Abstract
KCl cotransport (KCC) activity contributes to pathologic dehydration in sickle (SS) red blood cells (RBCs). KCC activation by urea was measured in SS and normal (AA) RBCs as Cl-dependent Rb influx. KCC-mediated volume reduction was assessed by measuring reticulocyte cellular hemoglobin concentration (CHC) cytometrically. Urea activated KCC fluxes in fresh RBCs to levels seen in swollen cells, although SS RBCs required lower urea concentrations than did normal (AA) RBCs. Little additional KCC stimulation by urea occurred in swollen AA or SS RBCs. The pH dependence of KCC in “euvolemic” SS RBCs treated with urea was similar to that in swollen cells. Urea triggered volume reduction in SS and AA reticulocytes, establishing a higher CHC. Volume reduction was Cl dependent and was limited by the KCC inhibitor, dihydro-indenyl-oxyalkanoic acid. Final CHC depended on urea concentration, but not on initial CHC. Under all activation conditions, volume reduction was exaggerated in SS reticulocytes and produced higher CHCs than in AA reticulocytes. The sulfhydryl-reducing agent, dithiothreitol, normalized the sensitivity of KCC activation to urea in SS RBCs and mitigated the urea-stimulated volume decrease in SS reticulocytes, suggesting that the dysfunctional activity of KCC in SS RBCs was due in part to reversible sulfhydryl oxidation.
Introduction
The KCl cotransporter (KCC) mediates electroneutral coupled transport of K+ and Cl− in a variety of cell types.1,2 KCC activation results in net efflux of KCl from cells with high potassium content, with accompanying water loss and volume reduction. KCC is active in reticulocytes and diminishes with red cell maturation.3,4 It is thought to function physiologically to reduce red blood cell (RBC) volume and establish the high cellular hemoglobin concentration (CHC) characteristic of mature cells. KCC is activated in vitro by cell swelling, acid pH, sulfhydryl oxidation/alkylation, and exposure to urea, but the relative importance of these stimuli in vivo is unknown.
In RBCs containing sickle hemoglobin (Hb S), KCC activity is high, in part because of the high percentage of reticulocytes.4 However, Hb S or Hb C in trait RBCs (AS or AC) with normal mean cell age, or after incorporation into normal RBC ghosts, appears to increase KCC activity.5,6 Expression of Hb S or C in transgenic mice also increases red cell KCC activity.7 Recently, we demonstrated an abnormal response to acid pH in SS RBCs that was partially corrected by incubation with the sulfhydryl reducing agent, dithiothreitol.8 These findings suggested that regulation of KCC is abnormal in SS RBCs.
As a volume regulator, KCC activity is responsive to CHC.9,10 KCC is activated on cell swelling, and, as KCl is lost, cell volume falls, cellular hemoglobin concentration increases, and transport activity diminishes. The resultant CHC thus reflects the physiologic “volume set point” of the system. The inactivation of KCC as the cell shrinks toward its new steady state is most likely a key factor in defining the volume regulatory function of the transporter, which in turn is crucial for establishing the steady state CHC for RBCs in vivo.
Volume reduction mediated by KCC has been demonstrated with RBC density measurements.7,11,12 However, these studies, like flux studies, are complicated by varying numbers of reticulocytes (or young cells) in unfractionated SS blood, which preclude comparisons of KCC-mediated volume reduction in SS and AA cells. We recently compared the RVD mediated by KCC and stimulated by cell swelling and acid pH in SS and AA reticulocytes by tracking the changes in reticulocyte CHC by means of density gradient analysis.8 Acid-stimulated volume reduction was exaggerated in SS reticulocytes and was diminished by sulfhydryl reduction, parallel to the behavior of KCC flux activity. However, despite the normal response of KCC flux activation to cell swelling in SS RBCs, swelling-stimulated volume reduction was markedly abnormal in SS reticulocytes and resulted in higher final CHC than in AA reticulocytes. Thus, volume reduction and KCC fluxes appear to reflect different functional aspects of the KCC system in RBCs and may be subject to different pathologic influences in SS RBCs.
Urea is a powerful activator of KCl cotransport in human,3,13-15 dog,16 sheep,17 and horse RBCs.18 Activation is dependent on urea concentration up to 1 M13,15,16 and is reversible,13,15 suggesting that protein denaturation is not involved. Gibson et al14 and Culliford et al15 first reported stimulation by urea of KCC in SS RBCs and demonstrated its abnormal response to deoxygenation compared with normal cells. Bize et al3 showed that stimulation of KCC in AA RBCs by urea was greater in low-density populations. However, there has been no comparison of the sensitivity of KCC to urea activation in SS and AA RBCs, and no evaluation of the effect of urea on the volume regulation system in reticulocytes. In this study, we examined the proportionate activation of KCC in SS and AA RBCs in response to urea and analyzed the resulting volume reduction in reticulocytes, using an improved method which measures reticulocyte CHC directly by means of the Advia 120 automated cell counter. This approach circumvents the difference in age distribution between SS and AA RBCs and permits direct comparison of an age-defined population of cells. Our studies reveal additional abnormalities in both KCC activity (SS RBCs) and KCC-dependent volume reduction (SS reticulocytes) in response to urea, and they implicate sulfhydryl oxidation as a contributor to this pathologic behavior.
Materials and methods
Blood samples
Blood was drawn into heparinized tubes with informed consent from adult volunteers who had not received a transfusion within 3 months, under a protocol approved by the Institutional Review Boards of both the University of Cincinnati and Cincinnati Children's Hospital Medical Center. Hematocrits were measured on oxygenated samples in microhematocrit tubes centrifuged 5 minutes at 13 000g. Hemoglobin concentration was assayed optically at 540 nm on a Beckman Instruments DU spectrophotometer (Hialeah, FL). Mean cellular hemoglobin concentrations (MCHCs) were derived from paired hematocrit and hemoglobin measurements. Reticulocyte cellular hemoglobin concentration (CHC) was determined on a cell-by-cell basis on an Advia 120 automated cell counter (Bayer Instruments, Tarrytown, NY), which reports a frequency distribution and mean value (CHCM) for this parameter and distinguishes reticulocytes from mature RBCs by fluorescent detection of RNA.19 The 3 designations for cellular hemoglobin concentration have subtly different meanings. MCHC refers to the value determined in a population of cells in whole blood or a suspension. CHC indicates the hemoglobin concentration of an individual cell, and CHCM reflects the mean of a distribution of CHC values.
Media and chemicals
Chemicals were obtained from Sigma/Aldrich (St Louis, MO) unless otherwise noted. HEPES (N-2-hydroxyethyl-piperazine-N′-2-ethane sulfonic acid)–buffered saline (HBS) contained 140 mM NaCl, 20 mM HEPES (pH 7.4 at 37°C with NaOH), 0.2 mM MgCl2, 0.1 mM EGTA (ethyleneglycotetraacetic acid), and 10 mM glucose. In HEPES-buffered Rb medium (HBRb), Rb salts replaced Na salts, and in Cl-free media, sulfamate salts replaced Cl salts, and MgSO4 replaced MgCl2. Ouabain was added as 10-mM stock solution in isotonic saline, and bumetanide was added as 1-mM stock in dimethyl sulfoxide. Urea was added as a solid to the desired concentration. Although the osmolality of solutions increased, RBC volume and MCHC were not altered by concentrations up to 1 M, because of the rapid equilibration of urea across the RBC membrane. However, when swollen cells that had been equilibrated with high concentrations of urea were added to large volumes of wash solutions that did not contain urea, we observed significant lysis that was presumably due to osmotic shock resulting from more rapid influx of water than efflux of urea from the cells. However, lysis was avoided in the experiments reported here by rapid addition of several small aliquots of urea-free media to the suspension to effect gradual, step-wise reduction in urea concentration, which permitted equilibration of urea across the membrane.
Nystatin treatment
Cation content and MCHC were altered by treatment with nystatin as described previously.8 Washed cells were resuspended at 0.02 (2%) hematocrit and incubated in media containing 140 mM KCl and 10 mM NaCl with nystatin (30 μg/mL suspension) at 0°C for 20 minutes, followed by 7 washes at 22°C with treatment buffer containing 1 mg/mL bovine serum albumin, and 2 washes with incubation buffer. Sucrose concentrations in treatment buffers were varied from 5 to 100 mM to yield final MCHCs in isotonic solutions of 300 to 380 g/L (30-38 g/dL); lower MCHC was achieved by using 5 mM sucrose and adding hypertonic KCl (up to an additional 100 mM) to the treatment suspension.12
Cation measurements and fluxes
KCC activity was measured as net Rb uptake in cells incubated under oxygenated conditions in HBS, as described previously.8 Briefly, washed cells were resuspended at 0.02 (2%) hematocrit in HBS containing 0.1 mM ouabain and 0.01 mM bumetanide. After warming to 37°C, prewarmed HBRb was added to give 24 mM external Rb. Triplicate samples (1 mL) were taken at 5 and 25 minutes into 15-mL conical polypropylene Falcon tubes (Becton Dickinson, Franklin Hills, NJ) containing 10 mL ice-cold isotonic Choline-Cl solution (180 mM, buffered to 7.0 with mM Tris-Cl) layered over immiscible oil composed of dibutyl phthalate to tetradecane (9:1 vol/vol). Centrifugation at 1000g at 4°C using a swinging bucket rotor in a GPR centrifuge (Beckman Instruments, Palo Alto, CA) pelleted the cells beneath the oil with less than 1% trapped extracellular volume.20 Tubes were then washed with distilled water without disturbing cells beneath the oil. After removing the wash water and oil, cells were lysed in 4 mM CsCl, and hemolysates were analyzed for hemoglobin by optical spectroscopy at 540 nm (Beckman DU Spectrophotometer) and for Rb by flame emission (Perkin Elmer model 370 spectrophotometer; Shelton, CT). Rb influx was computed as mmol/kg Hb per hour. KCC activity was calculated as the difference between Rb influx in Cl and sulfamate.3,11,21
Reticulocyte volume reduction
Whole blood was washed, and, if appropriate, RBCs were treated with nystatin, then suspended to 50% hematocrit and kept on ice until used. Cells were added at t = 0 to solutions prewarmed to 37°C to give 2% hematocrit. At timed intervals, 5-mL samples were added to 45 mL iced HBS, centrifuged, then resuspended in 300 μL HBS and kept on ice until assayed on the Advia 120 within 4 hours. When urea was present in the incubation, addition of wash medium was gradual, as described under “Media and chemicals.” Pilot experiments demonstrated that RBC and reticulocyte volumes were stable when held on ice over this time period. Reticulocyte counts were stable during incubations and were not altered by exposure to urea.
Dithiothreitol treatment
Washed cells were incubated in HBS at 2% hematocrit with 10 mM dithiothreitol (DTT) at 37°C for 1 hour. Cells were then washed with incubation media containing 1 mM DTT, and all other subsequent incubations (nystatin treatment and/or flux measurements) were performed in 1 mM DTT. Control cells were similarly incubated without DTT. We showed previously that SS RBCs maintain stable GSH (reduced glutathione) levels on incubation in vitro under oxygenated conditions even without DTT incubation, and incubation with DTT raised GSH levels only slightly higher compared with control (no DTT) samples.8
Statistics
Mean ± SEM are reported on experiments using different subjects and mean ± SD for replicate measurements in the same subject. Statistical comparisons were made by Student t test, either paired or unpaired as experimentally appropriate.
Results
SS RBCs are more sensitive than AA RBCs to KCC stimulation by urea
Urea stimulation of KCC activity as a percentage of the maximal KCC activation stimulated by cell swelling is illustrated in Figure 1A. Ouabain- and bumetanide-insensitive Rb influx was measured in isotonic media at pH 7.4 at various urea concentrations without prior adjustment of RBC cation content. Parallel measurements of the maximal volume-sensitive KCC flux (without urea) were made for each blood sample in cells swollen isotonically (nystatin method) to MCHC less than 270 g/L (27 g/dL), as described previously.8 Note that these measurements were made on “unfractionated” SS and AA RBCs that contained different percentages of reticulocytes. Accordingly, calculated flux values were 5- to 15-fold higher in SS than in AA blood (data not shown). However, expressing flux activity as the percentage of maximal swelling stimulated flux normalizes the different flux activities in SS and AA RBCs and permits direct comparison of proportionate KCC activation in the 2 cell types. Urea, at concentrations up to 1 M, activated KCC in both AA and SS RBCs as shown in Figure 1A. However, SS RBCs were more sensitive to activation by urea: at 400 mM urea, SS RBCs exhibited 62% ± 12% of maximal KCC activity compared with 24% ± 11% for AA RBCs (n = 4, P < .001 by Student t test). This difference persisted at 600 mM (84% ± 13% versus 43% ± 14%; n = 4, P < .02). The urea concentration for 50% activation, estimated graphically from Figure 1A, was 350 to 400 mM in SS RBCs compared with around 600 mM in AA RBCs. Thus, KCC in both AA and SS RBCs can be maximally activated by urea, but activation occurs at lower urea concentrations in SS RBCs.
Effect of urea on Rb influx in SS and AA RBCs. Fluxes were measured in unfractionated blood samples. (A) Rb influx versus urea concentration. Ouabain- and bumetanide-resistant (OBR) Rb influx was measured in HBS pH 7.4 in fresh RBCs (not swollen with nystatin) at various added urea concentrations and normalized to concurrently measured maximal volume-sensitive Rb influx in cells swollen to MCHC 270 g/L (27g/dL) or less by nystatin treatment. In Cl-free media, increasing urea concentration had no effect on OBR Rb influx (not shown). Symbols represent mean with error bars depicting SEM of 6 experiments. Curves were drawn by eye. The dashed line indicates 50% activation of KCC. (B) Rb influx versus urea concentration in swollen RBCs. Cells were swollen to initial MCHC 270 g/L (27 g/dL), and Rb influx was measured at various urea concentrations. Data are expressed as percentage of maximal volume stimulated flux ([urea] = 0). The 2 experiments shown with AA (open symbols) and SS (filled symbols) RBCs were independent of those in panel A. Lines were drawn by eye. (C) Rb influx versus MCHC in SS RBCs. Cells were swollen to various (initial) MCHC via nystatin treatment, and Rb influx was measured with 600 mM urea (▴) or without urea (▵). Maximal volume-stimulated flux was measured concurrently in cells with initial MCHC less than 270 g/L (27 g/dL) (without urea). Data are from 2 independent experiments. (D) Rb influx versus pH in SS RBCs. Rb influx was measured in fresh cells washed and incubated in HBS at various pH values. The acid-stimulated Rb influx is greater than 95% CI dependent and reflects KCC activity. When present, urea concentration was 600 mM (▴). Data are from a single experiment, representative of 3.
Effect of urea on Rb influx in SS and AA RBCs. Fluxes were measured in unfractionated blood samples. (A) Rb influx versus urea concentration. Ouabain- and bumetanide-resistant (OBR) Rb influx was measured in HBS pH 7.4 in fresh RBCs (not swollen with nystatin) at various added urea concentrations and normalized to concurrently measured maximal volume-sensitive Rb influx in cells swollen to MCHC 270 g/L (27g/dL) or less by nystatin treatment. In Cl-free media, increasing urea concentration had no effect on OBR Rb influx (not shown). Symbols represent mean with error bars depicting SEM of 6 experiments. Curves were drawn by eye. The dashed line indicates 50% activation of KCC. (B) Rb influx versus urea concentration in swollen RBCs. Cells were swollen to initial MCHC 270 g/L (27 g/dL), and Rb influx was measured at various urea concentrations. Data are expressed as percentage of maximal volume stimulated flux ([urea] = 0). The 2 experiments shown with AA (open symbols) and SS (filled symbols) RBCs were independent of those in panel A. Lines were drawn by eye. (C) Rb influx versus MCHC in SS RBCs. Cells were swollen to various (initial) MCHC via nystatin treatment, and Rb influx was measured with 600 mM urea (▴) or without urea (▵). Maximal volume-stimulated flux was measured concurrently in cells with initial MCHC less than 270 g/L (27 g/dL) (without urea). Data are from 2 independent experiments. (D) Rb influx versus pH in SS RBCs. Rb influx was measured in fresh cells washed and incubated in HBS at various pH values. The acid-stimulated Rb influx is greater than 95% CI dependent and reflects KCC activity. When present, urea concentration was 600 mM (▴). Data are from a single experiment, representative of 3.
Urea stimulation of KCC is minimal in swollen cells
Figure 1B depicts experiments in which cells were swollen with nystatin to MCHC 260 to 270 g/L (26-27 g/dL), then incubated with different urea concentrations as shown. Fluxes were normalized to the flux in the absence of urea, representing the maximal volume-stimulated KCC activity.8 In contrast to the dramatic stimulation of KCC activity observed in unswollen cells, fluxes in swollen cells were only enhanced by 25%.
Urea perturbs, but does not abolish, volume and pH responsiveness of KCC in SS RBCs
The activation of KCC by cell swelling represents a response to changing hemoglobin concentration.9,10 This relationship is illustrated in Figure 1C in which KCC fluxes were measured in SS RBCs at various initial MCHCs (calculated from hemoglobin and hematocrit measurements on unfractionated cell samples following nystatin treatment). Without urea, KCC activity varies inversely with MCHC, as previously shown.8,22-24 Urea treatment results in higher KCC activity at any given MCHC, but activity is clearly inhibited at high MCHCs. This behavior is consistent with the finding of Bize et al3 that urea stimulation of KCC occurred in hypertonic solutions (and therefore at high CHC). Nevertheless, it is apparent that KCC activity in urea-treated cells with MCHC of 360 g/L (36 g/dL) is similar to control SS cells swollen to 280 g/L (28 g/dL). Therefore, urea-treated RBCs with relatively high MCHCs behave as if they were swollen.
The characteristic biphasic response of KCC to pH is illustrated in Figure 1D, with control sickle cells showing a maximum at pH 6.9 and inhibition at more acidic and alkaline pH, as reported previously.8,12,22,23 Urea-treated SS RBCs show a different pattern, with shift of the pH maximum upward and toward alkaline pH, characteristic of the pH response of KCC in swollen cells.12 Thus, KCC in euvolemic, urea-treated SS RBCs responds to pH as if the cells were swollen.
Volume reduction in reticulocytes in response to cell swelling and urea exposure
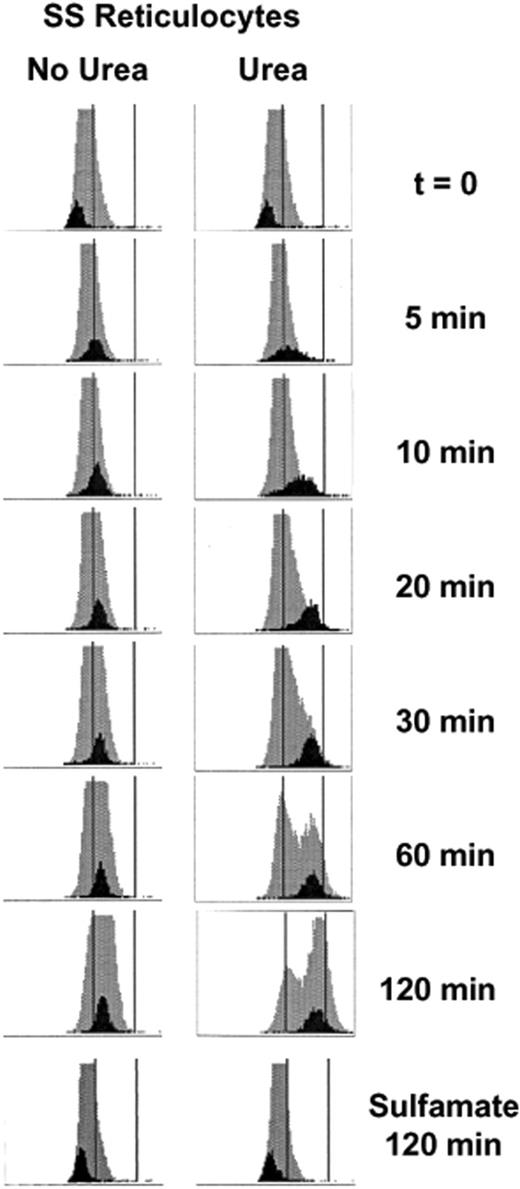
We demonstrated previously, using density gradient analysis to estimate MCHCs of reticulocyte populations, that swollen reticulocytes undergo a Cl-dependent volume reduction mediated by KCC which is greater in SS than AA cells.8,19 The studies reported here used the Advia 120 analyzer, which measures CHC directly in individual cells, and distinguishes reticulocytes from mature RBCs. In Figure 2, frequency distributions of CHCs are shown for both entire RBC (gray) and reticulocyte (black) populations during a 2-hour incubation in isotonic HBS (pH 7.4) without and with 600 mM urea. After nystatin treatment to swell the cells (t = 0), both mature and reticulocyte populations exhibit a tight, uniform distribution of CHCs. When no urea was present, the reticulocyte population rapidly shifts to higher CHCs, maintaining a relatively tight distribution and stabilizing within 60 minutes at a new mean CHC (CHCM). On exposure to urea, the reticulocyte CHC distribution initially broadens, suggesting heterogeneity of ion transport response within the reticulocyte population. However, as incubation proceeds, the CHC distribution tightens as a greater fraction of reticulocytes converges on the final, steady-state CHC, which is considerably higher than in the absence of urea. CHC distributions of cells incubated 2 hours in Cl-free sulfamate media (Figure 2 bottom) were stable, attesting to the Cl-dependence of volume reduction, both without and with urea. Removal of ouabain and bumetanide from the incubation medium did not affect the volume decrease (not shown); incubation at 0°C in HBS with or without urea resulted in no change in reticulocyte CHCs (not shown). Changes in nonreticulocyte CHC distribution are apparent in swollen cells in HBS and are dramatic in cells incubated with urea. This phenomenon will be explored in greater detail in a separate report.
Advia 120 cellular hemoglobin concentration (CHC) frequency distributions in SS reticulocytes. Histograms for reticulocytes (black) are shown against the backdrop of the entire RBC population (gray) measured over time. The x-axis depicts CHC on a scale of 0-500 g/L (0-50 g/dL), and 280 and 410 g/L (28 and 41 g/dL) are represented by vertical lines. Cells were initially swollen (nystatin method) to 240 g/L (24 g/dL), then incubated in isotonic HEPES-buffered saline (HBS, pH 7.4) without (control) or with (urea) 600 mM urea. Samples were taken at various times, washed in HBS, and stored on ice until analyzed on the Advia 120. CHC distributions for cells incubated 2 hours in Cl-free media (sulfamate) without and with urea are also shown.
Advia 120 cellular hemoglobin concentration (CHC) frequency distributions in SS reticulocytes. Histograms for reticulocytes (black) are shown against the backdrop of the entire RBC population (gray) measured over time. The x-axis depicts CHC on a scale of 0-500 g/L (0-50 g/dL), and 280 and 410 g/L (28 and 41 g/dL) are represented by vertical lines. Cells were initially swollen (nystatin method) to 240 g/L (24 g/dL), then incubated in isotonic HEPES-buffered saline (HBS, pH 7.4) without (control) or with (urea) 600 mM urea. Samples were taken at various times, washed in HBS, and stored on ice until analyzed on the Advia 120. CHC distributions for cells incubated 2 hours in Cl-free media (sulfamate) without and with urea are also shown.
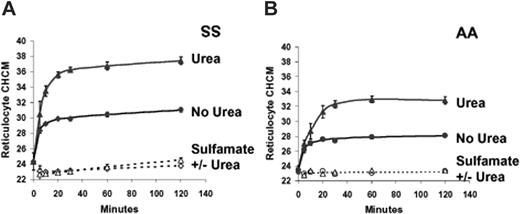
The Advia 120 analyzer reports the mean value (CHCM) obtained from the frequency distribution of reticulocyte CHCs. These values are plotted versus time of incubation in Figure 3A-B, for SS and AA reticulocytes. The difference between volume reduction in swollen SS and AA reticulocytes reported previously8 is apparent in the control (no urea) experiments. Urea exaggerates volume reduction to produce a much higher CHCM than swelling alone in both SS and AA reticulocytes, with SS cells again showing a more intense response. In Cl-free sulfamate media, CHCM is constant over the incubation period, and there is no effect of urea, demonstrating the Cl dependence of volume reduction. Figure 3C and 3D illustrate that the increase in SS and AA reticulocyte CHCMs in response both to swelling and to urea is substantially diminished by dihydro-indenyl-oxyalkanoic acid (DIOA), an inhibitor of KCl cotransport.25 These findings support the conclusion that both volume reduction in swollen reticulocytes and its augmentation by urea are mediated by KCl cotransport.
Effect of urea on volume reduction in sickle and normal reticulocytes. Cells were swollen by nystatin treatment and incubated in isotonic HBS pH 7.4 (no urea) or the same buffer plus 600 mM urea. (A-B) Sulfamate media were Cl free. Data points are means ± SEMs of 3 independent experiments. (C-D) Inhibition of DIOA. The concentration of KCC transport inhibitor, DIOA, when present, was 100 μM. Single experiments with SS and AA cells are depicted, representative of 2 others with both cell types.
Effect of urea on volume reduction in sickle and normal reticulocytes. Cells were swollen by nystatin treatment and incubated in isotonic HBS pH 7.4 (no urea) or the same buffer plus 600 mM urea. (A-B) Sulfamate media were Cl free. Data points are means ± SEMs of 3 independent experiments. (C-D) Inhibition of DIOA. The concentration of KCC transport inhibitor, DIOA, when present, was 100 μM. Single experiments with SS and AA cells are depicted, representative of 2 others with both cell types.
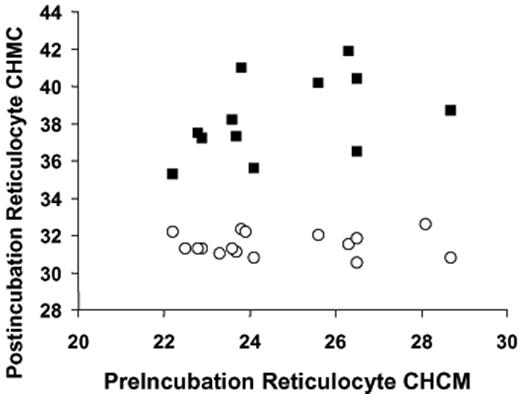
Table 1compares CHCM values for AA and SS reticulocytes under a variety of conditions, summarizing the results of experiments depicted in Figure 3 and comparing them to CHMC of fresh cells. As anticipated, CHCM of fresh SS reticulocytes (before nystatin treatment) was higher than that of fresh AA reticulocytes. Interestingly, for both cell types fresh reticulocyte CHCM was higher than the steady state CHCM that swollen reticulocytes achieved in vitro when incubated under physiologic conditions (isotonic HBS 7.4). This suggests that stimuli to KCC other than the simple “swelling” response (or other transport pathways) may be operative in vivo to activate RVD and establish reticulocyte CHC.
Reticulocyte CHCM (g/L) determined on fresh blood, after swelling and subsequent volume reduction (VR) in sickle and normal cells
| . | Fresh reticulocytes, before nystatin treatment . | Swollen reticulocytes, after nystatin treatment . | After VR in HBS 7.4, no urea . | After VR in HBS 7.4 plus 600 mM urea . | P, paired t test, no urea vs urea . |
|---|---|---|---|---|---|
| SS | 335 (10)* | 250 (20)† | 315 (07)† | 38.9 (21)† | < .001 |
| AA | 308 (17)‡ | 244 (18)§ | 275 (08)§ | 33.8 (22)§ | < .001 |
| P, unpaired t test, SS vs AA | < .01 | NS | < .001 | < .001 |
| . | Fresh reticulocytes, before nystatin treatment . | Swollen reticulocytes, after nystatin treatment . | After VR in HBS 7.4, no urea . | After VR in HBS 7.4 plus 600 mM urea . | P, paired t test, no urea vs urea . |
|---|---|---|---|---|---|
| SS | 335 (10)* | 250 (20)† | 315 (07)† | 38.9 (21)† | < .001 |
| AA | 308 (17)‡ | 244 (18)§ | 275 (08)§ | 33.8 (22)§ | < .001 |
| P, unpaired t test, SS vs AA | < .01 | NS | < .001 | < .001 |
Reticulocyte CHCM was measured in fresh blood within 4 hours of drawing into EDTA tubes. After washing in isotonic HBS, cells were treated with nystatin, washed as described in “Materials and methods,” under “Nystatin treatment,” and resuspended at 0.20 hematocrit in HBS 7.4. Samples were reserved on ice for measurement of initial CHCM (swollen reticulocytes) and then diluted to 0.02 hematocrit in 37°C buffer, either HBS 7.4 (control) or HBS 7.4 plus 600 mM urea. After 2 hours of incubation, samples were transferred to cold HBS 7.4, washed, and resuspended for Advia 120 analysis. Data shown are mean, with SEM shown in parentheses.
n = 6.
n = 12.
n = 7.
n = 13.
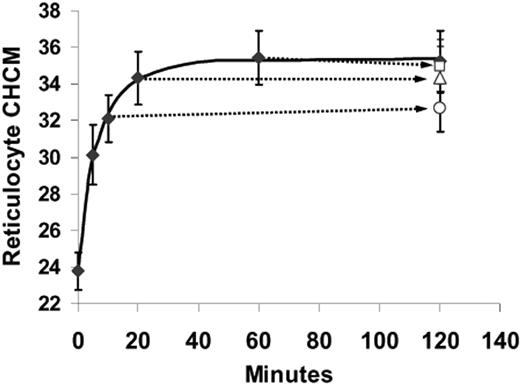
Although urea activation of KCC is reversible,13,15 the changes in CHCM induced by urea were not. Figure 4 shows the effect on SS reticulocyte CHCM of removing urea at various times during volume reduction. Cells exposed to urea continuously for 120 minutes showed a typical urea-stimulated volume reduction and achieved a new stable CHCM within 60 minutes of incubation. Cells that were withdrawn at 10, 20, and 60 minutes; washed; and incubated again without urea for the balance of the 120-minute period had the same CHCM at the end of the incubation as they had when urea was removed. For example, urea-stimulated volume reduction depicted in Figure 4 is only partially complete at 10 minutes, with SS reticulocytes attaining CHCM of 321 ± 22 g/L (32.1 ± 2.2 g/dL), statistically different from the CHCM achieved after 120 minutes of exposure to urea (358 ± 29 g/L [35.8 ± 2.9 g/dL]; P < .03 by paired t test).
Urea-stimulated volume reduction in SS reticulocytes is irreversible. Cells were treated with nystatin and incubated in HBS pH 7.4 plus 600 mM urea. The solid curve represents volume reduction in cells continuously exposed for 120 minutes. At 10, 20, and 60 minutes, samples were removed, centrifuged, and resuspended in HBS pH 7.4 without urea and incubated at 37°C for the duration of the 120-periond (open symbols, connected by arrows to the points representing their exposure time to urea). Data are means of 3 independent experiments, with error bars representing SEM.
Urea-stimulated volume reduction in SS reticulocytes is irreversible. Cells were treated with nystatin and incubated in HBS pH 7.4 plus 600 mM urea. The solid curve represents volume reduction in cells continuously exposed for 120 minutes. At 10, 20, and 60 minutes, samples were removed, centrifuged, and resuspended in HBS pH 7.4 without urea and incubated at 37°C for the duration of the 120-periond (open symbols, connected by arrows to the points representing their exposure time to urea). Data are means of 3 independent experiments, with error bars representing SEM.
However, in cells washed at 10 minutes and then incubated an additional 110 minutes in the absence of urea (Figure 4, ○), CHCM remained constant (327 ± 23 g/L [32.7 ± 2.3 g/dL], P > .30 versus CHCM at 10 minutes). Thus, cells did not recover from urea-induced perturbations of cell volume, at least over the time period of these in vitro incubations. Consistent with this behavior was the observation that repeated exposure of cells to urea produced progressive changes in reticulocyte CHCM up to the limit of steady state CHCM produced by continuous exposure (not shown).
Urea stimulates volume reduction in unswollen reticulocytes in a concentration-dependent manner
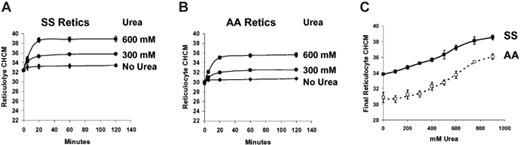
Figure 5 depicts a series of experiments in which RBCs were incubated without prior swelling with nystatin. The initial CHCM therefore reflects their in vivo “steady state” volume. As shown previously8 using density gradient analysis, there was little change in CHCM after incubation in isotonic media at pH 7.4 without urea. Addition of urea triggered volume reduction, with rapid establishment of a new steady state CHCM that was dependent on urea concentration. Figure 5C shows the reticulocyte CHCM of SS and AA cells after 2 hours of incubation at various urea concentrations. Even at low concentrations, urea stimulates volume reduction, especially in SS reticulocytes, in which there was an incremental increase in final reticulocyte CHCM with increasing urea concentration. Compared with incubation without urea, CHCM in SS reticulocytes was significantly increased at 300 mM (P < .005 by paired t test) and at 400 mM for AA cells (P < .01).
Concentration dependence of urea-stimulated volume reduction in unswollen reticulocytes. Fresh cells were washed and incubated without prior nystatin treatment in HBS pH 7.4, with no urea (control) or with urea at concentrations indicated. Initial CHCM values reflect in vivo CHMC. (Symbols represent means of 3 independent experiments, with error bars depicting SEM where larger than the symbol.) (A) SS reticulocytes. (B) AA reticulocytes. (C) Dependence on urea concentration of reticulocyte CHCM after 2 hours of incubation for SS (filled symbols) and AA (open symbols) cells.
Concentration dependence of urea-stimulated volume reduction in unswollen reticulocytes. Fresh cells were washed and incubated without prior nystatin treatment in HBS pH 7.4, with no urea (control) or with urea at concentrations indicated. Initial CHCM values reflect in vivo CHMC. (Symbols represent means of 3 independent experiments, with error bars depicting SEM where larger than the symbol.) (A) SS reticulocytes. (B) AA reticulocytes. (C) Dependence on urea concentration of reticulocyte CHCM after 2 hours of incubation for SS (filled symbols) and AA (open symbols) cells.
Final reticulocyte CHCM after KCC-mediated volume reduction is independent of initial CHCM
Figure 6 illustrates the relationship of final CHCM after volume reduction to nystatin-adjusted initial CHCM in SS reticulocytes swollen prior to incubation. Both with and without urea, final CHCM is independent of starting CHCM. Similar results were found with AA reticulocytes (not shown), although final CHCM both without and with urea was lower than in SS reticulocytes, as shown in Figure 3. These data are consistent with the notion that the final CHCM after KCC-mediated volume reduction represent a biologic set point for the KCl cotransporter, and that urea dramatically alters that set point.
Final CHCM after KCC-mediated volume reduction is independent of initial CHCM in SS reticulocytes. Cells were incubated in HBS pH 7.4 ± 600 mM urea. CHCM values less than 300 g/L (30 g/dL) were achieved by nystatin treatment (as in Figure 1).
Final CHCM after KCC-mediated volume reduction is independent of initial CHCM in SS reticulocytes. Cells were incubated in HBS pH 7.4 ± 600 mM urea. CHCM values less than 300 g/L (30 g/dL) were achieved by nystatin treatment (as in Figure 1).
Dithiothreitol treatment alters KCC response to urea
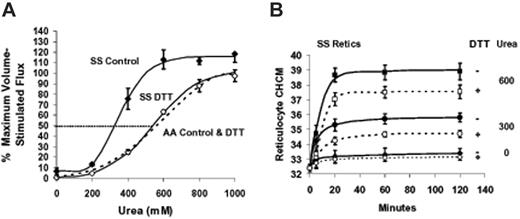
We previously demonstrated that DTT reduced KCC fluxes in SS RBCs (and AA RBCs) but did not alter swelling-stimulated volume reduction.8 Figure 7A demonstrates that DTT treatment normalized the concentration dependence of KCC activation by urea in SS RBCs. DTT preincubation did not alter urea stimulation of KCC in AA RBCs. These data strongly suggest that abnormal sensitivity of SS RBCs to urea stimulation of KCC results from reversible sulfhydryl oxidation. Figure 7B shows the effect of sulfhydryl reduction on KCC-mediated volume reduction in SS reticulocytes. In the absence of urea, there is little change in CHCM with or without DTT pretreatment. However, volume decrease stimulated by urea is significantly blunted by DTT pretreatment. Both the initial rate of change of CHCM (reflected in the 5-minute points) and the final steady state CHCM achieved by RVD are diminished by sulfhydryl reduction. At 600 mM urea, final CHCM was reduced from 389 ± 10 to 375 ± 8 g/L (38.9 ± 1.0 to 37.5 ± 0.8 g/dL) by DTT treatment (n = 3, P < .03 by paired t test). Note that the final CHCM in SS reticulocytes at 600 mM urea, even with DTT treatment, is substantially higher than that of AA reticulocytes exposed to the same urea concentration 375 ± 8 versus 356 ± 8 g/L (37.5 ± 0.8 versus 35.6 ± 0.8 g/dL, n = 3, P < .05, by unpaired t test). Thus, although DTT treatment normalizes the KCC flux response to urea, it does not completely correct the abnormal urea-stimulated volume reduction in SS reticulocytes.
Effect of DTT treatment on urea stimulation of KCC and volume reduction in reticulocytes. Fresh cells were washed and preincubated 1 hour without (control, filled symbols) or with 10 mM DTT (open symbols) as described in “Materials and methods,” under “Dithiothreitol treatment.” Points are mean ± SEM (n = 4); curves were drawn by eye. A parallel set of experiments with AA RBCs ± DTT are represented by a dashed line drawn by eye to fit the data points, which were omitted for clarity. (A) Rb influx measured at various urea concentrations in HBS pH 7.4. Parallel measurements in cells swollen to CHCM less than 270 g/L (27 g/dL) by nystatin treatment (± DTT pretreatment) provided a measure of maximal volume stimulated flux for normalizing data. Data are means ± SEM from 3 experiments independent from Figure 1. DTT pretreatment had no effect on urea stimulation of KCC in AA RBCs; data from cells incubated with and without DTT were combined to calculate the dashed line representing AA cells. (B) Effect of DTT and urea on volume reduction in SS reticulocytes. Unswollen cells were incubated as in Figure 4 at indicated urea concentrations, either without (filled symbols) or with (open symbols) DTT pretreatment. CHCM at 120 minutes was lower with DTT treatment at both 300 and 600 mM urea (P < .003 and P < .03, respectively, n = 3).
Effect of DTT treatment on urea stimulation of KCC and volume reduction in reticulocytes. Fresh cells were washed and preincubated 1 hour without (control, filled symbols) or with 10 mM DTT (open symbols) as described in “Materials and methods,” under “Dithiothreitol treatment.” Points are mean ± SEM (n = 4); curves were drawn by eye. A parallel set of experiments with AA RBCs ± DTT are represented by a dashed line drawn by eye to fit the data points, which were omitted for clarity. (A) Rb influx measured at various urea concentrations in HBS pH 7.4. Parallel measurements in cells swollen to CHCM less than 270 g/L (27 g/dL) by nystatin treatment (± DTT pretreatment) provided a measure of maximal volume stimulated flux for normalizing data. Data are means ± SEM from 3 experiments independent from Figure 1. DTT pretreatment had no effect on urea stimulation of KCC in AA RBCs; data from cells incubated with and without DTT were combined to calculate the dashed line representing AA cells. (B) Effect of DTT and urea on volume reduction in SS reticulocytes. Unswollen cells were incubated as in Figure 4 at indicated urea concentrations, either without (filled symbols) or with (open symbols) DTT pretreatment. CHCM at 120 minutes was lower with DTT treatment at both 300 and 600 mM urea (P < .003 and P < .03, respectively, n = 3).
Discussion
We have demonstrated that urea stimulates KCC activity in SS and AA RBCs in a concentration-dependent manner, with a maximal effect similar to cell swelling. Cells which are already swollen exhibit only slight additional stimulation of KCC by urea. KCC activity in urea-treated cells remains responsive to cell volume and pH, but with changes resembling those of swollen cells. SS RBCs at their in vivo steady state volume were more sensitive to urea stimulation of KCC than AA RBCs.
KCC activity is regulated by phosphatase/kinase equilibrium, and activation involves net dephosphorylation of the transporter (or other regulatory molecules) via a serine/threonine (S/T) phosphatase.16,26-31 The S/T kinase which keeps the transporter phosphorylated and inhibited has not been identified. Swelling (as well as other stimuli) appears to activate KCC by inhibiting this regulatory kinase, presumably because of a change in macromolecular crowding resulting from dilution of hemoglobin.32 Stimulation of the S/T phosphatase (probably PP1 or PP2A), may also increase KCC activity.26,28,29 Tyrosine phosphorylation modulates the system in complex ways, with some tyrosine kinase inhibitors activating KCC33 and others inhibiting.34,35 Some of these effects may be mediated by modulation of protein phosphatase activity by tyrosine phosphorylation36 and may explain the complex kinetics that have prompted more complicated models of KCC activation.1,37 Nevertheless, a 2-stage model involving activation by protein phosphatase(s) and inactivation by a volume-sensitive kinase explains most of the kinetics of KCC activation (for more detail, see Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Stimulation of KCl cotransport by urea was first reported in dog RBCs by Parker,16 in human AA RBCs by Kaji and Gasson,13 and in human SS RBCs by Culliford et al.15 Those studies noted concentration dependence up to 1 M urea, similar to that described here. Kaji and Gasson13 found that the delay time of KCC activation in AA RBCs increased with increasing urea concentrations, which suggested that urea inhibited the volume-sensitive kinase.29 At concentrations of urea above 600 mM, however, delay times increased substantially, whereas fluxes were only modestly elevated, suggesting a degree of phosphatase inhibition13 (see Document S1). Nevertheless, KCC was activated at all concentrations up to 1 M, indicating that inhibition of the kinase exceeded that of the phosphatase. Culliford et al15 noted no change in delay time for KCC activation in SS RBCs by 500 mM urea, but there was no comparison to AA RBCs. Bize et al3 noted a 20% to 40% increase in membrane-bound PP1 on urea stimulation of KCC in AA RBCs, but the increase was not proportional to KCC stimulation by urea (3- to 5-fold) and is difficult to reconcile with the kinetic data of Kaji and Gasson,13 suggesting phosphatase inhibition at higher urea concentrations. Inhibition of the regulatory kinase of KCC provides the simplest explanation of our data on urea stimulation of KCC in SS RBCs. Inhibition of this volume-sensitive kinase by both cell swelling and urea would explain why these stimuli are not additive and why urea-treated cells behave like swollen cells with respect to pH activation and changes in MCHCs (Figure 1). As Parker phrased it, the urea-treated cell acts “as if [the cell] were sensing its water content to be greater than it is.”16
The increased sensitivity of SS RBCs to activation of KCC by urea was a novel finding of this study, as previous studies of urea stimulation of KCC in SS RBCs lacked direct comparisons to normal cells.3,14,15 In the context of urea inhibition of the regulatory kinase of KCC, this increased sensitivity to activation could be explained by reduced activity of the kinase in SS RBCs (prior to urea exposure). The increased sulfhydryl oxidation known to exist in SS RBCs38 and the sensitivity of KCC activation to the sulfhydryl redox state8,11,39,40 are consistent with this idea. The fact that sulfhydryl reduction normalized the KCC sensitivity to urea activation in SS RBCs strongly implicates reversible sulfhydryl oxidation as its cause.
The functional effect of activating KCC in reticulocytes is volume reduction, a dynamic state in which KCC activity is high initially and presumably becomes progressively inactivated as CHC increases. A new steady state is reached when KCC activity returns to its original value, assuming other cation pathways are not altered. Thus, the process of volume reduction observed experimentally (Figure 3) reflects first the activation of the transporter and then its inactivation as final CHC is approached. The data presented here indicate that this process is relatively rapid in reticulocytes and is complete within about 30 minutes. We confirm our previous finding, based on density gradient analysis,8 that, when swollen, SS reticulocytes achieve higher steady state CHC via KCC activity than AA reticulocytes. Urea activation of KCC produces an exaggerated response in SS reticulocytes, resulting in even higher CHCs. Such behavior could theoretically result from higher numbers of transport sites on SS reticulocytes relative to AA reticulocytes. Indeed, Su et al41 have shown that immunoreactive KCC1 protein levels are higher in membranes of reticulocyte-rich samples of human RBCs. However, the explanation that abnormal KCC-mediated volume decrease results from increased transporter numbers due to the relative immaturity of SS reticulocytes is inconsistent with our previous finding that more mature SS reticulocytes (based on intensity of fluorescent reticulum staining) exhibited a greater volume decrease (higher final CHC) than immature reticulocytes,8 which would presumably have more KCC sites/cell. This suggests that abnormal volume regulation in response to cell swelling or urea stimulation of KCC likely resides in difference in activity of the regulators of KCC or in the response of the transporter to them.
Several mechanisms might be envisioned for such abnormal KCC regulation in SS RBCs. One possibility is that various isoforms of KCC are expressed in different ratios in SS and AA reticulocytes and that these isoforms respond differently to regulatory kinase/phosphatase activity. We recently demonstrated the presence of KCC1, KCC3, and KCC4 transcripts in mRNA from reticulocytes, as well as a splicing isoform of KCC1 (KCC1ex1b) coding for a truncated N-terminus of the protein.42 Mercado et al43 reported that splicing isoforms of KCC3 exhibited different responses to hypotonic swelling compared with full-length KCC3a. Casula et al44 reported that a N-truncation of KCC1 exhibited dominant inhibition of cotransfected KCC1 and KCC3 proteins, raising the intriguing possibility that one KCC isoform may affect the activity of another. This possible explanation of abnormal volume regulation in SS reticulocytes awaits further studies to quantitate the expression of KCC isoforms in RBCs and to define their functional activities and interactions.
The volume decrease stimulated by urea is remarkable on 3 accounts. First is the rapid and striking dehydration it produces in both AA and SS reticulocytes, even in cells at CHC levels found in vivo which exhibit little endogenous KCC activity and have stable CHCs in the absence of urea. This would be the expected consequence of inhibition by urea of the regulatory kinase, although effects, either direct or indirect, on the activating phosphatase cannot be definitively ruled out. Second, volume reduction in response to urea was exaggerated in SS versus AA reticulocytes. This, too, is consistent with reduced kinase activity (relative to phosphatase activity) in SS cells. In the context of the kinetic model for KCC activation by Jennings,30 both of these findings could be explained by reduced kinase activity in SS RBCs, coupled with urea inhibition of the kinase (see Document S1). Third, sulfhydryl reduction with DTT, decreased abnormal volume reduction of SS reticulocytes, consistent with the idea that, as with KCC flux activation by urea, this dysfunction results from reversible sulfhydryl oxidation.
Urea may be an important physiologic regulator of KCC activity and determinant of RBC volume in vivo, as originally suggested by Kaji and Gasson.13 Activation of KCC and stimulation of RVD occurs at concentrations of urea present in the renal medulla (100-600 mM).45 Activation of KCC by urea is reversible, although volume reduction is not, so that progressive volume loss and CHC increase could occur during repetitive circulation through the kidney. The increase in sensitivity of SS RBCs to urea activation and the exaggerated volume reduction of SS reticulocytes in response to urea may contribute to the elevated CHCs observed in both SS reticulocytes and RBCs compared with AA cells. The improvement in these abnormalities by DTT treatment provides additional rationale for use of sulfhydryl reducing agent to improve cellular hydration in individuals with sickle cell disease.46
Authorship
Contribution: C.H.J. designed the research, analyzed the data, and wrote the paper; R.K.R., M.J., and M.R. conducted research; R.S.F. designed the research, analyzed the data, and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Clinton H. Joiner, Cincinnati Comprehensive Sickle Cell Center, Children's Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: clinton.joiner@cchmc.org.
The online version of this article contains a data supplement
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Annette Lavender of the University of Cincinnati Adult Sickle Cell Program for her assistance in obtaining blood samples from individuals with sickle cell disease. We also thank Marlene Wall and the staff of the TriHealth Laboratory at Bethesda Oak Hospital, Cincinnati, for their enthusiastic help in performing the Advia 120 analyses.
This work was supported by the National Institutes of Health (U54 HL070871) (C.H.J. and R.S.F.).

![Figure 1. Effect of urea on Rb influx in SS and AA RBCs. Fluxes were measured in unfractionated blood samples. (A) Rb influx versus urea concentration. Ouabain- and bumetanide-resistant (OBR) Rb influx was measured in HBS pH 7.4 in fresh RBCs (not swollen with nystatin) at various added urea concentrations and normalized to concurrently measured maximal volume-sensitive Rb influx in cells swollen to MCHC 270 g/L (27g/dL) or less by nystatin treatment. In Cl-free media, increasing urea concentration had no effect on OBR Rb influx (not shown). Symbols represent mean with error bars depicting SEM of 6 experiments. Curves were drawn by eye. The dashed line indicates 50% activation of KCC. (B) Rb influx versus urea concentration in swollen RBCs. Cells were swollen to initial MCHC 270 g/L (27 g/dL), and Rb influx was measured at various urea concentrations. Data are expressed as percentage of maximal volume stimulated flux ([urea] = 0). The 2 experiments shown with AA (open symbols) and SS (filled symbols) RBCs were independent of those in panel A. Lines were drawn by eye. (C) Rb influx versus MCHC in SS RBCs. Cells were swollen to various (initial) MCHC via nystatin treatment, and Rb influx was measured with 600 mM urea (▴) or without urea (▵). Maximal volume-stimulated flux was measured concurrently in cells with initial MCHC less than 270 g/L (27 g/dL) (without urea). Data are from 2 independent experiments. (D) Rb influx versus pH in SS RBCs. Rb influx was measured in fresh cells washed and incubated in HBS at various pH values. The acid-stimulated Rb influx is greater than 95% CI dependent and reflects KCC activity. When present, urea concentration was 600 mM (▴). Data are from a single experiment, representative of 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/4/10.1182_blood-2006-04-018630/4/m_zh80040708020001.jpeg?Expires=1765970695&Signature=sWr~JckAjHDf3qGTPVTQAVXc99JIM7mYt1phPTaakjUd8P78XvFQ4Q5DP7kqhpUHgmp4sLhbjZVRVVKNR-LgtBdoHakysg8PRaIUVkAR2OLMZ054erhrC9tk8o~GcUMQ9GB7CkX9qAIDF2vaEdSJCFXxU5oM6QAAiwj9qpUa3-VEnzRGywIDWJLju56b5Cmjssjij8zibSAsuvLs8X1Jy2C2jysTLfmwwD6jHE2BRZcKC1Ro4BmwCJw4NkmJLQcxnxksNfmchtQnZihhi6J7WZp42msJpFu20mwnlvXXQCY4WkbGa0Fut9Nde6eO1woBvQpkj2-1Li9CsAATYQVmDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal