Abstract
The competence of the immune system of the developing fetus to act as a barrier to in utero hematopoietic-cell transplantation (IUHCT) has been a source of debate. Until now, comparisons of allogeneic and congenic engraftment have been inconclusive due to methodologic limitations resulting in minimal and inefficient engraftment. In this study, E14 fetal mice received transplants of either allogeneic or congenic bone marrow using a new intravascular technique that allows definitive administration of much higher doses of donor cells. Our results demonstrate that 100% of surviving recipients demonstrate engraftment at 1 week of age, but that 70% of allogeneic recipients lose engraftment by 1 month of age, and 80% ultimately fail to sustain long-term chimerism. In contrast, all congenic recipients maintain stable, long-term, multilineage chimerism. These results strongly support an immune barrier to allogeneic engraftment after IUHCT.
Introduction
The most compelling rational for in utero hematopoietic-cell transplantation (IUHCT) has always been the unique opportunity to achieve central tolerance.1 This rationale has been supported by the demonstration in animal models that IUHCT can result in donor-specific tolerance by a predominant mechanism of clonal deletion2,3 and that tolerance created by IUHCT can facilitate postnatal cellular and organ transplantation.1-5 Perhaps the most convincing argument that engraftment in the fetus is not limited by an immune barrier has been the repeated observation that there is no significant engraftment advantage for congenic compared to allogeneic cells.4-7 However, the reality remains that successful clinical IUHCT has only been achieved in severe combined immunodeficiency disease (SCID),8-10 and that inconsistencies of engraftment in allogeneic models remain unexplained. So the critical question of whether the immune system acts as a barrier to IUHCT remains open and subject to debate.1,11
In this study we reexamine this question using new methodology in the murine model that allows a much higher dose of donor cells to be transplanted with greater certainty of delivery.12,13 Using this methodology we demonstrate clearly, for the first time, that there are profound differences in the engraftment of congenic versus allogeneic cells and that allogeneic engraftment is lost after birth in the majority of recipients, supporting an adaptive immune barrier after IUHCT.
Materials and methods
Mice
Fourteen-day gestation Balb/c (H2d) and C57BL/6 (H2b, referred to as B6) mice were used as the fetal recipients (Jackson Laboratories, Bar Harbor, ME). Six- to 8-week-old C57BL/6TgN(act-EGFP)OsbY01 (H-2Kb, GFP+) mice provided by Dr Okabe (Osaka University, Genome Information Research Center) were used as donors. Animals were bred and time dated in our colony as previously described.6 The experimental protocols were approved by the Institutional Animal Care and Use Committee at the Children's Hospital of Philadelphia and followed guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Bone marrow harvest
Whole donor bone marrow (BM) was harvested from 6- to 8-week-old B6-GFP mice as previously described.6 After Ficoll gradient centrifugation, the low-density mononuclear cells (LDMCs) were counted and transplanted.
In utero transplantation
Under isoflurane anesthesia, a midline laparotomy was made and the uterine horns were exposed. Using a dissecting microscope, the vitelline vein was identified and each fetus was injected with 20 × 106 whole BM cells. A successful intravenous injection was confirmed by visualization of clearance of the blood in the vein by the injectate and the absence of extravasation at the site of injection. The uterus was returned to the maternal peritoneal cavity and the abdomen closed with 2 layers of absorbable 5-0 Vicryl suture.
Postnatal analysis
Peripheral blood (PB) from fetally injected mice was obtained at 1, 2, 4, and 6 months of age by retro-orbital puncture. A separate group of animals was harvested at 1 week of age (2 weeks after injection) to assess donor chimerism in PB and BM. Donor-cell chimerism was assessed as the percentage of CD45+ cells that were GFP+ by flow cytometry on a FACSCalibur (BD Biosciences, San Jose, CA). Analysis of allogeneic donor chimerism using the H2b marker in the B6-GFP into Balb/c strain combination in chimeric mice more than 4 months of age demonstrated good correlation with GFP expression confirming that GFP reliably represents all donor cells present in chimeric mice. At 4 months of age multilineage engraftment of donor cells was assessed as the percentage of GFP+ cells that were CD3+, B220+, Gr-1+, and CD11b+. All antibodies were phycoerythrin (PE) conjugated (BD Biosciences, San Diego, CA).
Statistical methods
Data are graphically represented as the mean of the respective group ± 1 SEM. Statistical comparisons between groups were performed with the Student t test for 2 samples assuming unequal variances. A 2-tailed P ≤ .05 was considered significant.
Results and discussion
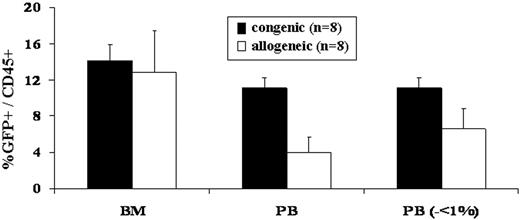
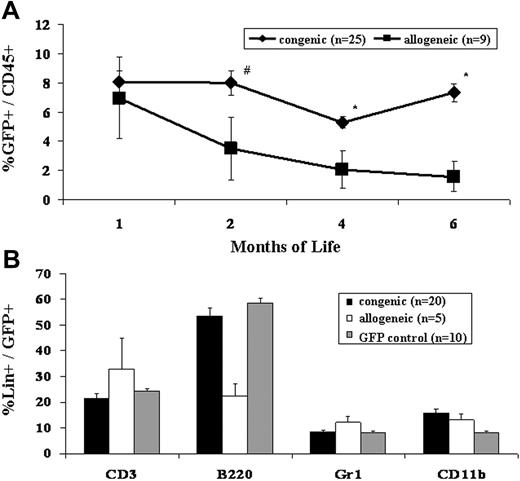
Our bias for many years has been that the competitive milieu of the early gestational fetus was the primary barrier to engraftment after IUHCT.1,11 This study refutes that bias. The most striking difference observed in this study, was the difference in frequency of engraftment in congenic compared to allogeneic recipients (Table 1)All congenic animals demonstrated sustained, multilineage chimerism after IUHCT, whereas only 19% of allogeneic animals showed sustained long-term engraftment. The second surprising finding of the study was that all of the fetuses, whether given allogeneic or congenic transplants, were similarly engrafted at 1 week of age (Figure 1). Thus, 70% of allogeneic recipients lost their engraftment between 1 week and 4 weeks of age (2-5 weeks after IUHCT) and an additional 3 of 9 allogeneic animals that were chimeric at 1 month of age ultimately lost their engraftment. Finally, the absolute levels of chimerism were initially similar in allogeneic compared to congenic recipients; however, there was a slow decline in levels of chimerism in allogeneic animals resulting in a significantly higher level of chimerism in congenic animals on late analysis (Figure 2).
Frequency of engraftment in congenic compared with allogeneic recipients
| . | Bone marrow . | Peripheral blood . | ||
|---|---|---|---|---|
| 1 wk of age . | 1 wk of age . | 1 mo of age . | 6 mo of age . | |
| Congenic, % (no./total) | 100 (8/8) | 100 (8/8) | 100 (25/25) | 100 (25/25) |
| Allogeneic, % (no./total) | 100 (8/8) | 100 (8/8) | 29 (9/31) | 19 (6/31) |
| . | Bone marrow . | Peripheral blood . | ||
|---|---|---|---|---|
| 1 wk of age . | 1 wk of age . | 1 mo of age . | 6 mo of age . | |
| Congenic, % (no./total) | 100 (8/8) | 100 (8/8) | 100 (25/25) | 100 (25/25) |
| Allogeneic, % (no./total) | 100 (8/8) | 100 (8/8) | 29 (9/31) | 19 (6/31) |
PB and BM engraftment efficiency and early chimerism levels following in utero allogeneic and congenic BM transplantation. The number of mice that were injected with either congenic or allogeneic BM that demonstrated PB and BM chimerism at 1 week of age and PB chimerism at 1 and 6 months of age was assessed (Table 1). Levels of donor PB and BM chimerism were assessed in mice humanely killed at 1 week of age. No significant difference was noted in BM chimerism levels between congenic and allogeneic recipients. When allogeneic recipients with PB donor chimerism levels less than 1% were removed from the analysis, assuming that these are the mice in which chimerism is lost at the time of 1 month analysis, there was no significant difference in PB donor chimerism between congenic and allogeneic recipients (analysis on the right of the x-axis). Error bars represent the mean ± SEM.
PB and BM engraftment efficiency and early chimerism levels following in utero allogeneic and congenic BM transplantation. The number of mice that were injected with either congenic or allogeneic BM that demonstrated PB and BM chimerism at 1 week of age and PB chimerism at 1 and 6 months of age was assessed (Table 1). Levels of donor PB and BM chimerism were assessed in mice humanely killed at 1 week of age. No significant difference was noted in BM chimerism levels between congenic and allogeneic recipients. When allogeneic recipients with PB donor chimerism levels less than 1% were removed from the analysis, assuming that these are the mice in which chimerism is lost at the time of 1 month analysis, there was no significant difference in PB donor chimerism between congenic and allogeneic recipients (analysis on the right of the x-axis). Error bars represent the mean ± SEM.
Engraftment levels following IUHCT of congenic and allogeneic BM. E14 fetal mice were given injections of either congenic or allogeneic whole BM. The PB of recipients was assessed at 1, 2, 4, and 6 months of life for total donor chimerism (A) #P < .1, *P < .05. The PB of fetal recipients of both congenic and allogeneic BM transplants was assessed for multilineage engraftment at 4 months of age (B). GFP control mice refer to age-matched naive GFP mice and chimerism levels are expressed as the percentage of GFP+ cells that express the indicated lineage marker. Error bars represent the mean ± SEM.
Engraftment levels following IUHCT of congenic and allogeneic BM. E14 fetal mice were given injections of either congenic or allogeneic whole BM. The PB of recipients was assessed at 1, 2, 4, and 6 months of life for total donor chimerism (A) #P < .1, *P < .05. The PB of fetal recipients of both congenic and allogeneic BM transplants was assessed for multilineage engraftment at 4 months of age (B). GFP control mice refer to age-matched naive GFP mice and chimerism levels are expressed as the percentage of GFP+ cells that express the indicated lineage marker. Error bars represent the mean ± SEM.
A number of previous studies have compared congenic to allogeneic engraftment following IUHCT with conflicting results. In an early study, Fleischman et al12 using transplacental injection, found increased engraftment for adult BM in MHC-matched versus -mismatched c-kit–deficient mice. In this study, however, engraftment could only be achieved in severely anemic mice, and the observation did not apply to fetal liver, which engrafted equally well independent of MHC mismatch. They attributed this difference to developmental acquisition of MHC restriction in adult hematopoietic stem cells (HSCs). In contrast Howson-Jan et al,13 using intraperitoneal injection into normal recipients, found a higher incidence of engraftment using allogeneic (5.2%) compared to congenic (0.7%) donors. The results of this study were limited by a low efficiency of engraftment in both groups and the fact that the engraftment was transient. Carrier et al5 demonstrated a slightly higher rate of polymerase chain reaction-detectable microchimerism in PB of congenic (25%) versus allogeneic (8%) recipients after IUHCT, but no differences in the frequency of organ engraftment. Recently, using mucopolysaccharidosis type VII (MPS-VII) mice, Barker et al14 demonstrated persistence of congenic cells using a histologic marker for the enzyme β-glucuronidase (GUSB) in peripheral tissues, but negligible levels of congenic or allogeneic chimerism in the PB. The minimal chimerism and low incidence of engraftment in these studies make interpretation of HSC engraftment difficult. In our own laboratory, with over a decade of experience in the murine model, we have never documented an advantage for congenic BM engraftment.7
The current study differs from previous studies because it compares the incidence and level of long-term donor allogeneic and congenic chimerism in a model where easily measurable levels of multilineage hematopoietic chimerism were achieved. We attribute this to improved methodology in the murine model allowing intravenous injection via the vitelline vein.15,16 This technique overcomes the volume limitation of intraperitoneal injection and allows delivery of much larger doses of cells. Whereas intraperitoneal injection was limited to a maximum of 5 × 106 cells/fetus at E14, intravenous injection allows higher volumes (and cell doses) to be administered, for example, 20 × 106 cells/fetus in this study. There is the additional advantage that the success of the injection can be visually monitored, removing the uncertainty of cell delivery that exists with the intraperitoneal approach. Thus, the higher cell doses and consistent delivery in this study unmasked a previously unrecognized, very clear difference in the incidence of chimerism between congenic and allogeneic donors.
We interpret these findings as strong evidence of an adaptive immune response that eliminates allogeneic cells in the majority of recipients after IUHCT. Although an innate immune barrier has not been ruled out, one would anticipate a more immediate loss of donor cells by innate immune mechanisms (natural killer cell or macrophage mediated). Future studies are required to mechanistically define the role of adaptive and innate immunity after in utero transplantation. Although the competitive barrier to donor-cell engraftment is formidable, the uniform engraftment of congenic cells in this study demonstrates that it can be consistently overcome by administration of massive doses of cells. We reconcile the findings of this study with our previous work documenting partial clonal deletion as the mechanism of tolerance after IUHCT with the following hypothesis. After IUHCT, donor-reactive cells escape thymic deletion due to inadequate or late presentation of donor antigen in the thymus. These cells either reject the graft or are subdued by a host peripheral regulatory response, analogous to the peripheral regulatory mechanisms that routinely control self-reactive clones that escape thymic deletion in healthy individuals.13 Understanding the immunologic mechanisms mediating engraftment after IUHCT will allow new strategies to be developed for clinical application.
Authorship
Contributions: W.H.P. designed the research, analyzed the data, performed research, and wrote the paper; M.E. performed research and contributed vital analytical skills; O.O.A. performed research and analyzed the data; and A.W.F. designed research, contributed vital analytical skills, analyzed data, and assisted in critically writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alan W. Flake, Department of Surgery, Children's Hospital of Philadelphia, Abramson Research Bldg, Rm 1116B, 3615 Civic Center Blvd, Philadelphia, PA 19104; e-mail: flake@email.chop.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants RO1 HL64715 and U54 HL070596-01 from the National Institutes of Health (A.W.F.). W.H.P. was supported by T32 HD046402 (A.W.F., Program Director). A.W.F. is also supported by funds from the Ruth and Tristram C. Colket Jr Chair of Pediatric Surgery.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal