby providing evidence of thrombocytopenia. This implies that low-PC mice are highly susceptible to early onset of DIC following LPS challenge. The observed DIC is most likely associated with the elevated levels of thrombin.
Evidence of more severe organ distress was demonstrated in low-PC mice compared to WT group. Among the maladies present was an early onset of cardiac damage, as indicated by up-regulation of CK, and by diminished heart rates in the challenged low-PC cohort. Moreover, hypoxia (data not shown) was noted in the hearts, likely a result of tissue ischemia due to severe thrombosis. This may account for the susceptibility of low-PC mice to LPS-induced cardiac dysfunction. The changes in plasma ALT were less dramatic between the low-PC and WT mice. Reduction of ALT levels in WT mice at later time points suggested that they were able to recover from the damage. In contrast, liver failure in low-PC mice was more severe and worsened with time. The vulnerability of low-PC mice to organ failure correlated with increased cell death in the kidneys, livers, and lungs. Such damage may reflect poor tissue irrigation due to LPS-induced hypotension.
Hypotension outcomes have been demonstrated in a cecal ligation and puncture (CLP) model of endotoxemia using PC+/− mice17 and in an LPS-induced endotoxemia model using low-EPCR mice.26 Moreover, aPC has been shown to prevent LPS-induced hypotension in rats.36 In support of these studies, our findings showed that changes in BP were more severe in low-PC mice as compared to WT controls. Because this is a lethal model for low-PC mice, and their survival rates varied considerably, we chose to analyze the BP data for each mouse separately to reflect the actual changes in BP instead of the average BP for all mice examined. From these analyses, it is clear that this relatively low dose of LPS only minimally affected BPs in WT mice, that is, only 43% of these mice were hypotensive. Importantly, the BP in these mice stabilized and they were able to overcome the hypotensive stress and survived. A similar pattern of BP change was also observed in PC−/−(PCTg527) mice expressing 18% of WT PC (data not shown). In contrast, PC−/−(PCTg785) mice suffered severe BP drops, and consequently died. These studies clearly demonstrated that the endogenous level of PC is critical in the maintenance of endotoxin-induced hypotensive shock. Further evidence for the role of aPC in LPS-induced hypotension was provided by the findings that administration of rhaPC significantly suppressed hypotension in low-PC mice, compared to vehicle mice treated with saline. Moreover, the majority of the hypotensive rhaPC-treated mice were able to recover their BP and generally live longer than the saline-treated mice.
In addition to severe DIC and organ damage, low-PC mice also had increased inflammation compared to WT mice. This is evidenced by the elevated TNF-α and IL-6 as well as higher leukocyte infiltration into various organs of low-PC mice than in WT mice. Furthermore, a considerable number of neutrophils adhering to the endothelium were noted in both large and small vessels of low-PC mice, and were not observed in WT mice. Evidence of increased neutrophil migration into the surrounding tissue was also present in low-PC mice. This observation suggests that aPC may be important for controlling neutrophil recruitment into the surrounding tissue. More vigorous migration of neutrophils would facilitate the onset of tissue injury observed in low-PC mice. A previous study has shown that rhaPC interfered with neutrophil migration into inflammatory sites.15 Our findings thus provide further support for a role of aPC in neutrophil migration in vivo and demonstrate in an overall sense that very low-PC mice were susceptible to inflammation following LPS challenge. These results corroborate with the previous observations using PC+/− mice challenged with a high dose of LPS and support the notion that the PC system is critical for determining disease outcome in LPS-induced endotoxemia. In addition, evidence is provided that a severe PC deficiency aggravates LPS-induced hypotensive shock and compromises survival following LPS challenge.
Although the importance of maintaining a functional PC system during sepsis is clear, aPC resistance due to the FV-Leiden (FVL) mutation in sepsis remains controversial.37 It is unclear whether aPC resistance increases or reduces survival in response to sepsis.38–40 A survival benefit in heterozygous FVL mice, due to elevated thrombin and increased aPC generation, has been suggested. Furthermore, the fact that homozygous FVL has a higher mortality than FVL heterozygotes indicated that a threshold of thrombin may be necessary in fine tuning the balance between coagulation and anticoagulation. Too much thrombin will offset coagulation as supported by our observation with the low-PC mice. Moreover, the finding that heterozygous FVL carriers benefit from aPC treatment41 implies that 50% activity of FVa is sufficient for optimal suppression of thrombin generation. These data also suggest that the anticoagulant activity of aPC may not in itself account for all the survival benefit. However, 2 studies failed to correlate a survival benefit with FVL heterozygotes.40,42 These discrepancies demonstrate that additional studies are required to elucidate how aPC improves sepsis survival in heterozygous FVL carriers. Of note, experiments designed to determine whether FVa has direct effects on the inflammatory response will be valuable and may reveal whether the role of aPC in inflammation is mediated through a FVa-dependent pathway.
In conclusion, this study demonstrates that PC plays a crucial role in response to LPS-induced endotoxemia. This is the first study that directly demonstrates an association between endogenous resting PC levels and endotoxin-induced mortality in mice. We show that severe deficiencies of PC predisposed mice to LPS-induced DIC, thrombocytopenia, hypotension, profound organ damage, and compromised survival. The early onset of thrombin-mediated coagulation in low-PC mice suggests that PC is important in preventing coagulation activation during acute inflammatory challenge. Furthermore, the preexisting inflammation associated with a PC deficiency accounted for enhanced inflammatory responses in low-PC mice. Reconstitution with rhaPC significantly improved survival outcomes through its impact on the control of coagulation, suppression of uncontrolled inflammation, and reversing hypotension. The finding that PC threshold strongly correlated with survival outcome is clinically significant. Determining the levels of PC/aPC in septic patients will be important for the diagnosis of disease severity and for predicting survival outcome.
Authorship
Contribution: A.J.L. designed and performed research, analyzed data, and wrote the manuscript; D.D. and M.-J.T. performed research; and F.J.C. mentored the overall study and edited all drafts of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francis J. Castellino, University of Notre Dame, W. M. Keck Center for Transgene Research, 230 Raclin-Carmichael Hall, Notre Dame, IN 46556; e-mail fcastell@nd.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants HL019982 and HL073750 from the National Institutes of Health and the Kleiderer-Pezold endowed professorship (F.J.C.).
We thank Dr Charles T. Esmon for the gift of HAPC1555 monoclonal antibody; Maeve Bonner, Mai Nguyen, and Mayra Sandoval-Cooper for histology assistance; Juan Fu for RNA analysis; and the staff of the Freimann Life Sciences Facility for mouse husbandry. We also thank Dr David Joyce for providing information on the rhaPC infusions in mice.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal