Abstract
A temporal decline in tissue stem cell functionality may be a key component of mammalian aging. The tumor suppressor p53 has recently been implicated as a potential regulator of aging. We examined age-associated hematopoietic stem cell (HSC) dynamics in mice with varying p53 activities. Reduced p53 activity in p53+/− mice was associated with higher numbers of proliferating hematopoietic stem and progenitor cells in old age compared with aged wild-type (p53+/+) mice. We also assessed HSC dynamics in a p53 mutant mouse model (p53+/m) with higher apparent p53 activity than wild-type mice. The p53 hypermorphic (p53+/m) mice display phenotypes of premature aging. Many aged p53+/m organs exhibit reduced cellularity and atrophy, suggesting defects in stem-cell regenerative capacity. HSC numbers from old p53+/m mice fail to increase with age, unlike those of their p53+/+ and p53+/− counterparts. Moreover, transplantation of 500 HSCs from old p53+/m mice into lethally irradiated recipients resulted in reduced engraftment compared with old wild-type p53+/+ and p53+/− HSCs. Thus, alteration of p53 activity affects stem-cell numbers, proliferation potential, and hematopoiesis in older organisms, supporting a model in which aging is caused in part by a decline in tissue stem cell regenerative function.
Introduction
Normal organ size and cellular composition represent a regulated balance between cell death and cell replacement through the proliferation and differentiation of immature cells. In many tissues, adult tissue stem cells are recruited to replace tissue lost to “natural turnover” or during damage and regeneration. Stem cells are defined as immature cells with the ability to self-renew and to produce more differentiated daughter cells.1 Despite the self-renewal capacity of stem cells, a number of studies suggest that over time adult tissue stem cells exhibit functional aging and gradually lose the ability to successfully regenerate tissue, thus driving tissue attrition and reduced regeneration, commonly accepted hallmarks of aging.2-5
A number of stem-cell aging studies have focused on the hematopoietic stem cell (HSC) compartment. The first studies to suggest stem-cell aging involved serial transplantation of whole bone marrow that supported only 4 to 5 rounds of transplantation.2,6 Given that the HSC compartment facilitates this regeneration, these findings suggested an exhaustion of the stem-cell pool. Later mouse studies revealed some interesting trends associated with HSC aging: the number of HSCs increased while their proliferative capacity decreased with age.7,8 Results from studies comparing HSCs in different mouse strains indicate that HSC functional decline can be correlated with lifespan; a negative correlation has also been shown between lifespan and proliferative capacity.9,10
Because aging in somatic cells is associated with an accumulation of DNA damage, gene products that regulate the DNA damage response are candidate regulators of aging. One such candidate is p53, a potent tumor suppressor that maintains genomic integrity by negatively regulating the cell cycle upon DNA damage. Mutation of p53 itself or alterations within the p53 signaling pathway are pivotal to the onset or progression of tumorigenesis and are present in at least 80% of all human cancers.11 The importance of p53 in cancer prevention was illustrated by the early tumors observed in mice lacking p53.12-14 Even in mice defective for a single p53 allele (p53+/−), tumor incidence is greatly increased.13,15
Recently, p53 has also been implicated as a potential regulator of longevity and aging.16,17 Several knockout and transgenic mouse lines with premature aging phenotypes exhibit increased p53 activity.18-24 In some cases, these aging phenotypes can be partially rescued by reduction of the p53 dosage.14,16,18,25 Our laboratory has produced a p53 mutant mouse (p53+/m) with apparent hyperactive p53 activity.23 Consistent with this, p53+/m mice have heightened tumor resistance compared with their wild-type counterparts. Surprisingly, these mice also display significantly reduced longevity compared with wild-type mice. Characterization of the p53+/m mice revealed a number of early aging phenotypes. In addition, these mice displayed reduced organ mass and cellularity beginning in middle age that became more accentuated in old age. Aged p53+/m mice had a reduced ability to regenerate peripheral white blood cells after ablation of hematopoietic progenitors with 5-fluorouracil.23 We hypothesized that reduced HSC functionality drove the diminished regenerative ability in the blood of p53+/m mice and that the opposite may be true for the p53-deficient animals, which may have increased stem-cell functionality. Studies of HSCs from young p53 null mice showed that these cells are more robust in their self-renewal and reconstitution functions,26-29 but the retention or loss of these properties in older p53-deficient HSCs has not been examined.
In this study, we show that the p53+/m mouse exhibits a reduction in the number of proliferating HSCs with age compared with wild-type mice. Reducing p53 levels shows the opposite result, with the p53+/− mouse displaying an increase in the number of proliferating HSCs with age. Additionally, we showed that the p53+/m HSCs have a reduced engraftment capability compared with wild-type and p53-deficient HSCs. Thus, increased p53 activity in the HSCs may contribute to the reduced functionality of the hematopoietic compartment in times of stress. To our knowledge, this is the first demonstration of p53 dosage effects on age-associated hematopoietic stem cell functionality.
Materials and methods
Mice
All p53 mutant mice used in this study were generated and reported previously by our laboratory.12,23 Wild-type and p53-deficient mice were produced by p53 heterozygote crossing and were genotyped by Southern blot analysis with the use of a murine p53, exon 5 to 10, cDNA probe to genomic tail DNA cleaved with BamHI, as described previously.12 p53+/m mice were obtained by crossing to wild-type mice, and the progeny were genotyped by PCR. Briefly, 500 ng genomic tail DNA was mixed with murine p53 exon 7 forward primer (5′-CGG CTC TGA GTA TAC CAC CAT CCA C-3′) and exon 8 reverse primer (5′-GCC TGC GTA CCT CTC TTT GCG CTC C-3′) and was subjected to 33 cycles of PCR in a standard reaction. Amplified PCR fragments were then incubated with StuI endonuclease for 2 hours at 37°C and separated by 2% agarose gel electrophoresis with ethidium bromide. Visualization of PCR fragments by UV illumination revealed either one 577-bp fragment (p53+/+) or 3 fragments measuring 577 bp, 507 bp, and 70 bp (p53+/m).
All p53 mutant mice were backcrossed at least 5 generations into C57BL/6. The mice were housed in a specific pathogen-free environment at Baylor College of Medicine and were harvested when young (4 months) or old (18-20 months). Founding C57BL/6-CD45.1 mice used in transplantation experiments were obtained from The Jackson Laboratory (Bar Harbor, ME), bred and maintained in a specific pathogen-free facility at Baylor College of Medicine, and used between 6 and 12 weeks of age.
Isolation and enumeration of bone marrow
For every animal, bone marrow was isolated from 4 hind limb bones. The bones were dissected, and the marrow was flushed through a 20-gauge needle into HBSS+ (Hanks balanced salt solution [Invitrogen, Carlsbad, CA]), 2% FBS (Invitrogen), and 10 mM HEPES. The cells were passed through the needle twice and filtered (45-μm filter) to ensure single-cell suspension. Nucleated cells were counted manually after the lysis of red blood cells with 3% acetic acid in methylene blue (Stem Cell Technologies, Vancouver, BC, Canada).
In vitro cobblestone area–forming cell assay
In each experiment, total bone marrow was flushed, and then pooled, from the 4 hind limb bones of 4 animals of the same genotype and same age. Each experiment was performed twice. The cobblestone area–forming cell (CAFC) assay was performed as previously described by de Haan et al.9 In brief, several 96-well plates were seeded with the stromal-cell line FBMD-1. Cells were allowed to grow for 10 to 14 days and to reach a confluent monolayer. After this, total whole bone marrow from young and old wild-type and p53 mutant mice was seeded onto the stromal cells at varying dilutions, specifically 81 000, 27 000, 9000, 3000, 1000, and 333 cells per well. Each complete set of dilutions was performed in triplicate, with each specific dilution value made in 20 wells. Individual wells were scored as positive or negative for the appearance of a cobblestone every week for 5 weeks. A cobblestone area was scored positive if the cells fit the predetermined phenotype of at least a 5-cell colony growing beneath the stromal layer, with a characteristic flattened or cobblestone appearance (as established by Ploemacher et al30 ). Cobblestone frequency estimates were calculated using maximum likelihood analysis,31 taking into consideration 3 cell doses that yielded positive and negative scores.
Flow cytometry to assess HSC number
After total bone marrow isolation, 108 cells were centrifuged and resuspended in 200 μL HBSS+. Cells were stained for 15 minutes on ice and protected from light with a cocktail of antibodies including 6 lineage markers: B220, GR-1, CD-8, Ter-119, CD4, and Mac-1 conjugated to PE-Cy5 (no. 15-0452, no. 15-5931, no. 15-0081, no. 15-5921, no. 15-0042, no. 15-0112, respectively; eBioscience, San Diego, CA). In addition, the cocktail contained anti–Sca-1-FITC (no. 553335; BD Biosciences PharMingen, San Diego, CA), anti–c-kit-APC (no. 553356; BD Biosciences PharMingen), and anti–Flk-2-PE (no. 553842; BD Biosciences PharMingen). After staining, the cells were washed and resuspended in HBSS+ containing 2 μg/mL propidium iodide. Analysis of the stained cells was performed on a triple-laser flow cytometer (MoFlow; Cytomation, Fort Collins, CO). HSCs were selected based on low or negative expression of the mature lineage markers (lin−/lo), dual-positive expression of Sca-1 and c-kit, and negative expression of Flk-2. Analysis was performed in triplicate; each analysis used 3 to 4 mice per genotype for young mice (n = 10) or 2 mice per genotype for old mice (n = 6).
Staining and analysis of side population cells
Staining and analysis of side population cells was carried out as previously described.32 In brief, bone marrow was isolated and enumerated as described under “Isolation and enumeration of bone marrow,” The cells were resuspended at 106 cells per milliliter in prewarmed DMEM+ (high-glucose Dulbecco modified eagle medium), 2% FBS (Invitrogen), and 10 mM HEPES. Hoechst 33342 (Bis-benzimide; Sigma, St Louis, MO) was added to a final concentration of 5 μg/mL, and the cell suspension was placed at 37°C for exactly 90 minutes. Cells were then centrifuged and resuspended in cold HBSS+ and kept on ice for all subsequent antibody staining and analysis.
Flow cytometry to assess HSC proliferation
Sixteen hours before the bone marrow was harvested, mice were injected intraperitoneally with 1 mg BrdU (no. 552598; APC-BrdU Flow Kit; BD Biosciences PharMingen). Bone marrow was isolated and prepared as described. After preparation, bone marrow was stained for 15 minutes and protected from light with a cocktail of antibodies including the 6 previously described lineage markers conjugated to PE-Cy5, anti–Sca-1-FITC, and anti–c-kit-PE (no. 553355; BD Biosciences PharMingen). For this assay, the Flk-2 antibody was omitted. After antibody staining, the APC-BrdU Flow Kit (no. 552598; BD Biosciences) was used according to the manufacturer's instructions to identify cycling cells. In brief, the cells were fixed, permeabilized, and fixed again before incubation with DNAse to expose the incorporated BrdU. Then cells were stained with anti–BrdU-APC (no. 552598; BD Biosciences PharMingen) for 20 minutes at room temperature. Finally, cells were washed and resuspended in HBSS+. Analysis of stained cells was again performed on a triple laser flow cytometer (MoFlow; Cytomation). Proliferating HSCs were selected based on lin−/lo, dual expression of Sca-1 and c-kit, and labeling with BrdU.
Total bone marrow competitive transplantation
Whole bone marrow was prepared, as described above, under “Isolation and enumeration of bone marrow,” from young and old “test” CD45.2 mice (p53+/+, p53+/−, p53−/−, and p53+/m) and from “donor” CD45.1 mice at 6 to 12 weeks. Five recipient CD45.1 mice were used for every donor, and at least 3 donor mice were used from each test genotype. A cell mixture was prepared for injection containing equal parts test and donor cells; 400 000 cells were injected in a 100-μL volume. The cell mixture was injected retro-orbitally into lethally irradiated CD45.1 recipient mice. Lethal irradiation was achieved by administering 10 Gy gamma irradiation in a split dose separated by 2 hours. Irradiation control mice were used in every experiment, and these mice died within approximately 10 days of treatment. Peripheral white blood cells of recipient mice were analyzed for CD45.1 or CD45.2 cell-surface markers 4 and 12 weeks after transplantation.
Limiting-dilution HSC transplantation
Whole bone marrow was prepared as described from old p53+/+, p53+/−, and p53+/m test CD45.2 mice. Three mice were used for each test genotype, and the marrow was pooled to obtain sufficient numbers of HSCs. All mice were examined for illness, tumors, or evidence of lymphoid hyperproliferation. No mice exhibiting any of these abnormalities were used as transplant donors. As an enrichment procedure for sorting and collecting HSCs, we used MAC beads according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). In brief, total bone marrow (108 cells/mL) was stained with anti–Sca-1-biotin (no. 557404; BD Biosciences PharMingen) for 15 minutes on ice, protected from light. The cells were washed with HBSS+ and resuspended in MACS buffer (PBS [pH 7.2], 0.5% BSA, 2 mM EDTA). The sample was incubated with anti–biotin MAC beads (no. 130-090-485; Miltenyi Biotec) for 15 minutes in the refrigerator. Excess beads were washed off, and the cells were resuspended in MACS buffer. Sca-1–positive marrow cells were isolated by magnetic separation and washed 3 times before elution. Cells were stained with Streptavidin-488 (no. S11223; Molecular Probes, Eugene, OR), washed, and resuspended in HBSS+. After this, cells were stained with the cocktail of antibodies used previously, 6 lineage markers conjugated to PE-Cy5, and anti–c-kit-APC and anti–Flk-PE. Cells were washed and resuspended in HBSS+ containing 2 μg/mL propidium iodide. Cells were sorted on a triple laser flow cytometer (MoFlow; Cytomation) into 1 mL HBSS+. LT-HSCs negative or low for expression of lineage markers, positive for Sca-1 and c-kit, and negative for Flk-2 were collected for injection into lethally irradiated CD45.1 recipient mice (prepared as described). Collected HSCs were injected at a 30- or 500-cell dose in conjunction with 100 000 nucleated, total marrow, CD45.1 cells. Six recipient CD45.1 mice were used for each test genotype and cell dose. Peripheral white blood cells of recipient mice were analyzed for CD45.1 or CD45.2 cell-surface markers at 4, 8, 12, and 24 weeks after transplantation.
Analysis of white blood cells in transplant recipients
At indicated time points, 100 μL heparinized blood was collected retro-orbitally from anesthetized transplant recipient mice. Red blood cells were removed by incubation in lysis buffer (0.15 M NH4Cl, 1 M KHCO3, 0.5 M EDTA at pH 8). White blood cells were washed and resuspended in 250 μL HBSS+. Cells were stained for 15 minutes on ice and protected from light with a cocktail of anti–CD45.1-PE (no. 553776; BD Biosciences PharMingen) and anti–CD45.2-FITC (no. 553772; BD Biosciences PharMingen). After staining, the cells were washed and resuspended in HBSS+ containing 2 μg/mL propidium iodide. Cells were analyzed on a FACScan dual-laser instrument (Becton Dickinson). Ten thousand events were collected for each sample.
Results
Aged p53+/m mice have reduced numbers of proliferating HSCs, whereas aged p53+/− mice have increased numbers of proliferating HSCs
Initial in vitro attempts to determine hematopoietic progenitor and stem-cell numbers included the cobblestone area-forming cell (CAFC) assay.8,30 With this assay we observed that whole bone marrow from old (18 to 20-month-old) p53+/− mice formed significantly higher numbers of progenitor cobblestone colonies than bone marrow from either old (18–20-month-old) p53+/+ or p53+/m mice marrow (data not shown). Detection of p53 genotype–specific differences in HSC numbers in vitro by the CAFC assay was not possible because of inadequate formation of colonies corresponding to long-term hematopoietic stem cells (LT-HSCs). We detected no p53 genotype–specific differences in HSC numbers in vitro by the CAFC assay, possibly because of the subtle differences in stem-cell number between the p53 genotypes and the error associated with reading a CAFC assay for stem-cell colonies.
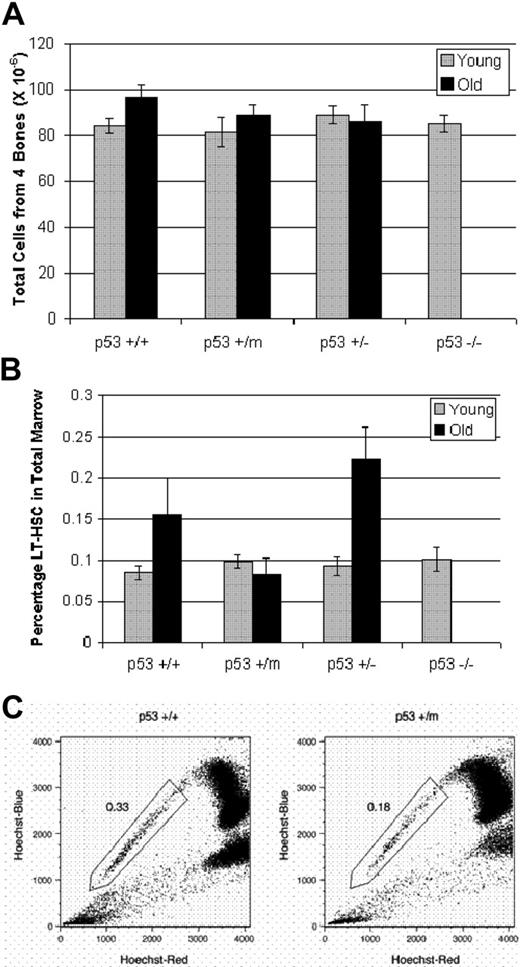
To further examine p53-regulated differences in HSC number and function in vivo with age, we compared the total number of cells found in the bone marrow of wild-type and the various mutant p53 mice at young and old ages. Isolation and enumeration of total bone marrow cells from 10 to 14 age-matched p53+/+, p53+/m, p53+/−, and p53−/− young (4 months) and old (18-20 months) mice revealed no difference in number of total cells (Figure 1A). Note that throughout this study, results from aged p53−/− mice were unavailable because of early death as a result of tumors.
Hematopoietic stem cells exhibit p53-dependent differences in stem-cell numbers in old mice. (A) Numbers of total bone marrow cells from young and old p53+/+, p53+/m, p53+/−, and p53−/− mice were obtained from hind limbs, counted manually and shown to be similar. Results represented are the mean ± SE of 10 to 14 mice per age/genotype. (B) LT-HSCs identified as lin−/lo, Sca-1+, c-kit+, and Flk-2− were selected by whole bone marrow antibody staining and subsequent flow cytometry. The experiments were performed in triplicate; n = 10 for young mice and n = 6 for old mice. Results shown represent the mean ± SE of the experiments. An increase in LT-HSC numbers is shown with age in p53+/+ and p53+/− bone marrow but not in p53+/m bone marrow. (C) Whole bone marrow was stained with Hoechst 33342 and enriched for Sca-1+ cells to identify the side population (SP) HSCs. SP-HSCs isolated from 12-month p53+/m bone marrow are reduced approximately 50% in number compared with SP-HSCs isolated from 12-month p53+/+ bone marrow. Numbers shown are for Sca-1+–enriched bone marrow, which enriches approximately 10-fold for SP cells.
Hematopoietic stem cells exhibit p53-dependent differences in stem-cell numbers in old mice. (A) Numbers of total bone marrow cells from young and old p53+/+, p53+/m, p53+/−, and p53−/− mice were obtained from hind limbs, counted manually and shown to be similar. Results represented are the mean ± SE of 10 to 14 mice per age/genotype. (B) LT-HSCs identified as lin−/lo, Sca-1+, c-kit+, and Flk-2− were selected by whole bone marrow antibody staining and subsequent flow cytometry. The experiments were performed in triplicate; n = 10 for young mice and n = 6 for old mice. Results shown represent the mean ± SE of the experiments. An increase in LT-HSC numbers is shown with age in p53+/+ and p53+/− bone marrow but not in p53+/m bone marrow. (C) Whole bone marrow was stained with Hoechst 33342 and enriched for Sca-1+ cells to identify the side population (SP) HSCs. SP-HSCs isolated from 12-month p53+/m bone marrow are reduced approximately 50% in number compared with SP-HSCs isolated from 12-month p53+/+ bone marrow. Numbers shown are for Sca-1+–enriched bone marrow, which enriches approximately 10-fold for SP cells.
Numbers of LT-HSCs were ascertained by antibody staining of whole bone marrow, and LT-HSCs were analyzed by flow cytometry. Our sorting criteria selected for cells negative or low (−/lo) for expression of mature hematopoietic lineage markers, including B220, Gr-1, CD-8, Ter-119, CD4, and Mac-1. Lin−/lo cells were further sorted based on their positive expression of 2 LT-HSC markers, Sca-1 and c-kit. Finally, we further sorted for the population of these cells negative for Flk-2 expression. Flk-2 negativity is reported on LT-HSCs; differentiation to short-term HSCs facilitates the switching on of Flk-2 expression.33,34 Results of this flow analysis performed in triplicate on a total of 10 mice per genotype revealed no difference in LT-HSC numbers among young p53+/+, p53+/m, p53+/−, and p53−/− mice (Figure 1B). Young LT-HSCs of all genotypes composed approximately 0.1% of the total bone marrow. With age, the p53+/+ animals showed approximately a 70% increase in LT-HSC number (P = .05; n = 6). LT-HSCs made up 0.16% of the old p53+/+ total bone marrow; this result is consistent with other studies that show increases in HSC numbers with age.2 Similarly, the aged p53+/− mice also showed more than a 2-fold increase in LT-HSC numbers (Figure 1B) (P < .05; n = 6), with 0.22% of the total bone marrow consisting of LT-HSCs. Interestingly, however, the numbers of LT-HSCs in the 6 p53+/m mice examined showed no alterations between young and old; rather, they maintained their numbers at 0.08% of the total bone marrow. Compared with age-matched old p53+/+ and p53+/− mice, the p53+/m mice showed approximately a 2-fold reduction in LT-HSC number (Figure 1B). Comparison of old p53+/m HSC numbers with age-matched p53+/− and p53+/+ HSC numbers by unpaired t test resulted in P = .004 and P = .08, respectively.
We used side population (SP) analysis as an alternative method to further investigate the reduction in HSC number in old p53+/m bone marrow compared with old p53+/+ marrow.32 SP cells were identified after staining whole bone marrow with Hoechst 33342 and analysis at 2 emission wavelengths in a flow cytometer. The SP population was further sorted for Sca-1+ cells. HSCs identified in this manner were phenotypically similar to those identified by the method of whole marrow antibody staining.35 Results from this analysis supported those obtained from the whole bone marrow antibody staining experiments, showing approximately a 2-fold reduction in HSC number in old p53+/m mice compared with their age-matched p53+/+ counterparts (Figure 1C). In fact, in the old p53+/+ mouse, HSCs measured by SP staining made up 0.33% of Sca-1–enriched bone marrow compared with only 0.18% in the old p53+/m mice.
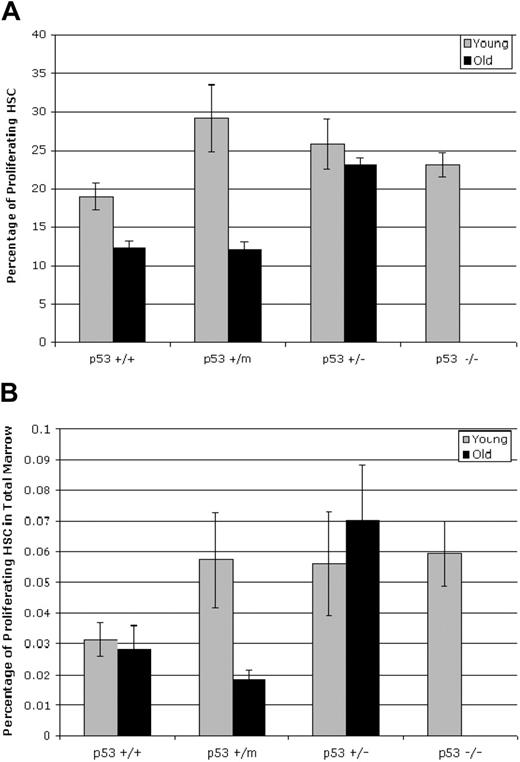
Proliferation of the HSCs was assessed with a BrdU-labeling technique and subsequent analysis of the lin−/lo, Sca+, c-kit+ HSC population by flow cytometry. These experiments were performed in duplicate on 5 young mice and 7 old mice, with modest increases in proliferative capacity of the HSCs obtained from young p53+/m, p53+/−, and p53−/− mice compared with those obtained from p53+/+ mice (Figure 2A). Aged p53+/+ and p53+/m mice showed significantly reduced fractions of proliferating HSCs (P = .03 and P = .001 respectively) compared with their young counterparts, whereas aged p53+/− mice retained rates of HSC proliferation similar to those observed in young mice (Figure 2A). Comparison of old p53+/m HSC BrdU incorporation values with age-matched p53+/− and p53+/+ HSC incorporation by unpaired t test resulted in P = .03 and P = .06, respectively.
Hematopoietic stem cells display p53-dependent differences in proliferation in old mice. (A) Whole bone marrow was labeled with BrdU for 16 hours before numbers of BrdU+, lin−/lo, Sca-1+, or c-kit+ HSCs were assessed by flow cytometry. BrdU incorporation was reduced in HSCs obtained from old p53+/+ and p53+/m mice but not in HSCs from old p53+/− mice. Results shown represent the mean ± SE of 2 experiments using a total of 5 (young) or 7 (old) mice. (B) The fraction of proliferating HSCs in the bone marrow of old mice is dependent on p53 status. The number of HSCs for each p53 genotype in Figure 2B was combined with the fraction of proliferating HSCs for each p53 genotype to determine the total number of proliferating HSCs in the bone marrow for each p53 genotype.
Hematopoietic stem cells display p53-dependent differences in proliferation in old mice. (A) Whole bone marrow was labeled with BrdU for 16 hours before numbers of BrdU+, lin−/lo, Sca-1+, or c-kit+ HSCs were assessed by flow cytometry. BrdU incorporation was reduced in HSCs obtained from old p53+/+ and p53+/m mice but not in HSCs from old p53+/− mice. Results shown represent the mean ± SE of 2 experiments using a total of 5 (young) or 7 (old) mice. (B) The fraction of proliferating HSCs in the bone marrow of old mice is dependent on p53 status. The number of HSCs for each p53 genotype in Figure 2B was combined with the fraction of proliferating HSCs for each p53 genotype to determine the total number of proliferating HSCs in the bone marrow for each p53 genotype.
Finally, we combined the total HSC numbers in each mouse bone marrow (Figure 1B) with the fraction of proliferating HSCs (Figure 2A) as an indicator of the mean total number of proliferating HSCs for each age/genotype of mice (Figure 2B). Overall, the number of proliferating HSCs in wild-type p53+/+ mice was relatively constant (approximately 0.03% of total marrow), despite considerable variation in number (up) and proliferation (down) with age. However, though the number of proliferating HSCs in the hypermorphic p53+/m mice was moderately high in young mice (approximately 0.05% of total marrow), these cells were dramatically reduced to levels significantly below those of wild-type mice in old age (approximately 0.02% of total marrow). Interestingly, proliferating HSC numbers were high in young p53+/− mice and remained high in old mice (approximately 0.06% of total marrow, both young and old) (Figure 2B). Thus, overall numbers of proliferating HSCs might not have changed much with age in wild-type mice, but in p53+/m and p53+/− mice, overall HSC activity in old age appeared to be inversely correlated with p53 dosage.
Limiting dilution of p53+/m HSCs has reduced engraftment and repopulation ability compared with that in p53+/+ and p53+/− cells
Initially, we attempted to assess the functionality of the various mutant p53 HSCs by performing total bone marrow competition assays. In these experiments we “competed” equal numbers of total bone marrow cells from young wild-type mice and young or old bone marrow cells from our various test mice (p53+/+, p53+/m, p53+/−, and p53−/−) by cotransplantation into lethally irradiated recipients. Relative reconstitution of each donor marrow was measured 4, 8, and 12 weeks after transplantation by flow cytometry measurement of WBC percentages containing the CD45.2 (on p53−/−, p53+/−, and p53+/m cells) or CD45.1 (on p53+/+ cells) antigen. These experiments showed no difference in the ability of wild-type or mutant p53 HSCs, young or old, to repopulate bone marrow and to contribute to peripheral blood (data not shown). The single exception was young p53−/− bone marrow competing with young p53+/+ bone marrow. p53−/− bone marrow “outcompeted” p53+/+ bone marrow by a 3-fold margin, but the recipients of p53−/− bone marrow succumbed to lymphoma within 8 to 12 weeks of transplantation (data not shown). Failure to observe differences in competitive engraftment between old and young p53 bone marrow might have occurred because this type of assay is too insensitive to detect subtle alterations in number or functionality of proliferative HSCs in the p53 mutant mice with age.
Limiting-dilution HSC transplantation enabled a more sensitive functional study of small numbers of HSCs after transplantation into marrow-ablated recipient mice. These experiments were performed in duplicate. We chose to inject either 30 or 500 LT-HSCs isolated from 3 old p53+/+, p53+/−, and p53+/m mice each per experiment. Bone marrow was pooled and enriched for HSC activity by magnetic separation of Sca-1+ cells from whole bone marrow. LT-HSCs were identified and isolated from this Sca-1–enriched population on the basis of negative or low expression of lineage-committed markers, negative expression of Flk-2, and dual-positive expression of Sca-1 and c-kit. CD45.2 LT-HSCs were injected into 6 lethally irradiated CD45.1 recipients (for each experiment) along with 100 000 total CD45.1 marrow cells; these latter cells supported the life of the recipient, whereas the LT-HSCs engraft and begin to contribute mature cells to hematopoiesis, though it is possible that donor LT-HSCs were reduced in their engraftment potential by the supporting bone marrow. Peripheral blood from recipient mice was analyzed at 4, 8, 12, and 24 weeks after transplantation.
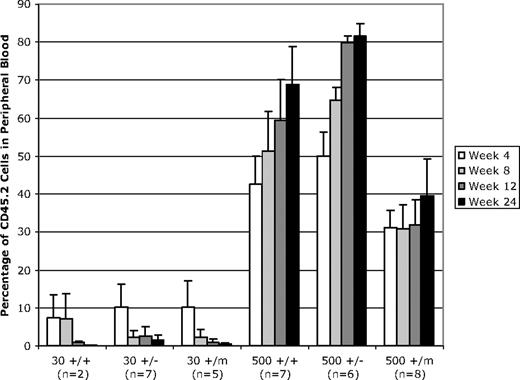
After injection of 30 LT-HSCs from aged mice, we observed no difference in engraftment or contribution to peripheral blood among mice of the p53+/+, p53+/−, and p53+/m genotypes at an old age (Figure 3). In fact, engraftment was poor overall. Four weeks after transplantation, the transplanted LT-HSCs contributed to less than 10% of the peripheral blood. This contribution to peripheral blood decreased to approximately 1% to 2% for all genotypes 12 weeks after transplantation (Figure 3). Results from the 500 LT-HSC transplants were more informative. Engraftment and contribution to peripheral blood was robust in all genotypes. The p53+/− HSCs from aged mice showed the highest levels of engraftment, with up to 79.7% of the white blood cells of the engrafted mice derived from the donor p53+/− genotype by 12 weeks after injection. Aged p53+/+ HSCs were moderately less successful in engraftment, making up approximately 59.3% of the recipient white blood cells 12 weeks after injection. The p53+/m LT-HSCs showed the least success in reconstituting peripheral blood compared with the p53+/+ and p53+/− cells. By 12 weeks after transplantation, the p53+/m LT-HSCs only composed 31.8% of the peripheral blood (Figure 3). This deficiency in p53+/m engraftment was significant (as determined by unpaired t test) compared with p53+/+ HSCs (P = .046) and p53+/− HSCs (P = .001). That these defects in p53+/m aged HSC engraftment were age specific was indicated by experiments in which 500 LT-HSCs derived from young (3-month-old) p53+/+, p53+/−, and p53+/m donors were transplanted into lethally irradiated recipients in a protocol identical to that for the aged donor LT-HSCs. Young LT-HSCs of all 3 p53 genotypes showed essentially identical engraftment potential, indicating no selective disadvantage for young p53+/m LT-HSCs (data not shown). These results indicated that the functionality of old HSCs, as measured by limiting-dilution reconstitution experiments, was dependent in part on p53 activity.
Limiting-dilution transplantation of 500 LT-HSCs (but not 30 HSCs) from old wild-type and p53 mutant mice results in differential white blood cell reconstitution in lethally irradiated recipients. LT-HSCs were isolated from CD45.2 donor mice based on their status as lin−/lo, Sca-1+, c-kit+, Flk-2−. Either 30 or 500 LT-HSCs from CD45.2 test mice were injected into marrow-ablated CD45.1 recipients. Recipient mice were analyzed 4, 8, 12, and 24 weeks after transplantation to assess the percentage of peripheral white blood cells produced from transplanted CD45.2 LT-HSCs. These experiments were performed in duplicate. For each experiment, 3 old donor mice of the same genotype were used, donor cells were pooled, and HSCs were isolated and injected into 6 irradiated recipient mice. Error bars indicate standard error of the mean.
Limiting-dilution transplantation of 500 LT-HSCs (but not 30 HSCs) from old wild-type and p53 mutant mice results in differential white blood cell reconstitution in lethally irradiated recipients. LT-HSCs were isolated from CD45.2 donor mice based on their status as lin−/lo, Sca-1+, c-kit+, Flk-2−. Either 30 or 500 LT-HSCs from CD45.2 test mice were injected into marrow-ablated CD45.1 recipients. Recipient mice were analyzed 4, 8, 12, and 24 weeks after transplantation to assess the percentage of peripheral white blood cells produced from transplanted CD45.2 LT-HSCs. These experiments were performed in duplicate. For each experiment, 3 old donor mice of the same genotype were used, donor cells were pooled, and HSCs were isolated and injected into 6 irradiated recipient mice. Error bars indicate standard error of the mean.
Discussion
The various p53 mutant mice that our laboratory has generated have been particularly useful in examining the role of p53 in numerous biologic processes. As summarized in Table 1, changes in p53 status can have profound effects on cancer incidence, longevity, and stem-cell function. The decline of stem-cell functionality with age may, in fact, be a key underlying mechanism in aging.36 Moreover, recent reports indicate that tumor suppressors may play a key role in stem-cell aging. Three groups have shown that p16INK4A, a cyclin-dependent kinase inhibitor, is increased in stem and progenitor cells with age, and this can inhibit various regenerative functions.37-39 This paper describes experiments to test whether p53 activity influences age-associated declines in stem-cell function. We assessed the dynamics and functionality of the HSC population in p53+/m, p53+/+, p53+/−, and p53−/− mice.
Relationships among p53 dosage, longevity, cancer, and HSC function
| p53 Genotype . | Median longevity,* wk . | Cancer incidence,† % . | Aged HSC no.,‡ % . | Aged HSC proliferation,§ % . | Aged HSC reconstitution,‖ % . |
|---|---|---|---|---|---|
| p53−/− | 18 | 100 | ND | ND | ND |
| p53+/− | 52 | 90 | 0.22 | 0.070 | 80 |
| p53+/+ | 118 | 45 | 0.15 | 0.029 | 59 |
| p53+/m | 96 | 6 | 0.08 | 0.019 | 31 |
| p53 Genotype . | Median longevity,* wk . | Cancer incidence,† % . | Aged HSC no.,‡ % . | Aged HSC proliferation,§ % . | Aged HSC reconstitution,‖ % . |
|---|---|---|---|---|---|
| p53−/− | 18 | 100 | ND | ND | ND |
| p53+/− | 52 | 90 | 0.22 | 0.070 | 80 |
| p53+/+ | 118 | 45 | 0.15 | 0.029 | 59 |
| p53+/m | 96 | 6 | 0.08 | 0.019 | 31 |
Age in weeks at which 50% of a p53 cohort died.
Percentage of mice in a p53 cohort that develop cancer in a lifespan.
Percentage of HSCs in total marrow cells from aged (18-20-month-old) mice (Figure 1B).
Percentage of proliferating HSCs in total marrow cells (Figure 2B).
Percentage of hematopoietic system reconstituted by 500 aged HSCs from donors of a given p53 genotype 12 weeks after transplantation (Figure 3).
Analysis of the HSC compartment in young wild-type and p53 mutant mice revealed no differences in number. However, analysis of old mice showed significant increases in HSC number in p53+/+ and p53+/− mice, consistent with earlier studies showing increased numbers of HSCs in older C57BL/6 mice.2 Interestingly, old p53+/m mice failed to exhibit age-associated increases in HSC number and, rather, showed almost 2-fold and 3-fold reductions in number compared with their age-matched p53+/+ and p53+/− counterparts, respectively. We also assessed HSC proliferation capacity in the various age/p53 genotype categories. In this assay, BrdU incorporation studies indicated that young proliferating HSC fractions did not differ greatly. However, old p53+/+ and p53+/m fractions showed similar rates of reduced proliferation, whereas p53+/− HSCs retained high levels of proliferation. When stem-cell numbers were combined with proliferating fraction numbers, the resultant values could be used to estimate the number of proliferating HSCs overall for each age/genotype (Figure 3B). With this assessment, overall HSC proliferation seemed to be inversely correlated with p53 activity.
Although the experiments suggested diminished HSC activity in the old p53+/m mice and increased HSC activity in the old p53+/− mice, a more definitive functional assay could be provided by various transplantation approaches. To measure functionality of the HSCs in a sensitive manner, we isolated LT-HSCs from old p53+/+, p53+/m, and p53+/− mice and transplanted small numbers (2 doses; 30 and 500 cells) into recipient mice (Figure 3). Transplantation of 30 cells showed little engraftment and contribution to the peripheral blood in all the genotypes. No differences could be noted, possibly because only a small fraction of the cells in our sorted HSC pools were viable bona fide LT-HSCs or because LT-HSCs had to compete against the coinjected bone marrow cells that served as a survival component for lethally irradiated recipient mice. However, injection of 500 LT-HSCs showed successful engraftment and diminished ability of the p53+/m LT-HSCs to engraft and reconstitute the peripheral blood of the animal over 4 to 24 weeks. Interestingly, 500 p53+/− HSCs showed a greater ability for engraftment and reconstitution than 500 p53+/+ HSCs. Transplantation studies suggest a cell-autonomous effect of p53 dosage on aged HSC functionality. What is unclear is the mechanism by which p53 could influence this reduction in proliferating HSC numbers and functionality. Explanations include (1) enhanced p53 stress response resulting in augmented apoptosis or senescence rates in p53+/m HSCs, (2) accelerated decay or atrophy of the p53+/m HSC niches (consistent with this, we see more accelerated overall tissue atrophy and senescence markers in the older p53+/m mice), (3) less favorable hormonal and growth factor environment, (4) distorted asymmetric divisions tending to favor differentiation over self-renewal (thus removing stem cells), and (5) slower overall rates of self-renewal (consistent with mouse embryo fibroblast studies showing a profound effect of p53 dosage on division rates40 ).
Preliminary attempts to determine the mechanistic effects of p53 dosage on stem-cell aging have been inconclusive; experiments to detect altered rates of senescence or apoptosis in aged HSCs have not revealed any p53-dependent differences (M.D., unpublished data, September 2004). Our current favored hypothesis is that altered p53 dosage may result in modest alterations in the overall self-renewal rates of LT-HSCs. Over time, these differences in HSC self-renewal rates will manifest themselves as biologically significant quantitative and qualitative functional differences in the hematopoietic compartments of the older p53 wild-type and p53 mutant mice. In the older p53+/m mice, the reduced self-renewal rates may result in reduced HSC numbers and transplantability and in reduced recovery from myeloablation agents. An additional effect would be a reduced tumor burden given the growing evidence that stem cells are cellular targets for transformation and tumor development.41 In fact, the p53+/m mice have a dramatically lower lifetime tumor incidence (6%) compared with their p53+/+ counterparts (47%) (Table 1), and a spontaneous hematopoietic cancer has never been observed in the p53+/m mice.23 In the p53+/− mouse, the relaxed p53 response might manifest itself as increased HSC numbers, transplant engraftment, and increased susceptibility to hematopoietic cancers. Indeed, some of the most frequently occurring cancers in p53+/− mice are lymphoid malignancies.42
If such p53-associated antiproliferative effects occur in other stem-cell compartments as well as in HSCs, the array of early aging phenotypes and reduced longevity in the p53+/m mice may be in part a result of a global accelerated decline in stem-cell functionality with age. We are testing this by examining other stem-cell compartments in the p53+/m mice. We hope to establish that p53 is an important regulator of organismal aging and to better understand the mechanisms by which it may function in longevity and aging.
Authorship
Contribution: M.D. performed most of the experiments, wrote the first manuscript draft, and contributed to writing the subsequent manuscript drafts. L.M. performed some of the limited dilution transplantation experiments in Figure 3. S.M.C. performed the experiments shown in Figure 2C. H.G. supervised and contributed to the CAFC experiments by providing key advice, reagents, and protocols. G.V.Z. supervised cobblestone assays and contributed to writing the manuscript. M.A.G. provided numerous mice, helped design transplantation experiments (Figure 3) and SP experiments (Figure 2C), provided continuous advice, mice, reagents, protocols, and HSC expertise, and contributed to writing the manuscript. L.A.D. was the overall director of the research, provided funds for the research and impetus for the experiments, and contributed to much of the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lawrence A. Donehower, Department of Molecular Virology and Microbiology, Baylor College of Medicine, One Baylor Plaza, Houston, TX 77030; e-mail: larryd@bcm.tmc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by National Institutes of Health grants AG19693 (L.A.D.), U01 DK63588 (M.A.G.), and AG20917 (G.V.Z.). M.A.G. is a scholar of the Leukemia and Lymphoma Society. S.M.C. was supported by National Institute for Aging training grant AG000183.
We thank Cathy Gatza for help with the breeding and husbandry of animals. We thank Mike Cubbage, Chris Threeton, and Tatiana Goltsova for assistance with flow cytometry.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal