Abstract
The IL-7 cytokine promotes the survival of a diverse T-cell pool, thereby ensuring an efficient immune response. Moreover, IL-7 induces the proliferation of recent thymic emigrants (RTEs) in neonates. Here, we demonstrate that the survival and proliferative effects of IL-7 on human RTEs can be distinguished on the basis of dose as well as duration of IL-7 administration. A dose of 0.1 ng/mL IL-7 is sufficient to promote viability, whereas cell-cycle entry is observed only at doses higher than 1 ng/mL. Moreover, a short 1-hour exposure to high-dose IL-7 (10 ng/mL) induces long-term survival but continuous IL-7 exposure is necessary for optimal cell-cycle entry and proliferation. We find that distinct signaling intermediates are activated under conditions of IL-7–induced survival and proliferation; STAT5 tyrosine phosphorylation does not correlate with proliferation, whereas up-regulation of the glucose transporter Glut-1 as well as increased glucose uptake are markers of IL-7–induced cell cycle entry. Glut-1 is directly regulated by PI3K and, indeed, inhibiting PI3K activity abrogates IL-7–induced proliferation. Our finding that the survival and proliferation of RTEs are differentially modulated by the dose and kinetics of exogenous IL-7 has important implications for the clinical use of this cytokine.
Introduction
The IL-7 cytokine is essential for early T-cell development and proliferation1,2 and plays a role in peripheral T-cell survival and expansion.3 In contrast to cytokines such as IL-2, which may be restricted by the expression profile of their receptors, IL-7 is likely to have widespread effects due to receptor expression on the vast majority of peripheral T lymphocytes. The IL-7R is composed of a 75-kDa IL-7Rα subunit that is associated with the IL-2R common γ chain (γc).4 Under conditions of lymphopenia, IL-7 supports the homeostatic proliferation of peripheral T lymphocytes, promoting the expansion of T cells with a diverse T-cell receptor (TCR) repertoire.5-8 Exogenous IL-7 therapy can enhance these effects, promoting thymic expansion and short-term proliferation of peripheral T cells.5,8-15
The ensemble of these properties has lead to the possibility that recombinant IL-7 be used as an adjuvant immune therapy, improving T-cell reconstitution in lymphopenic patients as well as augmenting vaccine responses. Indeed, exciting preliminary work assessing the effects of IL-7 in patients has recently been published, demonstrating transient increases in peripheral CD4+ as well as CD8+ T cells.16 These findings are consistent with preclinical primate studies showing that exogenous IL-7 results in significant increases in cell-cycle entry of T lymphocytes.13,15,17 As observed in these primate studies, the effects of IL-7 in humans were transient, with proliferation and T-cell counts returning to baseline within a short period after cessation of cytokine therapy.
Recent thymic emigrants (RTEs) show increased proliferation to IL-7 compared with long-term resident peripheral T cells.18-23 Thus, this subset of peripheral T cells is an interesting target of IL-7 treatment in vivo. RTES are greatly enriched in neonates and umbilical cord blood compared with adult blood, as demonstrated by high levels of TCR excision circles in the former.19 Moreover, the survival of these immature umbilical cord (UC) lymphocytes is limited and their viability is much more dependent on IL-7 than that of resident naive or memory T cells.18,24 Importantly, the concentration of IL-7 available to RTEs in vivo is in constant fluctuation, likely varying with proximity to sites of IL-7 production. It is thus probable that, in vivo, RTEs periodically encounter high levels of IL-7, followed by phases of relative withdrawal, both in healthy individuals as well as in lymphopenic patients.
The effects of varying the duration and concentration of IL-7 on RTEs is therefore of physiologic and therapeutic relevance. We now report that the viability of CD4+ RTEs is significantly enhanced by exposure to low doses of IL-7, and this response does not require the continuous presence of IL-7. In contrast, optimal proliferation of CD4+ RTEs is only observed following continuous stimulation with high-dose IL-7 (10 ng/mL) over 5 days. This latter response is associated with surface induction of the CD71 transferrin receptor and the Glut-1 glucose transporter. These cell-surface markers are indicative of an increased metabolic activity of these RTEs as demonstrated by a significantly enhanced glucose uptake. Glut-1 has been shown to be directly regulated by PI3K and, indeed, inhibiting PI3K activity abrogates the induction of this downstream effector as well as proliferation but does not significantly decrease signal transducers and activators of transcription (STAT) STAT5 tyrosine phosphorylation and survival. Thus, the dose and kinetics of exogenous IL-7 administration alter the fate of CD4+ RTEs.
Materials and methods
T-cell isolation and culture
Umbilical cord (UC) blood, obtained immediately after delivery of full-term infants, was collected in heparinized tubes. CD4+ T cells were purified by negative selection using tetrameric complexes in which one antibody recognizes a surface antigen on B cells, monocytes, natural killer (NK) cells, or CD8+ cells and the other recognizes glycophorin A on the surface of red blood cells (RosetteSep; StemSep Technologies, Vancouver, BC, Canada). Non-CD4+ T cells were then pelleted upon ficoll-hypaque separation. The purity of the selected cells was monitored after each isolation on a FACSCalibur (BD Pharmingen, San Jose, CA) following staining with FITC-conjugated αCD3 and PE-conjugated αCD4 monoclonal antibodies (mAbs; Beckman Coulter, Marseille, France). The purity of the selected cells was greater than 90%.
Lymphocytes were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; BioWest, Paris, France), penicillin, and streptomycin. Cells were cultured in the presence of recombinant IL-7 (Cytheris, Vanves, France) at concentrations ranging from 0.01 to 100 ng/mL, as indicated. In experiments where recombinant IL-7 (rIL-7) was not maintained during the entire 120-hour culture period, cells were cultured for 1, 24, or 72 hours in the presence of rIL-7 (10 ng/mL), washed twice after dilution in 10-fold volumes of PBS, and then cultured in the absence of IL-7 for an additional 119, 96, and 48 hours, respectively. In some experiments, PI3K activity was blocked with LY294002 (Sigma, St Louis, MO). LY294002 was dissolved in DMSO and stored at −80°C prior to use. LY294002 was added to cultures 1 hour prior to IL-7 stimulation at concentrations ranging from 1.5 to 15 μM, and the equivalent respective volumes of DMSO were used as controls. Additional controls included LY294002-treated RTEs cultured in the absence of IL-7 stimulation.
Flow cytometry for surface markers
To detect surface expression of CD127 (IL-7R α-subunit receptor), CD25, CD69, CD71, CXCR4, and VLA-4, cells were incubated for 20 minutes on ice with the appropriate fluorochrome-conjugated mAbs (Beckman Coulter). Background fluorescence was measured using isotype-matched irrelevant antibodies. Cells were washed with PBS and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). Data analyses were performed using CellQuest Pro (Becton Dickinson) or FlowJo (TreeStar, Ashland, OR) software.
Surface expression of Glut-1 was monitored by binding to a recombinant HTLV-2 envelope receptor-binding domain (HRBD) fused to the enhanced green fluorescence protein–coding sequence (HRBDEGFP) as previously described.25-27 Briefly, HRBDEGFP was produced by transfecting 293T cells with the truncated envelope expression vector using the calcium phosphate method, and supernatant was harvested, filtered through a 0.45-μm pore–size filter, and stored at −20°C until use. Glut-1–binding assays were carried out by incubating 5 × 105 T cells with 300 μL of HRBDEGFP or control supernatants for 30 minutes at 37°C. Cells were then washed and analyzed.
Cell-cycle and proliferation analyses
For cell-cycle analyses, CD4+ T cells were fixed and permeabilized in PBS containing 2% paraformaldehyde and 0.05% saponin (Sigma) for 20 minutes at room temperature. Following extensive washes, cells were stained with a mAb recognizing the nuclear antigen Ki67 (Dako, Trappes, France). Alternatively, the percentage of cells in the S/G2/M phases of the cell cycle was assessed by propidium iodide (PI) staining. At the indicated time points, cells were resuspended in PI (50 μg/mL) diluted in PBS with 5% glycerol and 0.1% triton X-100 and incubated on ice for 15 minutes prior to analysis. Cell cycle was analyzed on the FL2-A wavelength following doublet discrimination. To detect nonviable cells, lymphocytes were incubated with PI (50 μg/mL) without permeabilization.
To precisely assess the generation of daughter cells, CD4+ T cells (2 × 106 cells/mL) were labeled with the 5-carboxyfluorescein diacetate succinimidyl ester (CFSE) fluorochrome (Molecular Probes, Eugene, OR) for 3 minutes at room temperature at a final concentration of 2.5 μM. Labeling was terminated by the addition of FCS (30% of final volume), and cells were washed twice and then cultured as indicated.
Quantitative analysis of IL-7Rα mRNA levels
Quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) analyses were performed as previously described.28 Briefly, at the indicated time points, 1 × 106 cells were lysed and total RNA was extracted by GenElute mammalian total RNA miniprep kit (Sigma). cDNAs were prepared by reverse transcription and quantitative PCR was performed using the Quantitect SYBR green PCR Master Mix (Qiagen, Valencia, CA). Primers for IL-7Rα were 5′-TACCGTGAGCGACAAAGATG-3′ (forward) and 5′-GCTGAATCATTGGGTCACCT-3′ (reverse). Primers for GAPDH were 5′-ACACCCACTCCTCCACCTTT-3′ (forward) and 5′-TCCACCACCCTGTTGCTGTA-3′ (reverse). The cycling conditions for IL-7Rα and GAPDH cDNAs comprised a denaturation step for 15 minutes at 95°C, followed by 40 cycles of denaturation (95°C for 15 s), annealing (59°C for IL-7Rα or 62°C for GAPDH for 20 s), and extension (72°C for 15 s). After amplification, melting-curve analysis was performed with denaturation at 95°C for 5 seconds, then continuous fluorescence measurement from 70°C to 95°C at 0.1°C/s. Each sample was amplified in duplicate.
STAT5 tyrosine phosphorylation analysis
At the indicated time points, cells were prepared for intracellular staining by fixation (Cytofix; BD Pharmingen) and permeabilization (PhosFlow Perm III; BD Pharmingen). The tyrosine phosphorylation state of STAT5 was assessed using an anti–phospho-STAT5 (Y694) antibody coupled to Alexa Fluor 647, according to the manufacturer's instructions (BD Pharmingen). Control fluorescence was analyzed using Alexa Fluor 647–coupled control IgG antibodies (BD Pharmingen).
Glucose uptake
CD4+ T cells (5 × 105) were incubated at 37°C in serum-free RPMI for 30 minutes, then washed and incubated for an additional 30 minutes in 500 μL serum/glucose-free RPMI. Uptake was initiated by adding labeled 2-deoxy-D[1-3H]glucose (Amersham Biosciences, Sunnyvale, CA) to a final concentration of 0.1 mM (2 μCi/mL [0.074 MBq/mL]). Cells were incubated for 45 minutes at 37°C, washed in cold serum/glucose-free RPMI, and solubilized in 500 μL 0.1% SDS. Radioactivity was measured by liquid scintillation and statistical analyses were performed using Student t test.
Results
The dose and duration of rIL-7 stimulation allows the survival and proliferative responses of RTEs to be distinguished
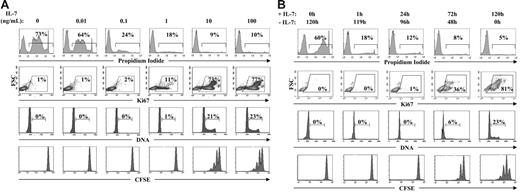
Umbilical cord CD4+ lymphocytes were used as a source of RTEs in this study. As indicated in the “Introduction,” cord blood cells are defined as RTEs on the basis of their high T-cell receptor excision circle (TREC) content.19 At the single-cell level, mature CD4+ lymphocytes expressing CD31 (platelet endothelial cell adhesion molecule-1) have been shown to be RTEs and this marker is lost upon proliferation.29,30 In agreement with these observations, the vast majority of UC CD4+ T cells are CD31+ (Stockinger et al31 and data not shown). To determine whether the IL-7–induced survival and proliferation of these CD4+ RTEs can be discriminated, freshly isolated UC CD4+ lymphocytes were cultured in the presence of varying concentrations of rIL-7 for 5 days. This time point was chosen based on previous work showing that cell-cycle entry is optimal at this time point.18-20 As previously shown, the vast majority of RTEs progressed into cycle and underwent 1 to 3 divisions following culture in the presence of 10 ng/mL IL-7. Notably, this pattern of division was not modified in the presence of higher concentrations of IL-7 (Figure 1A). At a 10-fold lower concentration of IL-7 (1 ng/mL), approximately 10% of cells entered into cycle, as monitored by expression of the nuclear antigen Ki67, which is induced during the mid-G1 phase of the cell cycle.32 Nevertheless, at this lower IL-7 concentration, RTEs were not able to complete the cell cycle as demonstrated by an absence of cells with more than 2N DNA and an absence of CFSE-diluted daughter cells. In agreement with previous reports, the vast majority of RTEs died in the absence of any exogenous cytokines.18,24 Importantly, however, the survival of these RTEs was promoted at concentrations that were 2-logs lower than that required for optimal cell division (0.1 ng/mL; Figure 1A). Thus, low doses of IL-7 promote the survival of RTEs, whereas significantly higher doses are required for cell-cycle entry and progression to mitosis.
IL-7–induced survival and cell-cycle entry of RTEs can be distinguished by the dose and duration of rIL-7 stimulation. (A) CD4+ RTEs were isolated from UC blood by negative selection and cultured in the presence of recombinant IL-7 at doses ranging from 0.01 to 100 ng/mL. After 5 days of culture, cell viability was monitored by propidium iodide (PI) labeling and the percentages of dead cells are indicated in each histogram. Cell-cycle progression was monitored by expression of the Ki67 proliferation marker and DNA content, and the direct proliferation of daughter cells was monitored by dilution of the CFSE fluorochrome. In these latter assays, analyses were performed after gating on live cells. The percentages of cells in the indicated gates are noted. FSC indicates forward scatter. (B) CD4+ RTEs from UC blood were cultured for 120 hours as described above, but the time of exposure to rIL-7 was varied. Following either a 1-hour, 24-hour, or 72-hour culture in the presence of rIL-7 (10 ng/mL), cells were washed and incubations were then continued in the absence of rIL-7 for the remainder of the 120-hour period. Cell viability, cell-cycle progression, and division were assessed at 120 hours as described above. Results are representative of data obtained in 8 independent experiments.
IL-7–induced survival and cell-cycle entry of RTEs can be distinguished by the dose and duration of rIL-7 stimulation. (A) CD4+ RTEs were isolated from UC blood by negative selection and cultured in the presence of recombinant IL-7 at doses ranging from 0.01 to 100 ng/mL. After 5 days of culture, cell viability was monitored by propidium iodide (PI) labeling and the percentages of dead cells are indicated in each histogram. Cell-cycle progression was monitored by expression of the Ki67 proliferation marker and DNA content, and the direct proliferation of daughter cells was monitored by dilution of the CFSE fluorochrome. In these latter assays, analyses were performed after gating on live cells. The percentages of cells in the indicated gates are noted. FSC indicates forward scatter. (B) CD4+ RTEs from UC blood were cultured for 120 hours as described above, but the time of exposure to rIL-7 was varied. Following either a 1-hour, 24-hour, or 72-hour culture in the presence of rIL-7 (10 ng/mL), cells were washed and incubations were then continued in the absence of rIL-7 for the remainder of the 120-hour period. Cell viability, cell-cycle progression, and division were assessed at 120 hours as described above. Results are representative of data obtained in 8 independent experiments.
It has long been postulated that in vivo stimulation of a T lymphocyte by IL-7 results in the transmission of a signal promoting the survival of that particular cell. Nevertheless, it is not clear as to whether optimal responsiveness requires the continuous presence of cytokine and, as indicated in the “Introduction,” it is highly likely that in vivo the concentrations of IL-7 to which an RTE is exposed are constantly fluctuating. It was therefore important to determine the fate of RTEs following short or long exposures to IL-7. Importantly, even a short 1-hour exposure to IL-7 (10 ng/mL) significantly increased the survival of RTEs assessed after 120 hours of culture (Figure 1B). Nevertheless, cell-cycle progression and, to a greater extent, division required the continuous presence of IL-7. First-generation daughter cells were not observed unless lymphocytes were exposed to IL-7 for at least 72 hours of the 120-hour culture period, and the emergence of 2 to 3 divisions required that IL-7 be maintained during the entire culture period (Figure 1B). The ensemble of these data demonstrates that the IL-7 “threshold” required for survival is significantly lower than that required for proliferation.
Continuous down-regulation of IL-7Rα surface levels and gene expression requires high-dose long-term IL-7 stimulation
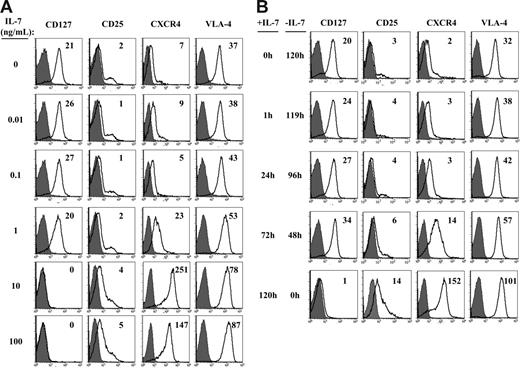
Down-regulation of the IL-7 receptor, either via internalization of IL-7Rα or transcriptional repression of this subunit,28,33 would render the cell unable to optimally respond to further IL-7 stimulation. Indeed, we have previously shown that high-dose IL-7 (10 ng/mL) results in significant receptor down-regulation on adult peripheral blood (APB) T cells within 1 hour of its addition.28 In the experiments performed here, we determined that upon stimulation by lower doses of cytokine, IL-7Rα expression was not significantly altered (Figure 2A). Additionally, surface IL-7Rα expression was rapidly up-regulated, as demonstrated by the reappearance of IL-7Rα within 48 hours after cessation of high-dose IL-7, to levels equivalent or superior to that detected in freshly isolated RTEs (Figure 2B). This was of interest, as the down-regulation of IL-7Rα on mature adult peripheral T cells as well as murine T cells is regulated at the transcriptional level with significant decreases in IL-7Rα transcription following IL-7 stimulation.28,33
Expression of cell-surface markers is modulated by the dose and duration of IL-7 stimulation. CD4+ RTEs were cultured for 120 hours in the presence of rIL-7 doses ranging from 0.01 to 100 ng/mL (A). Alternatively, the time during which cells were exposed to rIL-7 (10 ng/mL) during the 120-hour incubation was varied, ranging from 0 hours to 120 hours (B). Following the 120-hour culture, cell-surface expression of CD127 (IL-7Rα), CD25 (IL-2Rα), the CXCR4 chemokine receptor, and the VLA-4 integrin was assessed using the appropriate fluorochrome-conjugated mAbs. Histograms depicting fluorescence (open histograms) relative to control IgG (filled histograms) are shown and the delta mean fluorescence intensities (ΔMFIs) relative to control staining are indicated in the top right of each panel. Results are representative of data obtained in 2 to 5 independent experiments.
Expression of cell-surface markers is modulated by the dose and duration of IL-7 stimulation. CD4+ RTEs were cultured for 120 hours in the presence of rIL-7 doses ranging from 0.01 to 100 ng/mL (A). Alternatively, the time during which cells were exposed to rIL-7 (10 ng/mL) during the 120-hour incubation was varied, ranging from 0 hours to 120 hours (B). Following the 120-hour culture, cell-surface expression of CD127 (IL-7Rα), CD25 (IL-2Rα), the CXCR4 chemokine receptor, and the VLA-4 integrin was assessed using the appropriate fluorochrome-conjugated mAbs. Histograms depicting fluorescence (open histograms) relative to control IgG (filled histograms) are shown and the delta mean fluorescence intensities (ΔMFIs) relative to control staining are indicated in the top right of each panel. Results are representative of data obtained in 2 to 5 independent experiments.
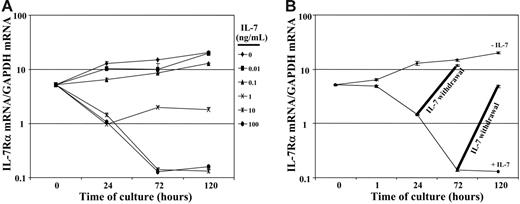
To determine whether the observed changes in IL-7Rα surface levels reflected the transcription of this gene, we proceeded to assess the level of IL-7Rα transcripts in these RTEs following IL-7 stimulation. Real-time qRT-PCR analyses revealed significant changes in IL-7Rα mRNA levels with a 1- and 2-log decrease at 72 hours after stimulation with doses of 1 ng/mL and 10 ng/ml of rIL-7, respectively (Figure 3A). Upon conditions wherein the exposure time to rIL-7 (10 ng/mL) was modulated, IL-7Rα gene expression decreased by 1-log within 24 hours and descended to a nadir at 72 hours after stimulation (2-log decrease). Interestingly, IL-7Rα mRNA levels increased rapidly following cessation of rIL-7 stimulation. When rIL-7 was withdrawn following a short 24-hour exposure to rIL-7, receptor mRNA levels increased to 80% of baseline by 72 hours (Figure 3B). Following longer continuous stimulation with rIL-7 (72 h), increases in IL-7Rα gene expression were less rapid, with levels reaching 25% of baseline 48 hours later (120 h of culture). Nevertheless, given the 2-log decrease, these data indicate that IL-7Rα mRNA levels were augmented by 25-fold within the 48-hour period after cessation of rIL-7 stimulation (Figure 3B). Thus, it appears that the re-emergence of IL-7Rα at the cell surface of RTEs is regulated, at least in part, at the level of transcription. The maintenance of high IL-7Rα mRNA and protein levels on RTE lymphocytes exposed to suboptimal IL-7 concentrations and its rapid re-expression following cessation of high-dose IL-7 stimulation strongly suggests that these cells will be able to respond anew upon further contact with this cytokine.
IL-7 stimulation suppresses IL-7Rα gene expression, and mRNA levels increase upon cessation of cytokine treatment. (A) The levels of IL-7Rα transcripts were assessed in CD4+ RTEs stimulated with doses of rIL-7 ranging from 0.01 to 100 ng/mL. After 0, 24, 72, and 120 hours of culture, total RNAs were extracted and reverse transcribed. cDNAs were amplified with primers specific for IL-7Rα and GAPDH. The relative level of mRNA transcripts was assessed by real-time PCR in duplicate samples and the mean number of molecules of IL-7Rα (±SD) relative to GAPDH at each time point are shown on semilogarithmic graphs. (B) IL-7Rα mRNA levels were monitored in nonstimulated and IL-7–stimulated (10 ng/mL) RTEs after 0, 1, 24, 72, and 120 hours of culture. After 24 hours and 72 hours of culture in the presence of rIL-7 (10 ng/mL), some cultures were washed and incubations were continued for an additional 48 hours in the absence of rIL-7. IL-7Rα mRNA was assessed at the indicated time points and is labeled as “IL-7 withdrawal.”
IL-7 stimulation suppresses IL-7Rα gene expression, and mRNA levels increase upon cessation of cytokine treatment. (A) The levels of IL-7Rα transcripts were assessed in CD4+ RTEs stimulated with doses of rIL-7 ranging from 0.01 to 100 ng/mL. After 0, 24, 72, and 120 hours of culture, total RNAs were extracted and reverse transcribed. cDNAs were amplified with primers specific for IL-7Rα and GAPDH. The relative level of mRNA transcripts was assessed by real-time PCR in duplicate samples and the mean number of molecules of IL-7Rα (±SD) relative to GAPDH at each time point are shown on semilogarithmic graphs. (B) IL-7Rα mRNA levels were monitored in nonstimulated and IL-7–stimulated (10 ng/mL) RTEs after 0, 1, 24, 72, and 120 hours of culture. After 24 hours and 72 hours of culture in the presence of rIL-7 (10 ng/mL), some cultures were washed and incubations were continued for an additional 48 hours in the absence of rIL-7. IL-7Rα mRNA was assessed at the indicated time points and is labeled as “IL-7 withdrawal.”
Phenotype of IL-7–stimulated RTEs is modulated by the dose and duration of IL-7 stimulation
The expression profile of various cell-surface markers modulates the fate of T lymphocytes with respect to homing as well as proliferation and apoptosis. While IL-7 stimulation has not been shown to induce an extensive change in cell phenotype, with RTEs clearly retaining their CD45RA+RO− naive phenotype,18,24,34,35 some modifications have been reported.22,36,37 As previously described, IL-7 induced minimal changes in expression of activation markers with an absence of CD69 expression and a low-level induction of the IL-2Rα subunit, CD25.18,24,36 Indeed, up-regulation of CD25 was only observed when high IL-7 doses (10 ng/mL) were maintained for the duration of the 120-hour culture (Figure 2). We also chose to assess surface levels of the CXCR4 chemokine receptor and the VLA-4 integrin receptor, as these 2 molecules play an important role in the homing and trafficking of RTEs in vivo.38 CXCR4 has previously been shown to be augmented in response to γc cytokines,39 and we found that CXCR4 was up-regulated at lower doses or exposure times of IL-7 (1 ng/mL or a 72-h exposure) compared with those required for CD25 induction (Figure 2). Although IL-7 does not increase VLA-4 on murine thymocytes,40 we found that expression of this integrin receptor was strongly induced on our human RTEs in response to high-dose (10 ng/mL) continuous IL-7 (Figure 2). Thus, a low-level up-regulation of the CXCR4 and VLA-4 receptors is observed at suboptimal IL-7 doses and expression is significantly augmented in response to continuous high-dose IL-7.
Enhanced metabolism of IL-7–stimulated RTEs correlates with cell-cycle progression
The augmented energy demands of an activated T cell are met by an increase in glucose transport and metabolism.41-44 The main functional glucose transporter isoform in T lymphocytes appears to be Glut-1.45 Interestingly, however, Glut-1 is not expressed on quiescent T cells but is induced upon T-cell receptor activation.27,45-47 We therefore monitored surface Glut-1 expression under conditions wherein IL-7 stimulation resulted in either the survival or proliferation of RTEs. Importantly, surface Glut-1 was detected only under conditions of RTE proliferation (Figure 4).
Induction of metabolic markers correlates with IL-7–induced cell-cycle progression of CD4+ RTEs. CD4+ RTEs were cultured for 120 hours in the presence of rIL-7 doses ranging from 0.01 to 100 ng/mL (A). Alternatively, the time during which cells were exposed to rIL-7 (10 ng/mL) during the 120-hour incubation was varied, ranging from 0 hours to 120 hours (B). Surface expression of the Glut-1 glucose transporter was assessed using an EGFP-HRBD fusion protein that specifically binds Glut-1,26 whereas expression of the CD71 transferrin receptor was monitored with a fluorochrome-coupled mAb. Histograms depicting fluorescence (open histograms) relative to control staining (filled histograms) are shown. FSC/side scatter (SSC) profiles, a measure of blast formation, are also shown for each culture condition, and the live cell gates used for the above analyses are shown in each plot. Data are representative of results obtained in 4 independent experiments.
Induction of metabolic markers correlates with IL-7–induced cell-cycle progression of CD4+ RTEs. CD4+ RTEs were cultured for 120 hours in the presence of rIL-7 doses ranging from 0.01 to 100 ng/mL (A). Alternatively, the time during which cells were exposed to rIL-7 (10 ng/mL) during the 120-hour incubation was varied, ranging from 0 hours to 120 hours (B). Surface expression of the Glut-1 glucose transporter was assessed using an EGFP-HRBD fusion protein that specifically binds Glut-1,26 whereas expression of the CD71 transferrin receptor was monitored with a fluorochrome-coupled mAb. Histograms depicting fluorescence (open histograms) relative to control staining (filled histograms) are shown. FSC/side scatter (SSC) profiles, a measure of blast formation, are also shown for each culture condition, and the live cell gates used for the above analyses are shown in each plot. Data are representative of results obtained in 4 independent experiments.
The CD71 transferrin receptor is a marker of high metabolic activity in the thymus48 and we have recently determined that its expression is closely correlated with that of Glut-1 on human thymocytes.49 Indeed, CD71 was detected on IL-7–stimulated RTEs upon culture in those IL-7 culture conditions resulting in surface Glut-1 expression (Figure 4). CD71 was also detected on a small percentage of RTEs cultured under suboptimal proliferation conditions (exposure to high-dose IL-7 [10 ng/mL] for 72 hours of the 120-hour culture period; Figure 4B). Furthermore, increases in cell size and granularity, indicative of T-cell metabolic activity,50 were also restricted to conditions resulting in cell-cycle progression and proliferation. Glut-1 and CD71 are therefore both expressed under conditions of IL-7–mediated proliferation of RTEs, and CD71 appears to be maintained for a longer time period upon withdrawal of IL-7.
To evaluate whether the cell-surface expression of Glut-1 on IL-7–stimulated RTEs has a physiologic impact, RTEs cultured in the presence of various IL-7 concentrations and conditions were assessed for their ability to uptake glucose. Following 120-hour culture in the presence of various IL-7 concentrations, glucose uptake was measured by the ability to uptake nonhydrolyzable 2-deoxy-D[1-3H]glucose. Importantly, increased glucose uptake was only detected under culture conditions resulting in significant proliferation. Uptake was augmented by 5- to 10-fold at high IL-7 concentrations (10 ng/mL; Figure 5A) and this increase required continuous exposure of RTEs to IL-7 during the 120-hour stimulation period (Figure 5B). In some experiments, however, an increase in glucose uptake above basal levels could be detected at lower doses (1 ng/mL), likely due to variability between independent samples with low cell-cycle entry at this suboptimal IL-7 dose (data not shown). These results indicate that the presence of cell surface Glut-1 on IL-7–stimulated RTEs specifically promotes nutrient transport in these cells. The enhanced glucose metabolism is likely to be crucial to the ability of these cells to undergo division.
Glut-1 expression on IL-7–stimulated RTEs reflects increased glucose uptake. CD4+ RTEs were cultured for 120 hours in the presence of rIL-7 doses ranging from 0.01 to 100 ng/mL (A). Alternatively, the time during which cells were exposed to rIL-7 (10 ng/mL) during the 120-hour incubation was varied, ranging from 0 hours to 120 hours (B). Glucose uptake was assayed by incubating cells (5 × 105) with 2-deoxy-D[1-3H]glucose (0.1 mM) for 45 minutes at 37°C. Uptake is expressed as mean counts per minute (CPM) for triplicate samples; error bars indicate SD. Data are representative of results obtained in 4 independent experiments.
Glut-1 expression on IL-7–stimulated RTEs reflects increased glucose uptake. CD4+ RTEs were cultured for 120 hours in the presence of rIL-7 doses ranging from 0.01 to 100 ng/mL (A). Alternatively, the time during which cells were exposed to rIL-7 (10 ng/mL) during the 120-hour incubation was varied, ranging from 0 hours to 120 hours (B). Glucose uptake was assayed by incubating cells (5 × 105) with 2-deoxy-D[1-3H]glucose (0.1 mM) for 45 minutes at 37°C. Uptake is expressed as mean counts per minute (CPM) for triplicate samples; error bars indicate SD. Data are representative of results obtained in 4 independent experiments.
Activation of signaling intermediates required for IL-7–mediated proliferation of RTEs
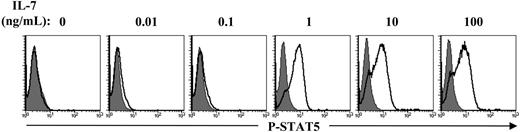
Signaling pathways activated via γc cytokines are transduced via Janus kinases (JAKs) and STATs. IL-7 is known to activate JAK1 and JAK3 as well as STAT5, which translocates to the nucleus, where it functions as a transcription factor (reviewed in Leonard and O'Shea51 ). Indeed, STAT5 has been shown to play an important role in the differentiation of human as well as murine thymocytes.52-54 Analysis of STAT5 tyrosine phosphorylation, monitored by intracellular staining with an antibody recognizing the Y694-phosphorylated form of this protein, revealed optimal STAT5 tyrosine phosphorylation at a dose of 1 ng/mL IL-7 (Figure 6A). Notably, this concentration of IL-7 resulted in only minimal cell-cycle progression (Figure 1A). Moreover, naive CD4+ T lymphocytes from APB, which undergo few or no cell divisions following in vitro IL-7 stimulation,19,20,35 demonstrate equivalent levels of STAT5 tyrosine phosphorylation as that detected in the naive UC population assessed here (Swainson et al,28 Park et al,33 and data not shown). The ensemble of these data suggests that IL-7–stimulated STAT5 tyrosine phosphorylation does not in itself result in proliferation.
IL-7–induced STAT5 phosphorylation is not a marker of T-cell proliferation. CD4+ RTEs were cultured in the presence of 0.01 to 10 ng/mL rIL-7 for 120 hours and STAT5 phosphorylation was then assessed. Cells were permeabilized and stained with the Alexa Fluor 647–conjugated polyclonal antibody recognizing the Tyr694-phosphorylated form of STAT5 (P-STAT5). Representative histogram plots depicting staining (open histograms) relative to control IgG fluorescence (shaded histograms) are shown. Data are representative of results obtained in 3 independent experiments.
IL-7–induced STAT5 phosphorylation is not a marker of T-cell proliferation. CD4+ RTEs were cultured in the presence of 0.01 to 10 ng/mL rIL-7 for 120 hours and STAT5 phosphorylation was then assessed. Cells were permeabilized and stained with the Alexa Fluor 647–conjugated polyclonal antibody recognizing the Tyr694-phosphorylated form of STAT5 (P-STAT5). Representative histogram plots depicting staining (open histograms) relative to control IgG fluorescence (shaded histograms) are shown. Data are representative of results obtained in 3 independent experiments.
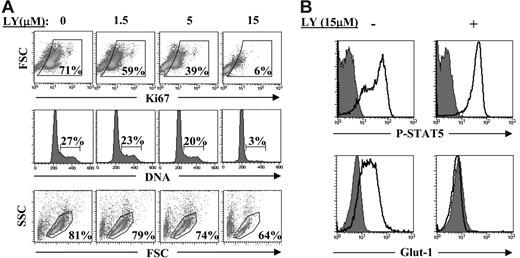
We therefore assessed the importance of the phosphatidylinositol 3-kinase (PI3K) pathway in the IL-7–induced proliferation of RTEs. PI3K is a major effector of IL-7 in T-cell acute lymphoblastic leukemia cells55 and appears to mediate the IL-7–induced survival and proliferation of human thymocytes52,56 and primary human T cells prestimulated via the TCR.57 Incubation of IL-7–stimulated RTEs with the PI3K inhibitor LY294002 resulted in a complete abrogation of cell-cycle progression at a dose of 15 μM (Figure 7A). Importantly, cell viability was only minimally affected at this LY294002 dose, and a similar small reduction in viability was observed when RTEs were treated with the LY294002 inhibitor in the absence of IL-7, indicating that this minor modulation in survival was independent of IL-7 (Figure 7A and data not shown). Moreover, IL-7–induced surface Glut-1 expression, but not tyrosine phosphorylation of STAT5, was abrogated in the presence of LY294002 (Figure 7B); these data indicate that only the former response is inhibited by the PI3K pathway. Therefore, activation of the PI3K pathway is required for induction of surface Glut-1 and subsequent proliferation of RTEs in response to the IL-7 cytokine.
Activation of the PI3K pathway is required for IL-7–induced cell-cycle progression and surface Glut-1 expression. (A) rIL-7–stimulated CD4+ RTEs (10 ng/mL) were cultured in the continuous presence of varying concentrations of the PI3K activity inhibitor LY294002 (0-15 μM). Cell-cycle progression, assessed by Ki67 expression and DNA content, was monitored at 96 hours after gating on viable cells. Viability, monitored by FSC/SSC profiles, was assessed on the total ungated population of lymphocytes. Data are representative of results obtained in 2 independent experiments. (B) rIL-7–stimulated CD4+ RTEs (10 ng/mL) were cultured in the absence or presence of LY294002 (15 μM) for 5 days. Tyr694 phosphorylation of STAT5 and surface Glut-1 expression were assessed and representative histogram plots, relative to control fluorescence (shaded histograms), are shown.
Activation of the PI3K pathway is required for IL-7–induced cell-cycle progression and surface Glut-1 expression. (A) rIL-7–stimulated CD4+ RTEs (10 ng/mL) were cultured in the continuous presence of varying concentrations of the PI3K activity inhibitor LY294002 (0-15 μM). Cell-cycle progression, assessed by Ki67 expression and DNA content, was monitored at 96 hours after gating on viable cells. Viability, monitored by FSC/SSC profiles, was assessed on the total ungated population of lymphocytes. Data are representative of results obtained in 2 independent experiments. (B) rIL-7–stimulated CD4+ RTEs (10 ng/mL) were cultured in the absence or presence of LY294002 (15 μM) for 5 days. Tyr694 phosphorylation of STAT5 and surface Glut-1 expression were assessed and representative histogram plots, relative to control fluorescence (shaded histograms), are shown.
Discussion
This study demonstrates the importance of the duration and concentration of IL-7 on the survival and proliferation of RTEs. The finding that IL-7R levels are rapidly up-regulated at the mRNA and protein level following removal of IL-7 and are maintained when RTEs are exposed to suboptimal concentrations of IL-7 strongly suggests that these cells will be able to respond anew upon further contact with this cytokine. In vivo, this has important clinical implications, as RTEs will periodically encounter high levels of IL-7 followed by phases of relative withdrawal from the effects of this cytokine. Upon administration of exogenous IL-7, it is still likely that there will be fluctuations in the relative availability of this cytokine for a given lymphocyte but the overall exposure will be higher. The data presented here suggest that at low effective concentrations, IL-7 will promote the survival of the RTE pool. Moreover, our data indicate that exposure to high concentrations of IL-7 over an extended time period (> 72 h) will result in a homeostatic proliferation. Nevertheless, it is important to note that significantly lower levels of endogenous IL-7 (pg/mL) in the peripheral circulation of individuals with lymphopenia have been found to be associated with T-cell proliferation.3,58-60 The discrepancy between these observations is likely due to the presence of TCR/self-peptide/major histocompatibility complex (MHC)–derived signals in vivo but not in the ex vivo experiments performed here wherein cells expressing MHC were eliminated. Indeed, in vivo, homeostatic proliferation of naive T cells is regulated by the combined actions of IL-7 and self-MHC/peptide ligands (reviewed in Surh and Sprent61 ). An additional possibility is that the level of IL-7 in the peripheral circulation is significantly lower than that present in the microenvironment of lymph nodes wherein stromal cells are secreting IL-7. Irrespective of the relative contribution of these factors, the data presented here indicate that high-dose IL-7 results in significant proliferation of RTEs in the absence of any MHC/peptide signals. Moreover, high-dose continuous IL-7 stimulation resulted in a strong up-regulation of the VLA-4 and CXCR4 homing molecules on RTEs, and as such this cytokine is likely to promote naive T-cell migration to lymphoid tissues via binding to the extracellular matrix fibronectin38 and endothelial-expressed CXCL12.62
PI3K is one of the major downstream effectors of IL-7 stimulation. Various studies have shown an essential role for PI3K activation in almost all IL-7–induced responses including those related to viability, metabolism, and proliferation via destabilization of p27Kip1.52,55,56,63-65 Nevertheless, the vast majority of these studies were performed in transformed thymocyte cell lines or T-acute lymphoblastic leukemia cells. As such, responses downstream of PI3K in primary T cells, whose metabolic properties and cell-cycle status differ significantly from those of transformed cells, remain to be elucidated. The data presented here indicate a crucial role for PI3K in the proliferation of primary human CD4+ RTEs. The minimal effect of this pathway on RTE survival is in agreement with those reported by Rathmell et al66 showing that the IL-7–induced survival of primary murine lymphocytes is mediated via a PI3K-independent pathway. Thus, the signals induced downstream of PI3K are likely to be modulated by the differentiation and transformation state of a T lymphocyte.
A PI3K-dependent response that is observed in response to diverse stimuli is the activation of the Ser/Thr kinase Akt with a subsequent increase in glucose uptake. This metabolic effect has been described following activation of cells by insulin,67-70 CD28,47 IL-3,50 as well as IL-7.27,55,71 The data presented here demonstrate that IL-7–mediated activation of the glucose transporter Glut-1 and subsequent increases in glucose uptake are observed only under conditions wherein IL-7 stimulation results in the proliferation of RTEs. Moreover, expression of the CD71 transferrin receptor, which mediates iron delivery via transferrin endocytosis and is a marker of metabolic activity in the thymus,48,49,72 is observed under the same conditions. Finally, we determined that IL-7–induced surface Glut-1 expression as well as proliferation of RTEs are mediated by the PI3K pathway, as both these downstream responses were blocked by PI3K inhibitors.
In other populations as well, IL-7/PI3K–mediated induction of Glut-1 appears to be specific to those cells wherein activation of PI3K results in proliferation: IL-7–induced Glut-1 expression and glucose uptake has been reported in proliferating thymocytes, T-ALL cells, and RTEs27,55,71 but has not been detected in human APB T cells, which cycle only minimally in response to ex vivo IL-7 stimulation.27 Similarly, glucose metabolism is not augmented following ex vivo IL-7 stimulation of primary murine T cells, which, like their human counterparts, cycle only minimally under these conditions.66 Moreover, IL-7 stimulation does not induce expression of other metabolic receptors such as CD71 on these mature APB T cells,35 whereas a high induction is observed on cycling RTEs (Figure 4). The ensemble of these data strongly supports the conjecture that PI3K-mediated activation of metabolic markers is indicative of a proliferative state.
The role of STAT5 in IL-7–mediated responses of RTEs is less clear. This transcription factor was activated under low-dose IL-7 conditions that did not result in division of RTEs. Furthermore, high continuous IL-7 stimulation of mature primary T cells resulted in STAT5 tyrosine phosphorylation to levels equivalent to that observed in RTEs despite minimal cell-cycle progression. This factor is an unlikely candidate for promoting survival, as tyrosine phosphorylation was not detected at IL-7 concentrations resulting in increased RTE viability (0.1 ng/mL). This is also the case in thymocytes where IL-7–induced STAT5 tyrosine phosphorylation is important for differentiation but is not required for viability.52 Indeed, a STAT-independent signaling cascade appears to be responsible for the viability observed following stimulation with other γc cytokines; IL-4 prevents apoptosis of resting T cells via a STAT-independent manner.73
Exciting preliminary data from the first phase 1 trial administering recombinant IL-7 to patients16 point to important potential benefits of this adjuvant therapy. The ability to differentially modulate survival, proliferation, and phenotype of RTEs by the dose and duration of IL-7 treatment will have significant ramifications. In conclusion, we find that survival is induced by minimal IL-7 contact but more sustained interactions are required for induction of homing receptors, metabolic markers, and proliferation. Notably, surface IL-7Rα is up-regulated following cessation of IL-7 treatment and this may be of clinical consequence, as the reappearance of IL-7Rα on activated T cells is associated with a survival advantage conferring protective immunity.74 This is a crucial point because in addition to enhancing homeostatic proliferation under lymphopenic conditions, IL-7 has been shown to be a potent vaccine adjuvant.75 In conclusion, activation of distinct signaling pathways and metabolic effectors, due to fluctuations in IL-7 concentrations, will result in diverse fates of IL-7–stimulated RTEs.
Authorship
Contribution: L.S. was the principal participant and designed and performed research, analyzed data, and contributed to the writing of the manuscript; S.K. and C.M. performed research, analyzed data, and contributed to the preparation of the manuscript; M.S. and T.H. performed initial experiments and analyzed data; and N.T. was responsible for the overall study, designed research, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
S.K. and C.M. contributed equally to this study and are listed in alphabetical order.
Correspondence: Naomi Taylor, Institut de Génétique Moléculaire de Montpellier, 1919 Route de Mende, 34293 Montpellier, Cedex 5, France; e-mail: naomi.taylor@igmm.cnrs.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by grants from the European Community (contract LSHC-CT-2005-018914 “ATTACK”), the ANRS, SIDACTION, and Fondation de France. L.S. has been supported by successive fellowships from the ANRS and SIDACTION. C.M. was supported by SIDACTION and the Fondation de France. S.K. and N.T. are supported by CNRS and INSERM, respectively.
We are indebted to the staff at Clinique St Roch, without whose assistance this study would not have been possible. We are grateful to C. Mackall and colleagues for sharing unpublished data and E. Verhoeyen for extensive discussions. We thank the scientists at Cytheris for generously providing rIL-7. O. Adjali, M. Dao, J. Hernandez, C. LeSaout, S. Mennechet, A. Montel, R. Vicente, and V. Zimmerman have all provided important support.


![Figure 5. Glut-1 expression on IL-7–stimulated RTEs reflects increased glucose uptake. CD4+ RTEs were cultured for 120 hours in the presence of rIL-7 doses ranging from 0.01 to 100 ng/mL (A). Alternatively, the time during which cells were exposed to rIL-7 (10 ng/mL) during the 120-hour incubation was varied, ranging from 0 hours to 120 hours (B). Glucose uptake was assayed by incubating cells (5 × 105) with 2-deoxy-D[1-3H]glucose (0.1 mM) for 45 minutes at 37°C. Uptake is expressed as mean counts per minute (CPM) for triplicate samples; error bars indicate SD. Data are representative of results obtained in 4 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/3/10.1182_blood-2006-06-027912/5/m_zh80030707450005.jpeg?Expires=1767749748&Signature=YksWRMeW0COI2HHsjE~W3QzDPhYat6kimi4mFOLlZRuXXWJZ3F009lwDYO7Eni8edfvoNr0GWdFxNoJuKv8kP5tgn4c4IDtEuBPYtSWKS-u24NWnokuQhr6iB~-oGRTJPiRrsP3PlVj4ksoLIwSNlcpL5z6eoQbLqArjVKKfLJU2~wV7rpz9lrUZjk-oSUYpeVWRj0a05lLhxKQTF5thQPDrB42HcBCeNmacllchm1YceKqVIi-n989m3I4nGRetwAb6VqsacKeKpJs267q7eWBw6ViXTggJ9SCs2HN6B03TI3kTMmmR7DFvoKH1pOEJZuHfyaMSE--VMT7lYGnZgw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal