Abstract
Epstein-Barr virus (EBV) is associated with posttransplant lymphoproliferative disease (PTLD), which is a leading cause of cancer death in recipients of transplants. We investigated the role of plasmacytoid dendritic cells (PDCs) in the development of EBV infection and the onset of lymphoproliferative disease (LPD) in humanized NOD-SCID mice and studied the effect of EBV on PDC function. NOD-SCID mice reconstituted with PDC-depleted peripheral blood mononuclear cells (PBMCs) from EBV IgG+ human donors had significantly enhanced mortality from disseminated EBV infection (median survival, 43 days) compared to PBMC-only mice (median survival, 72 days; log-rank P < .05). Mice reconstituted with PDC-enriched PBMCs challenged with EBV exhibited delayed mortality from EBV-LPD (median survival, 80 days) compared to PBMC-only mice challenged with EBV (median survival, 50 days; log-rank P < .05). EBV-stimulated pDCs produced interferon α (IFN-α) and promoted the activation of natural killer cells and IFN-γ–producing CD3+T cells. PDC activation of CD3+T cells in response to EBV stimulation was dependent on cell-to-cell contact, in part mediated by toll-like receptor 9 (TLR-9) signaling that was inhibited by chloroquine and TLR-9 inhibitory CpG. Thus, PDCs play an important role in anti-EBV cellular immune responses that may be targets for manipulation in novel strategies for the treatment of PTLD.
Introduction
Epstein-Barr virus (EBV) is a ubiquitous, potentially oncogenic human herpes virus affecting up to 95% of the adult population worldwide. Primary infection often occurs in childhood1 as asymptomatic or mild self-limiting illness or as infectious mononucleosis in adults.2 In immunocompromised patients with impaired cell-mediated immunity, acute EBV infection is associated with the development of lymphoproliferative disease (LPD) with mortality rates between 10% and 100%.3 In recipients of solid organ transplants, the incidence of posttransplant lymphoproliferative disease (PTLD) ranges from 1% to 15%, with the highest risk in EBV IgG-seronegative recipients receiving a graft from EBV IgG-seropositive donors.4
Dendritic cells (DCs) are a rare, heterogenous population of antigen-presenting cells (APCs) that initiate and regulate both innate and adaptive immune responses against invading pathogens.5-7 There are at least 2 circulating blood DC subsets including myeloid DC (MDCs) and plasmacytoid DC (PDCs).8,9 Although both DC subsets are involved in the initial response against infectious agents, PDCs play a key role in antiviral immunity. PDC precursors (pre-PDCs) express distinct lymphoid markers including pre-Tα, λ5, Ig1-like 14.1, and Spi-B; blood DC antigen 2 (BDCA-2) and BDCA-4 (neuropilin-1)11 ; and toll-like receptor 7 (TLR-7) and TLR-9.12,13 Viral stimulation of TLR-7 and TLR-9 expressed on PDCs results in production of type I interferons (IFNs),12,13 key cytokines in antiviral response.10,14,15
Humanized severe combined immunodeficiency (SCID) mice are a well-established model of spontaneous EBV-induced B-cell lymphoma.16 Nonobese diabetic (NOD)–SCID mice possess additional immunologic defects (eg, decreased murine natural killer [NK] cell function) that permit greater engraftment of human cells following injection of human peripheral blood mononuclear cells (PBMCs).17
In a previous study, we demonstrated that circulating pre-PDCs are severely reduced in the peripheral blood of renal transplant recipients resulting in impaired IFN-α production by PBMCs following viral stimulation.18 We hypothesized that the reduction in circulating pre-PDCs observed in transplant recipients plays a crucial role in the risk of viral infection, and in particular EBV-related infection and the onset of LPD. In the present study, we address this hypothesis by assessing the impact of a reduction in the numbers of circulating PDCs in the development of EBV-related infection and LPD using a humanized NOD-SCID mouse model as a surrogate model of human EBV infection. We demonstrate that reduction in PDCs enhances the development of EBV-related infection and LPD, whereas the supplementation of extra PDCs delays or even prevents the development of EBV-related LPD in humanized NOD-SCID mice. We now show for the first time that PDCs are involved in anti-EBV immunity by the secretion of IFN-α and activation of T cells mediated by TLR-9 pathways. Thus, manipulating PDCs may provide novel therapeutic options in the prevention or treatment of EBV-related PTLD.
Materials and methods
Reagents
Fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, and cychrome (CyC)–conjugated mouse antihuman monoclonal antibodies (mAbs) were used to detect cell surface HLA-DR (clone G46-6), CD3 (clone SP34), CD14 (clone MY4-FITC), CD20 (clone 2H7), CD40 (clone mAb89), CD56 (clone NCAM16.2), CD80 (clone L307.4), CD83 (clone HB15A), CD86 (clone FUN1), CD11c (clone B-ly6), CD123 (IL-3 receptor, clone 7G3), and BDCA-2 (clone AC144). Isotype-matched controls used were IgG2b (clone 27-35) and IgG2a (clone G155-178). All reagents were from BD PharMingen (San Diego, CA), except for anti-CD14 (Beckman Coulter, Hialeah, FL), CD40 and CD83 (Immunotech, Marseilles, Cedex, France), and BDCA-2 (Miltenyi Biotec, Bergisch Gladbach, Germany). Anti–human IFN-α mAb was purchased from Mabtech (Nacka Strand, Sweden). Pre-PDCs, pre-MDCs, NK cells, and T cells were isolated using BDCA-4 isolation kit, BDCA-1 isolation kit, CD56, and CD3 or CD8 microbeads, respectively (Miltenyi Biotec). Serum or culture supernatant levels of IL-12p70 (BD Biosciences, North Ryde, New South Wales, Australia), IL-10 (BD Biosciences), and IFN-α (R&D Systems, Minneapolis, MN) were determined using enzyme-linked immunosorbent assay (ELISA) kits, according to manufacturer's instructions, with levels of detection of 7.8 pg/mL, 7.8 pg/mL, and 10 pg/mL, respectively. Ficoll-Hypaque was purchased from Amersham Biosciences (Brown Deer, WI). Fluorescence-activated cell sorting (FACS) lysing solution was from BD PharMingen. Fetal calf serum (FCS) was obtained from JRH Biosciences (Lenexa, KS) and rabbit serum from ICN Pharmaceuticals (Costa Mesa, CA). Buffers used included staining buffer (PBS with 1% vol/vol FCS and 0.1% wt/vol sodium azide [Sigma Aldrich, St Louis, MO]) and running buffer (PBS with 0.5% vol/vol FCS and 2 mM EDTA [Sigma Aldrich]). RPMI 1640 (Sigma Aldrich) was supplemented with 10% vol/vol FCS, 2 mM l-glutamine (MultiCel, Trace Scientific, Noble Park, Victoria, Australia), sodium pyruvate (ICN Pharmaceuticals), penicillin-streptomycin (MultiCel, Trace Scientific), and sodium bicarbonate (Amresco, Solon, OH) and referred to as complete medium. Chloroquine was purchased from Sigma Aldrich, inhibitory CpG oligonucleotide (ODN TTAGGG) from InvivoGen (San Diego, CA), interferon-α2a from Roche Products (Dee Why, New South Wales, Australia), and phorbol 12-myristate 13-acetate (PMA) and ionomycin both from Sigma Aldrich. For NK cell cytolytic assays, 51 Cr was purchased from Amersham Biosciences (Piscataway, NJ) and Triton X from Bio-Rad Laboratories (Hercules, CA). Detection of IFN-γ–producing cells was performed by enzyme-linked immunospot assay (ELISPOT; Mabtech). Both marmoset B95.8 and human K562 cell lines were obtained from American Type Culture Collection (Rockville, MD). EBV supernatant containing live EBV was generated from B95.8 marmoset cell line incubated in complete medium for 14 days and a single batch of supernatant was used for all experiments. Free virus was demonstrated in EBV supernatant by electron microscopy.
Humanized NOD-SCID mouse model of EBV infection and LPD
Fifty-six 4- to 8-week-old NOD-SCID mice (Institute of Medical and Veterinary Science, Adelaide, Australia) of both sexes were housed at the University of Adelaide Animal House Facility under strict pathogen-free conditions. This study was approved by the institutional animal ethics committee of the Queen Elizabeth Hospital and University of Adelaide in Adelaide.
Human PBMCs were isolated from buffy coats of 3 healthy EBV IgG+ healthy human blood donors (Australian Red Cross Blood Service, South Australia, Australia) using Ficoll-Hypaque density gradient separation. Mice were humanized by intraperitoneal injection of 1 × 108 PBMCs (resuspended in 0.5 mL RPMI) in either the left or right iliac fossa of each mouse. EBV immune status of blood donors was assessed using a commercial assay for serum antibody against EBV capsid antigen. The mice were separated into 2 groups—the latent EBV infection (mice reconstituted with EBV IgG+ PBMCs without exposure to active EBV) and the active EBV infection group (mice reconstituted with EBV IgG+ PBMCs and simultaneously challenged with active EBV). The latent EBV infection group included 4 groups of 8 mice reconstituted with human PBMCs alone, PDC-depleted PBMCs, PDC-enriched PBMCs, and IFN-α–pulsed PBMCs. The active EBV infection group consisted of 3 groups of 8 mice, reconstituted with human PBMCs and EBV supernatant (0.2 mL), PDC-enriched PBMCs and EBV (0.2 mL), and IFN-α–pulsed PBMCs and EBV (0.2 mL). In all groups, PBMCs were from EBV IgG+ human blood donors. PDC reduction was achieved by immunomagnetic bead depletion using automated immunomagnetic bead separator (Automacs, Miltenyi Biotec) prior to injection into PDC-depleted PBMC mice. Flow cytometric analysis of PDC-depleted PBMC fraction stained with anti–human CD123+ mAbs confirmed approximately 50% reduction in the proportion of PDCs of total PBMCs approximately corresponding to the reduced proportion of PDCs in the peripheral blood of renal transplant recipients.18 PDC-enriched PBMC mice received a further 1 × 105 autologous PDCs from the same blood donor (positively isolated using Automacs) injected into each mouse in addition to PBMCs. The depletion and enrichment of PDCs were performed using BDCA-4+ isolation kit and cells separated by Automacs as described.18 In the IFN-α–pulsed PBMC group, PBMCs were cultured overnight at a concentration of 1 × 108 in 10 mL complete medium supplemented with IFN-α (1000 U/mL). Because all mice were reconstituted with PBMCs from the same blood donors, observed differences were not attributed to interindividual donor risk of EBV infection.
Mice were observed for signs of distress on alternate days. All mice were examined for postmortem evidence of tumor. To assess for the presence of EBV, tissues were examined for the evidence of viral particles by electron microscopy (EM, Hitachi H-600 transmission electron microscope, Tokyo, Japan 1983) and stained for EBV-related latent-membrane protein 1 (LMP-1; Dako, Glostrup, Denmark) by immunoperoxidase. Tumors were stained for the presence of PDCs and B cells using anti–human BDCA-2 and anti–human CD20 mAbs, respectively, by immunofluorescence or immunoperoxidase techniques. Images were obtained by Olympus BX40 camera, Nikon Coolpix 4500 with numerical aperture 0.75.
Cytokine production and costimulatory molecule expression by PDCs following EBV stimulation
Cell-surface expressions of MHC class II and costimulatory molecules (CD40, CD80, CD86) were determined on freshly isolated PDCs and following stimulation with EBV (10% vol/vol) for 48 hours by 2-color flow-cytometric analysis using a FACScan flow cytometer with CellQuest software (BD Immunocytometry Systems, San Jose, CA).
PDCs (1 × 106 cells/mL) isolated from healthy blood donors were cultured in complete medium and stimulated for 20 hours with EBV supernatant (10% vol/vol) or with herpes simplex virus-1 (HSV-1; Kos strain, a gift from Dr Keryn Williams, Ophthalmology Department, Flinders Medical Centre, Adelaide, Australia) at a concentration of 5 plaque-forming units per cell. Concentrations of IFN-α, IL-12p70, and IL-10 were determined in the tissue culture supernatant by ELISA as per manufacturer's instructions. The results were simultaneously compared to pre-MDCs isolated from the same healthy blood donors cultured under identical conditions. IFN-α production by PDCs (1 × 106 cells/mL) following stimulation in culture for 20 hours with EBV supernatant (10% vol/vol) and in the presence of anti–IL-10 mAb (100 ng/mL; a gift from Dr Greg Goodall, Hanson Centre, Adelaide, Australia) were determined. Both pre-PDCs and pre-MDCs were positively selected from PBMCs using BDCA-4 and BDCA-1 isolation kits, respectively, by an immunomagnetic bead isolation technique with purity of more than 95%, as assessed by flow cytometry (Figure 1). In addition, highly purified CD123high PDCs were isolated from immunomagnetic bead-isolated BDCA-4+ PDCs using a high-flow cell sorter, cultured with EBV for 20 hours and amount of IFN-α quantified by ELISA.
DC purity. Purity of PDCs and MDCs following immunomagnetic bead isolation counterstained with CD123 (97.5%) and CD11c (99.2%) mAbs, respectively, are shown.
DC purity. Purity of PDCs and MDCs following immunomagnetic bead isolation counterstained with CD123 (97.5%) and CD11c (99.2%) mAbs, respectively, are shown.
NK cytolytic activity in response to EBV-stimulated PDCs determined by 51Cr-release assay
PDCs and NK cells (purity of > 95%) were positively selected from EBV IgG+ blood donors using immunomagnetic beads by Automacs. 51 Cr-labeled target K562 cells were prepared by incubating 100 μCi (3.7 MBq) 51 Cr with 1 × 107 cells at 37°C for 1 hour. Cells were washed with PBS and adjusted to cell density of 2.0 × 105 cells/mL complete medium. K562 cells (2 × 104 cells/well) were plated in 96-well round bottom plates (Techno Plastic Products, Trasadingen, Switzerland) with decreasing concentrations of NK cells of 2 × 105, 1 × 105, and 5 × 104 cells/well (ie, ratios of 1:10, 1:5, and 1:2.5, respectively) and incubated for 4 hours. Autologous PDCs were added to NK and K562 cells cocultured with and without EBV supernatant (10% vol/vol) and PDCs adjusted to maintain a ratio of 1:4 between PDCs and NK cells irrespective of NK cell numbers. Stimulation of NK cells with IFN-α (1000 U/mL) was used as a positive control.19 Spontaneous and maximal release of 51Cr by K562 cells was determined in each experiment by culturing 2 × 104 K562 cells alone in complete medium or in the presence of 2.5% vol/vol of the detergent Triton X, respectively. Cell suspensions were incubated in a humidified CO2 incubator (37°C for 4 hours), and then centrifuged at 3000g to precipitate the cell pellet. Supernatant (25 μL) was removed and mixed thoroughly with 150 μL Opti-phase supermix (EG&G Wallac, Wellesley, MA). Release of 51Cr was determined using a Microbeta Trilux liquid scintillation counter (EG&G Wallac). Chromium release was expressed as number of lytic units per 107 effector cells.
Induction of IFN-γ–producing T and NK cells by EBV-stimulated PDCs determined by ELISPOT
PDCs and NK, CD3+, and CD8+ T cells (purity > 95%) were isolated from EBV IgG+ healthy blood donors using Automacs separation. PDCs (1 × 105) were cultured with autologous NK cells (1 × 106) or CD3+ and CD8+ T cells (1 × 106) resuspended in 1 mL culture medium with or without the presence of EBV supernatant (10% vol/vol) for 20 hours in a humidified CO2 incubator at 37°C. Positive controls for IFN-γ production by CD3+ and CD8+ T and NK cells were stimulation by PMA (10 ng/mL)/ionomycin (5 μL/mL) and IFN-α (1000 U/mL), respectively. Analysis of IFN-α–producing cells was performed on MultiScreen-IP high protein-binding 96-well plates (Millipore, Bedford, MA) using an ELISPOT kit according to manufacturer's instructions. The reactions were terminated and spots were counted in an ELISPOT reader (Mabtech).
To determine if cell contact between PDCs and autologous target cells (NK cells and T cells) was necessary for immunologic effect, polycarbonate membrane Transwell inserts (Costar, Corning, NY) with a membrane pore size of 0.4 μm were used to separate cell populations of interest. PDCs (1 × 105) with or without EBV supernatant (10% vol/vol) were resuspended in 100 μL culture medium and added to Transwell inserts in a 24-well plate (Techno Plastic Products), and 1 × 106 autologous NK cells or T cells resuspended in 600 μL complete medium were added to the lower chamber allowing only soluble factors secreted from PDCs to pass through to the plate well. The cells were cultured for 20 hours in a CO2 incubator at 37°C and the number of IFN-γ–producing NK and T cells were determined by ELISPOT.
Influence of TLR- 9 inhibition on the induction of IFN-γ-producing T and NK cells
We assessed the ability of EBV-stimulated PDCs to generate IFN-γ–producing T or NK cells in the presence of chloroquine (nonspecific) or inhibitory CpG ODN TTAGGG (specific) agents, which inhibit TLR-9 signaling pathway. PDCs and autologous T or NK cells were cultured for 20 hours in the presence of 0.02 and 0.05 mM chloroquine or 0.5 and 1.0 μM of inhibitory CpG and the number of IFN-γ–producing NK and T cells were determined by ELISPOT.
Statistical analyses
Data are expressed as means ± SD or SEM. The Student t test and Kaplan-Meier log-rank test were used to compare data where appropriate, with P values below .05 considered significant. Statistics were performed using GraphPad Instat version 3.00, GraphPad Software (San Diego, CA).
Results
Depletion of PDCs enhances mortality from disseminated EBV infection in the latent EBV infection model
We established a humanized immunodeficient NOD-SCID mouse model to study the effects of the alterations in the proportion of PDCs in the peripheral blood in the development of EBV-related diseases. In the latent EBV infection model (Table 1), the median survival of PDC-depleted PBMC mice was 43 days (range, 31-58 days). Mice reconstituted with PBMCs alone (median survival 72 days, range 33-120 days or longer) or IFN-α–pulsed PBMC mice (median survival 65 days, range 51-160 days) had similar survival to PDC-enriched PBMC group (median survival of 81 days, range 39-197 days, log-rank P = .7). The addition of 1 × 105 extra autologous PDCs did not prolong survival compared to the PBMC group or IFN-α–pulsed PBMC group (Table 1). Only 1 of 8 mice (12.5%) in the PBMC group had evidence of intra-abdominal tumors at postmortem examination. Mice reconstituted with PDC-depleted PBMCs died from disseminated EBV infection with evidence of positive LMP-1–staining cells in tissue sections (liver, spleen) and extensive plasma cell infiltrates in lymphoid organs. Unlike PDC-depleted mice, around 20% of the remaining mice had no evidence of LMP-1 staining in tissues postmortem. There was no evidence of tumor infiltrate in these mice but lymphadenopathy with or without splenomegaly were present in 2 (25%) of 8 mice in each group at autopsy.
Actual survival, in days, of NOD-SCID mice in latent infection model
| NOD-SCID latent infection model . | Survival, d . | Log-rank P compared to PDC-depleted PBMCs group . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | Median . | ||
| PDC-depleted PBMCs | 31 | 35 | 36 | 37 | 49 | 49 | 51 | 58 | 43 | |
| PBMCs alone | 33 | 51 | 51 | 68 | 76* | 100 | > 120 | > 120 | 72 | .02 |
| PDC-enriched PBMCs | 39 | 44 | 51 | 71 | 90 | 123 | 193 | 197 | 80 | .01 |
| IFN-α-pulsed PBMCs | 51 | 52 | 58 | 65 | 65 | 69 | 128 | 160 | 65 | .03 |
| NOD-SCID latent infection model . | Survival, d . | Log-rank P compared to PDC-depleted PBMCs group . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | Median . | ||
| PDC-depleted PBMCs | 31 | 35 | 36 | 37 | 49 | 49 | 51 | 58 | 43 | |
| PBMCs alone | 33 | 51 | 51 | 68 | 76* | 100 | > 120 | > 120 | 72 | .02 |
| PDC-enriched PBMCs | 39 | 44 | 51 | 71 | 90 | 123 | 193 | 197 | 80 | .01 |
| IFN-α-pulsed PBMCs | 51 | 52 | 58 | 65 | 65 | 69 | 128 | 160 | 65 | .03 |
n = 8 for each group; total n = 32.
Evidence of tumors at autopsy.
PDC enrichment significantly delayed mortality from EBV lymphoma in the active infection model
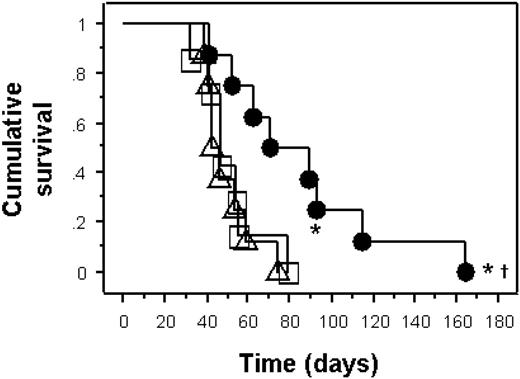
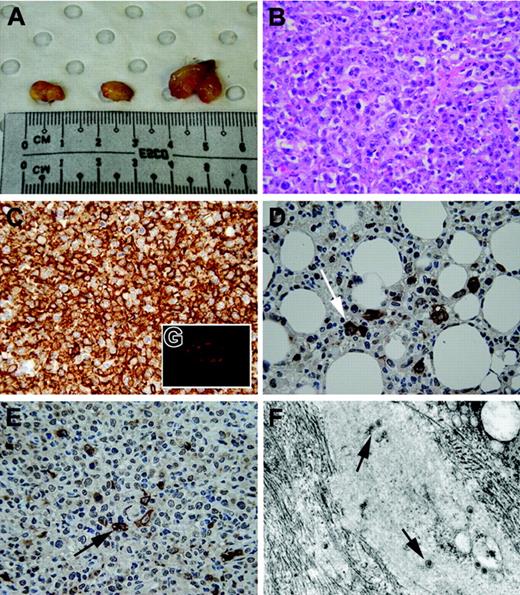
In contrast to the latent infection model, exposure of mice to EBV at the time of reconstitution with PBMCs substantially enhanced the development of tumors (Figure 2). Mice reconstituted with PBMCs and EBV died from tumors with a median survival of 50 days (range, 32-111 days). Mice reconstituted with IFN-α–pulsed PBMCs with EBV survived for a median of 44 days (range, 39-74 days; log-rank P = .9 compared to PBMC and EBV group). However, when mice were reconstituted with extra autologous PDCs (PDC-enriched PBMC and EBV group), their survival was significantly prolonged (median survival, 80 days; range 41-164 days; log-rank P < .01 compared to PBMC and IFN-α–pulsed PBMC groups; Table 2). Furthermore, 2 (25%) of these 8 mice in the PDC-enriched PBMC and EBV group showed no evidence of tumor nor evidence of lymphadenopathy. Autopsies of the mice affected by tumor showed extensive infiltrative tumors intra-abdominally involving the peritoneal wall, bowel, liver, spleen, and even kidneys (Figure 3A). Microscopically, these tumors were high-grade large cell lymphomas with high mitotic rates and varying degrees of tissue necrosis (Figure 3B). Immunohistochemical staining confirmed that all tumors were of B-cell origin (CD20+ cells; Figure 3C) and staining for LMP-1 was universally positive in all tumors (Figure 3D-E). Electron microscopy confirmed the presence of viral particles within tumors (Figure 3F). In addition, the presence of PDCs within tumors was determined by immunofluorescence (Figure 3G). These histological findings of the tumors are typical of human EBV-associated B-cell lymphoma typical of the monomorphic type of PTLD.20 We next sought to determine the mechanisms underlying the observed differences in survival of the mice by studying the in vitro effects of EBV on PDCs in culture.
Survival of humanized NOD-SCID mice in a model of active EBV infection. Kaplan-Meier survival curve of NOD-SCID mice in an active infection model. Mice reconstituted with PDC-enriched (extra 1 × 105 autologous PDCs) PBMCs (1 × 108) from IgG+ human blood donors with EBV (n = 8, •) had significantly delayed mortality from EBV-associated lymphoma compared to mice reconstituted with PBMCs and EBV (n = 8, □) or IFN-α-pulsed PBMC and EBV (n = 8, ▵; log-rank P = .004). Two mice in the PDC-enriched PBMC group had no evidence of EBV lymphoma (*).
Survival of humanized NOD-SCID mice in a model of active EBV infection. Kaplan-Meier survival curve of NOD-SCID mice in an active infection model. Mice reconstituted with PDC-enriched (extra 1 × 105 autologous PDCs) PBMCs (1 × 108) from IgG+ human blood donors with EBV (n = 8, •) had significantly delayed mortality from EBV-associated lymphoma compared to mice reconstituted with PBMCs and EBV (n = 8, □) or IFN-α-pulsed PBMC and EBV (n = 8, ▵; log-rank P = .004). Two mice in the PDC-enriched PBMC group had no evidence of EBV lymphoma (*).
Actual survival, in days, of humanized NOD-SCID mice reconstituted with EBV or EBV and PDCs
| NOD-SCID active infection model . | Survival, d . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | ||
| PDC-enriched PBMCs + EBV | 41 | 52 | 62 | 71 | 89 | 93† | 115 | 164†‡ | |
| PBMCs + EBV* | 32 | 41 | 47 | 47 | 54 | 55 | 79 | 82 | |
| IFN-α-pulsed PBMCs + EBV* | 39 | 40 | 42 | 42 | 46 | 53 | 59 | 74 | |
| NOD-SCID active infection model . | Survival, d . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | ||
| PDC-enriched PBMCs + EBV | 41 | 52 | 62 | 71 | 89 | 93† | 115 | 164†‡ | |
| PBMCs + EBV* | 32 | 41 | 47 | 47 | 54 | 55 | 79 | 82 | |
| IFN-α-pulsed PBMCs + EBV* | 39 | 40 | 42 | 42 | 46 | 53 | 59 | 74 | |
n = 8 for each group.
P < .05 compared to PDC-enriched PBMC + EBV group.
No evidence of lymphoma at autopsy.
Mouse was killed.
Macroscopic and histological appearance of EBV-induced tumors. (A) Typical appearance of intra-abdominal tumors in one NOD-SCID mouse at autopsy. Tumors were large (0.5-1.0 cm) and solid and contained necrotic foci on cross-section. (B) Histological appearance of tumors in NOD-SCID mice. On light microscopy, tumors were high-grade large-cell lymphomas with high mitotic rates, with varying degrees of tissue necrosis. (C) Immunohistochemical staining confirmed that all tumors were of B-cell origin (brown color indicates CD20+ cells; original magnification ×400). (D-E) Immunohistochemical staining of tumors for LMP-1 marker (brown staining; arrows) of EBV lymphoma in both extranodal (D) and nodal (E) regions. (F) Electron microscopy revealing presence of virus (arrows) within intra-abdominal tumor mass (original magnification ×45 000). (G) Immunofluorescent staining of tumors demonstrated the presence of PDCs (red fluorescence) within the tumor periphery using anti–human BDCA-2 mAbs. Images in panels B-E were taken with a 10×/0.30 NA objective; image in panel G was taken with a 40×/0.75 NA objective.
Macroscopic and histological appearance of EBV-induced tumors. (A) Typical appearance of intra-abdominal tumors in one NOD-SCID mouse at autopsy. Tumors were large (0.5-1.0 cm) and solid and contained necrotic foci on cross-section. (B) Histological appearance of tumors in NOD-SCID mice. On light microscopy, tumors were high-grade large-cell lymphomas with high mitotic rates, with varying degrees of tissue necrosis. (C) Immunohistochemical staining confirmed that all tumors were of B-cell origin (brown color indicates CD20+ cells; original magnification ×400). (D-E) Immunohistochemical staining of tumors for LMP-1 marker (brown staining; arrows) of EBV lymphoma in both extranodal (D) and nodal (E) regions. (F) Electron microscopy revealing presence of virus (arrows) within intra-abdominal tumor mass (original magnification ×45 000). (G) Immunofluorescent staining of tumors demonstrated the presence of PDCs (red fluorescence) within the tumor periphery using anti–human BDCA-2 mAbs. Images in panels B-E were taken with a 10×/0.30 NA objective; image in panel G was taken with a 40×/0.75 NA objective.
EBV promotes the maturation of and production of IFN-α and IL-10 by PDCs
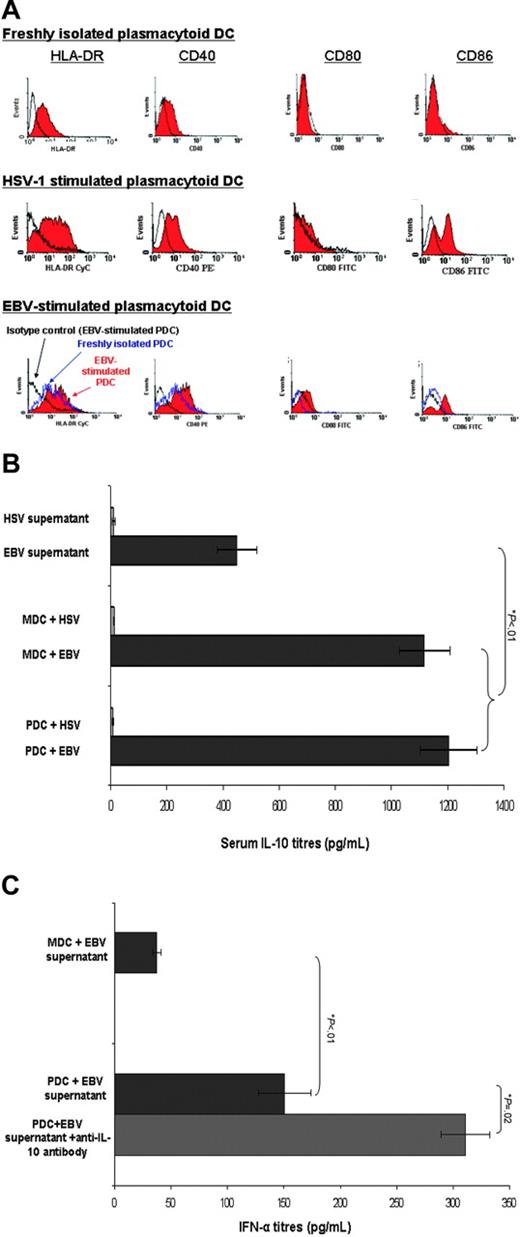
Freshly isolated PDCs were phenotypically immature with low expression of costimulatory molecules (eg, CD40, CD80, CD86) as determined by flow cytometric analysis. Exposure to EBV induced phenotypic maturation of PDC with up-regulation of CD86 and to a lesser extent MHC class II and other costimulatory molecule (CD40, 80) expression (Figure 4A). HSV-1–stimulated PDCs were shown for comparison.
Functional analysis of PDCs in response to EBV stimulation. (A) Cell-surface expression of costimulatory molecules on immunomagnetic bead-isolated PDCs before and after stimulation with EBV. Flow cytometric analysis revealed up-regulation of cell surface expressions of CD86 and to a lesser extent MHC class II and other costimulatory molecules (CD40, CD80) following EBV stimulation in vitro for 48 hours. An HSV-1–stimulated PDC is shown as the positive control. Open black profiles denote isotype controls, dotted line profiles denote freshly isolated unstimulated PDCs, and solid filled in histograms represent EBV-matured PDCs. Isotype controls of EBV-stimulated PDCs and freshly isolated PDCs were similar and hence only the former histogram was shown. Representative histograms of 3 separate experiments. (B) Cytokine production by PDCs and MDCs in response to EBV stimulation measured by ELISA. Both PDCs (1203.1 ± 100.5 pg/mL) and MDCs (1116.4 ± 90.5 pg/mL) produced significantly greater amounts of IL-10 following EBV stimulation (10% vol/vol) for 20 hours compared to the amount present in the EBV supernatant (449.5 ± 71.4 pg/mL, Student t test P < .01). For comparison, HSV-1–stimulated BDCA-4+ PDCs and BDCA-1+ MDCs did not produce measurable IL-10 (data not shown). PDCs cultured in complete medium did not produce any detectable IL-10. Results are expressed as mean ± SEM. (C) PDCs produced IFN-α following EBV stimulation that is enhanced in the presence of anti–IL-10 mAb. PDCs cultured in complete medium did not produce any detectable IFN-α. Results are expressed as mean ± SEM.
Functional analysis of PDCs in response to EBV stimulation. (A) Cell-surface expression of costimulatory molecules on immunomagnetic bead-isolated PDCs before and after stimulation with EBV. Flow cytometric analysis revealed up-regulation of cell surface expressions of CD86 and to a lesser extent MHC class II and other costimulatory molecules (CD40, CD80) following EBV stimulation in vitro for 48 hours. An HSV-1–stimulated PDC is shown as the positive control. Open black profiles denote isotype controls, dotted line profiles denote freshly isolated unstimulated PDCs, and solid filled in histograms represent EBV-matured PDCs. Isotype controls of EBV-stimulated PDCs and freshly isolated PDCs were similar and hence only the former histogram was shown. Representative histograms of 3 separate experiments. (B) Cytokine production by PDCs and MDCs in response to EBV stimulation measured by ELISA. Both PDCs (1203.1 ± 100.5 pg/mL) and MDCs (1116.4 ± 90.5 pg/mL) produced significantly greater amounts of IL-10 following EBV stimulation (10% vol/vol) for 20 hours compared to the amount present in the EBV supernatant (449.5 ± 71.4 pg/mL, Student t test P < .01). For comparison, HSV-1–stimulated BDCA-4+ PDCs and BDCA-1+ MDCs did not produce measurable IL-10 (data not shown). PDCs cultured in complete medium did not produce any detectable IL-10. Results are expressed as mean ± SEM. (C) PDCs produced IFN-α following EBV stimulation that is enhanced in the presence of anti–IL-10 mAb. PDCs cultured in complete medium did not produce any detectable IFN-α. Results are expressed as mean ± SEM.
Freshly isolated PDCs stimulated with EBV produced greater amounts of IFN-α than MDCs (449.5 ± 71.4 pg/mL compared to 37.5 ± 3.5 pg/mL, Student t test P = .01). However, this was significantly less than IFN-α production by PDCs following HSV-1 stimulation (2000 ± 50.0 pg/mL, Student t test P < .01). To confirm this, highly purified PDCs stimulated with EBV produced IFN-α. Neither PDCs nor MDCs produced detectable IL-12p70 in response to EBV stimulation (data not shown). IL-10 was detectable in EBV supernatant indicating an intrinsic production of IL-10 by EBV. Nonetheless, EBV-stimulated, but not HSV-1–stimulated MDCs and PDCs, produced equivalent amounts of IL-10 in excess of the amount produced by background EBV (573.4 ± 20.3 pg/mL produced by PDCs compared to 522 ± 44.1 pg/mL produced by MDCs, Student t test P = .9; Figure 4B). Neutralization of IL-10 activity with anti–IL-10 mAbs significantly enhanced IFN-α production by PDCs (P = .02; Figure 4C) suggesting that the reduction in IFN-α secretion of PDCs by EBV was related to the effect of IL-10.
EBV-stimulated PDCs activate NK and T cells by cell-to-cell contact
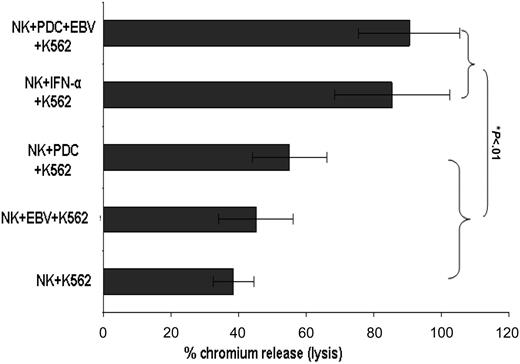
Having determined that PDCs produce IFN-α in response to EBV, the effect of PDCs on effector cell populations of autologous NK and T cells was investigated. Although freshly isolated resting NK cells induced low-level lysis of target K562 cells with and without the presence of PDCs, NK cells stimulated by PDCs in the presence EBV significantly enhanced NK cell cytotoxicity to the same extent as exogenous IFN-α (Student t test P < .01; Figure 5). Similarly, a greater number of IFN-γ–producing NK cells were evident when NK cells were exposed to EBV in the presence of PDCs than with unstimulated PDCs with NK cells, EBV-stimulated NK cells, or NK cells alone (194 ± 18 compared to 117 ± 10, 41 ± 11, and 31 ± 5 spots/3 × 105 NK cells, respectively; Student t test P < .01). In contrast to a previous report,21 exposure of NK cells to exogenous IFN-α was sufficient to promote IFN-γ production in NK cells. In addition, EBV-stimulated PDCs significantly augmented the development of IFN-γ–producing CD3+ T cells compared to unstimulated PDC (249.3 ± 18.5 compared to 23.0 ± 8.5 spots/3 × 105 T cells, Student t test P < .001). The ability of T cells to produce IFN-γ in response to PDC activation appeared to be dependent on the presence of CD4+ T cells because identical experiments using only CD8+ T cells in the coculture with PDCs and EBV were insufficient to induce the production of IFN-γ–producing CD8+ T cells. Measurements of IFN-α in the culture supernatants of PDCs and NK and T cells were consistently less than 80 pg/mL.
EBV-stimulated PDCs increase NK cell-mediated cytolysis. EBV-stimulated PDCs induce lysis of 51Cr-labeled target K562 cells compared to unstimulated PDCs with NK cells, EBV-stimulated NK cells, or NK cells alone. Results expressed as percentage chromium release at a ratio of NK to target K562 cells of 1:2.5. Results are expressed as mean ± SEM.
EBV-stimulated PDCs increase NK cell-mediated cytolysis. EBV-stimulated PDCs induce lysis of 51Cr-labeled target K562 cells compared to unstimulated PDCs with NK cells, EBV-stimulated NK cells, or NK cells alone. Results expressed as percentage chromium release at a ratio of NK to target K562 cells of 1:2.5. Results are expressed as mean ± SEM.
To address whether EBV-stimulated PDC activation of NK and CD3+ T cells required cell-to-cell contact or was mediated by soluble factors secreted by PDCs in response to EBV, we separated PDCs and NK/T cells by a semipermeable membrane using a Transwell system. The induction of IFN-γ–producing cells by EBV-stimulated PDCs was observed only when both PDCs and NK/T cells were cultured together, indicating that cell contact, rather than PDC-secreted soluble factors, was the dominant factor in this response (Figure 6). Furthermore, the addition of anti–human IFNα mAbs (10 μg/mL) had no impact on the ability of PDCs to promote IFN-γ–producing NK cells, further indicating the importance of cell-to-cell contact between PDCs and NK cells necessary for the induction of IFN-γ–producing NK cells.
Cell-to-cell contact dependence required for PDC activation of IFN-γ–producing CD3+ T cells. Immunomagnetic bead-isolated PDCs were separated from CD3+ T cells using a Transwell filter with membrane pore size of 0.4 μm. Cell contact between PDCs and CD3+ T cells in the presence of EBV generated significantly greater number of IFN-γ–producing CD3+ T cells compared to the soluble factors secreted by PDCs in response to EBV (155.0 ± 7.1 compared to 27.0 ± 2.8 spots/3 × 105 T cells, Student t test P < .01). Results are expressed as mean ± SEM.
Cell-to-cell contact dependence required for PDC activation of IFN-γ–producing CD3+ T cells. Immunomagnetic bead-isolated PDCs were separated from CD3+ T cells using a Transwell filter with membrane pore size of 0.4 μm. Cell contact between PDCs and CD3+ T cells in the presence of EBV generated significantly greater number of IFN-γ–producing CD3+ T cells compared to the soluble factors secreted by PDCs in response to EBV (155.0 ± 7.1 compared to 27.0 ± 2.8 spots/3 × 105 T cells, Student t test P < .01). Results are expressed as mean ± SEM.
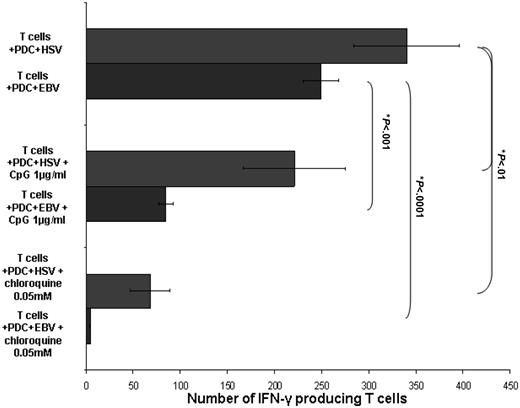
Inhibition of TLR-9 signal pathways blocks EBV-induced IFN-γ production
Having established that PDCs induce the production of IFN-γ–producing NK and CD3+ T cells following EBV stimulation, we then analyzed whether this response was dependent on TLR-9 recognition of EBV. The ability of PDCs to produce IFN-γ–producing CD3+ T cells (but not NK cells) was inhibited in the presence of chloroquine and confirmed with the specific TLR-9 inhibitory CpG ODN TTAGGG indicating the dependence of TLR-9 signaling in the PDC response to EBV (Figure 7). The lack of NK cell response to TLR-9 inhibition suggests that other pathways may be important for PDC/NK cell interaction.
TLR-9 dependence of PDC response to EBV. PDC response to EBV is dependent on TLR-9 signaling. The presence of chloroquine 0.05 mM (4.7 ± 0.6 spots/3 × 105 T cells) and an inhibitory CpG oligonucleotide 1 μg/mL (84.7 ± 7.6 spots/3 × 105 T cells) in culture significantly inhibited the ability of EBV-stimulated PDCs to induce the generation of IFN-γ–producing CD3+ T cells (249.3 ± 18.5 spots/3 × 105 T cells, *Student t test P < .001). HSV-1–stimulated PDCs with CD3+ T cells alone or in the presence of chloroquine and inhibitory CpG oligonucleotide are shown for comparison. Results are expressed as mean ± SEM.
TLR-9 dependence of PDC response to EBV. PDC response to EBV is dependent on TLR-9 signaling. The presence of chloroquine 0.05 mM (4.7 ± 0.6 spots/3 × 105 T cells) and an inhibitory CpG oligonucleotide 1 μg/mL (84.7 ± 7.6 spots/3 × 105 T cells) in culture significantly inhibited the ability of EBV-stimulated PDCs to induce the generation of IFN-γ–producing CD3+ T cells (249.3 ± 18.5 spots/3 × 105 T cells, *Student t test P < .001). HSV-1–stimulated PDCs with CD3+ T cells alone or in the presence of chloroquine and inhibitory CpG oligonucleotide are shown for comparison. Results are expressed as mean ± SEM.
Discussion
In these studies, we analyzed the role of PDCs in EBV-related diseases including infection and the onset of lymphoproliferative disease. We and others have previously shown that circulating precursor PDCs are reduced in renal and cardiac transplant recipients.18,22,23 PDCs isolated from recipients of renal transplants were functionally normal in response to viral stimulation, suggesting that the defect in antiviral response in renal transplant recipients was a reflection of the reduction in circulating precursor PDCs rather than an intrinsic abnormality of PDCs. Thus, the present studies were performed to address the consequence of reduced PDCs in the pathogenesis of EBV-related diseases using a NOD-SCID mouse lymphoma model.
In humanized NOD-SCID mice, PDC depletion significantly enhanced mortality from EBV infection in the latent infection model. The low incidence of tumors in our latent infection model compared to other reported studies may reflect heterogeneity of LPD development in our model, depending on differences in cytokine genotypes of reconstituted PBMCs.24 The addition of EBV-containing tissue culture supernatant resulted in the development of LMP-1 and CD20+ B-cell lymphoma in 100% of control NOD-SCID mice. PDC augmentation did not affect EBV-related infection in the latent infection model, but significantly delayed mortality from EBV-induced tumor in the active infection model. This indicates that PDCs exhibit antiviral properties in the presence of free virus, but not during the steady state (ie, latent infection) when the virus is predominantly located intracellularly. This is consistent with the fact that EBV-related PTLD is most prevalent in EBV-seronegative individuals who encounter the virus before they have acquired protective immunity.25 Nevertheless, PDCs appear to have a role in maintaining viral immunity during latent EBV infection because the depletion of these cells accelerated mortality from EBV infection. IFN production is a key component of the body's innate immune defense against viral pathogens. The crucial role of type I IFN is the ability to induce a number of genes that are responsible for inhibiting viral replication and preventing spread at various phases of the viral life cycle.26 In addition, type I IFN may activate MDCs and NK cells, stimulate IgG production by B cells, and prime cytotoxic T-lymphocytes (CTLs).27,28 Interferon-α has been used in the treatment of EBV-associated PTLD, in chronic viral infections including hepatitis B and C, and in several other malignancies (eg, melanoma) with variable success.29,30 However, addition of IFN-pulsed PBMCs at the time of reconstitution of mice was insufficient to overcome the acute EBV challenge, with all mice developing tumors at a comparable time point to control mice. One possible explanation is that this strategy may not have generated antigen-specific immune cells necessary for viral immunity as shown by the in vitro observation that cell-to-cell contact between PDCs and effector cells was required for full effector cell function.
Having established that PDCs were involved in EBV-associated diseases, the underlying mechanisms responsible for this response were investigated. For the first time, we established that PDCs, in part through TLR-9 recognition of EBV, activate both the innate and adaptive immune responses to this virus. Consistent with reports of other human herpes viruses (eg, HSV-1 and -2),13,31 EBV promoted the phenotypic maturation of PDCs and induced IFN-α production by these cells. However, the lack of significant up-regulation of all costimulatory markers on PDCs following EBV stimulation may be influenced by the differential induction of PDC maturation to multiple stimuli including EBV, IL-10, and IFN-α and future experiments to clarify the significance of these individual factors in the induction of PDC maturation markers are continuing.
EBV-stimulated PDCs produce IL-10, an immunosuppressive cytokine, which may be a strategy used by the virus to evade immune recognition and establish viral chronicity. This observation explains in part the lower secretion of IFN-α produced following EBV stimulation compared to other herpes viruses.32 There have been no studies demonstrating production of IL-10 by PDCs in response to viral stimulation (eg, HSV-1) and our finding likely reflects the intrinsic ability of EBV to produce this cytokine. The EBV viral genome encodes a cytokine and cytokine receptor (EBV BCRF1) that shares 70% genetic homology with mammalian IL-10 and mimics its suppressive activities.33 Recent reports have suggested that serum IL-10 concentration may be useful in the early diagnosis and monitoring of EBV-associated PTLD.34,35 Thus, EBV-related IL-10 production may provide a means by which EBV inhibits protective PDC-mediated antiviral immunity.
The present studies also show that PDC promotion of IFN-γ–producing T cells was dependent on cell-to-cell contact, rather than through cytokine production. Although IFN-α has been shown to activate T cells efficiently, suboptimal concentrations of IFN-α generated by PDCs in response to EBV infection may be insufficient to activate viral-specific T cells. This in combination with reduced circulating PDCs in transplant recipients may contribute to the increased risk of EBV-related PTLD. Further experiments indicated that the ability of T cells to produce IFN-γ in response to PDC activation depended on the presence of CD4+ T cells because identical experiments using only CD8+ T cells (without CD4+ T cells) in the coculture with PDCs and EBV was insufficient to induce the production of IFN-γ–producing CD8+ T cells. This finding suggests that activation of CD8+ T cells (which were likely to include CD8+ memory T cells as cells were derived from EBV IgG+ blood donors) may be dependent on CD4+ T-cell help or that CD4+ T cells are capable of producing IFN-γ following viral stimulation, both of which have been demonstrated in previous studies.36,37 It is clearly established that CD4+ T cells are critical during acute viral infection and are able to induce DCs to prime CD8+ T cells and promote CD8+ T-cell memory, further indicating the importance of CD4+ T cells in generating a CD8+ T-cell response.37,38
NK cells play an important role in controlling the course of a primary EBV infection. NK cell numbers are increased during primary EBV infection and their activation may be mediated by cytokines including IFN-α. Activated NK cells stimulate antigen-specific T cells through their production of IFN-γ but are also capable of directly lysing EBV-infected cells.39 In our in vitro experiments, PDCs initiated NK cell activation in response to EBV and enhanced NK cell cytotoxicity and production of IFN-γ. However, the induction of NK cell response by PDCs depended on cell contact rather than via IFN-α production alone (and not dependent on IL-12). The fact that the addition of anti–human IFN-α mAbs had no effect on the ability of PDCs to promote IFN-γ–producing NK cells further indicates that cell-to-cell contact between PDCs and NK cells is necessary to activate NK cells.40 We were surprised to find that exogenous IFN-α (1000 U/mL) promoted the generation of IFN-γ–producing NK cells because studies have shown that IFN-α induced no significant IFN-γ secretion in NK cells.40,41 However, these studies analyzed IFN-γ levels in the culture supernatants rather than detecting IFN-γ–producing NK cells by ELISPOT. Others have shown that IFN-α was capable of promoting resting NK cells and NK cell lines to produce IFN-γ and enhance IFN-γ gene expression in NK cells.42,43
DCs may interact with NK cells through the expression of NK cell activating receptor ligands and the up-regulation of costimulatory molecules by mature DCs.44 This interaction has not previously been shown between PDCs and NK cells. Unlike T cells, the activation of NK cells by PDCs appears to be independent of TLR-9 signaling, suggesting the possibility that EBV may interact and activate PDCs by a non-TLR dependent pathway. The suppressive cytokine, IL-10, may directly activate NK cells (and therefore enhance IFN-γ production)45,46 and this may explain why EBV-stimulated PDCs induce IFN-γ–producing NK cells in the presence of TLR-9 inhibitors. Furthermore, the amount of IFN-α produced by EBV-stimulated PDCs in the PDC/NK cell culture experiments was less than 80 pg/mL and may be another reason why NK cell activation was not influenced by TLR-9 inhibition as described by others.40,41 Recent indirect evidence that PDCs and NK cells may interact include in vitro demonstration that viral-induced chemokine production by PDCs (eg, CCL4, CXCL10) preferentially recruit NK and activated T cells.47 In addition, PDCs and NK cells express similar homing molecules (eg, CCR7 on activation) and thus may colocalize in lymphatic tissues.
These studies indicate that the interaction between EBV and PDCs is in part mediated through TLR activation. The presence of chloroquine48 impairs the ability of PDCs to induce the production of IFN-γ–producing T cells. This suggests that the interaction between EBV and PDCs is mediated through TLR-7 or TLR-9, both of which are present on PDCs.49,50 Further analysis suggests that it is most likely that TLR-9 is responsible in the recognition of the virus because a similar impairment of PDC function to activate T cells was observed when TLR-9 signaling was inhibited using a specific inhibitory CpG oligonucleotide.
Because EBV-associated PTLD remains a major cause of mortality in recipients of cellular and solid organ transplants, newer treatment modalities are needed to improve the cure rates of this disease without jeopardizing the graft function. Adoptive immunotherapy using in vitro-generated EBV antigen-specific CTLs and DC-based therapies are effective but require significant time for production and may even be ineffective in seronegative patients.51,52 Our studies have identified that PDCs play a role in the pathogenesis of EBV infection53 and may be a potential target for future manipulation for the prevention or treatment of PTLD by increasing circulating PDCs using commercially available agents such as G-CSF54 or fms-like tyrosine kinase (Flt-3) ligand.55
Authorship
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Correspondence: Patrick Toby Hewlett Coates, Department of Nephrology and Transplantation Services, Queen Elizabeth Hospital, 28 Woodville Rd, Woodville, South Australia, Australia 5011; e-mail: toby.coates@nwahs.sa.gov.au.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by financial assistance from Kidney Health Australia, Jacquot Research Establishment Award of Royal Australasian College of Physicians, and the Muriel T. Gunn Foundation. W.H.L. is a recipient of the National Health Medical Research Council Postgraduate Medical Research Scholarship of Australia. The authors would like to acknowledge assistance of Dr John Edward Cooper and John Brealey from the Histopathology Department of Queen Elizabeth Hospital and from Dr Chen Au Peh and Dr Plinio Hurtado from the Hanson Institute, Adelaide, South Australia, Australia.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal