To the editor:
Imatinib substantially changed the course and prognosis of chronic myeloid leukemia (CML). As a consequence, several female CML patients decided to become pregnant. In this setting, an unsolved clinical question regards the potential exposure of the offspring to therapeutic doses of imatinib through breast feeding. We report here the results obtained in a 40-year-old CML patient who delivered a healthy male baby after 38 weeks of gestation. The patient decided to breastfeed her son. During the fourth week of lactation, plasma and milk samples were obtained from the patient, after receiving written informed consent, and collected immediately before and at 1, 2, 3, 4, and 9 hours after the daily oral administration of 400 mg of the drug. Imatinib and its main active metabolite N-desmethyl derivative (CGP 74588) were extracted from milk and plasma using a protein precipitation procedure with acetonitrile1 and quantified by a liquid chromatography coupled to tandem mass spectrometry method (HPLC/MS/MS). The mass spectrometer was operated in the multiple-reaction monitoring mode, and following HPLC separation, the peak areas corresponding to the mass-charge ratio (m/z) 494_394 reaction for imatinib and m/z 480_394 for CGP 74588 were measured. The limit of quantitation in milk samples was 30 ng/mL for both analytes, and the recovery rate was greater than 85%.
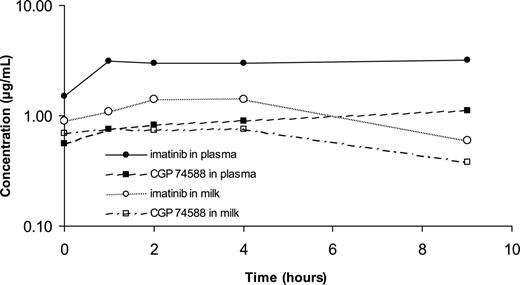
Figure 1 reports the concentration profiles over time obtained in plasma and milk. Imatinib and CGP 74588 achieved plasma steady-state concentrations ranging from 3.0 to 3.2 and 0.8 to 1.1 μg/mL, respectively.
Concentrations of imatinib and CGP 74588 in plasma and milk at steady state.
Both the parent drug and the metabolite distributed in human milk. The milk-plasma ratio reached 0.5 for imatinib and 0.9 for CGP 74588. Therefore CGP 74588 showed relatively higher concentrations in milk, reaching a steady-state level of 0.8 μg/mL, quite close to the concentrations of the parent drug, which ranged between 1.1 and 1.4 μg/mL. Similar results were obtained in samples obtained during the second month of breastfeeding.
Milk intake in infants is known2 to average 728 to 777 mL/d (with a range of 450 to 1165 mL/d). Considering the combined concentration of imatinib and CGP 74588 and the maximum milk intake described, it is unlikely that infants will receive more than 3 mg/d. Even when considering infant weight in the first months of life, this amount is quite far from therapeutic ranges, and the exposure might correspond to about 10% of a therapeutic dose.
Therefore, these results, although obtained in a single patient and needing confirmation by larger series, suggest that mothers with CML could safely breast-feed their children. Given the important biologic and psychological value of breast-feeding, such information will be useful for CML patients considering a pregnancy and for the hematologists caring for them. However, the effects of even low-dose, chronic exposure of infants to imatinib are not known. Accordingly, in such a case mothers would want to discuss this option with their pediatrician.
Authorship
Correspondence: Carlo B. Gambacorti-Passerini, Internal Medicine, University of Milano Bicocca and San Gerardo Hospital, via Cadore 48, 20052 Monza, Italy; e-mail:carlo.gambacorti@unimib.it.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal