Abstract
The combined blockade of the IL-6R/STAT3 and the MAPK signaling pathways has been shown to inhibit bone marrow microenvironment (BMM)–mediated survival of multiple myeloma (MM) cells. Here, we identify the molecular chaperones heat shock proteins (Hsp) 90α and β as target genes of both pathways. The siRNA-mediated knockdown of Hsp90 or treatment with the novel Hsp90 inhibitor 17-DMAG attenuated the levels of STAT3 and phospho-ERK and decreased the viability of MM cells. Although knockdown of Hsp90β—unlike knockdown of Hsp90α—was sufficient to induce apoptosis, this effect was strongly increased when both Hsp90s were targeted, indicating a cooperation of both. Given the importance of the BMM for drug resistance and MM-cell survival, apoptosis induced by Hsp90 inhibition was not mitigated in the presence of bone marrow stromal cells, osteoclasts, or endothelial cells. These observations suggest that a positive feedback loop consisting of Hsp90α/β and major signaling pathways supports the survival of MM cells. Finally, in situ overexpression of both Hsp90 proteins was observed in most MMs but not in monoclonal gammopathy of undetermined significance (MGUS) or in normal plasma cells. Our results underpin a role for Hsp90α and β in MM pathogenesis.
Introduction
Multiple myeloma (MM) is a common hematologic disorder in which expansion of a malignant plasma-cell (PC) clone in the bone marrow (BM) leads to osteolytic bone destruction, impaired hematopoiesis, and renal failure.1 Despite therapeutic progress that has been achieved through the optimization of chemotherapeutic regimens through the introduction of novel drugs, such as bortezomib and lenalidomide, most MM patients currently succumb to their disease.2
Clinically apparent MM is assumed to evolve through a multistep transformation process leading to oncogenic deregulation of signaling pathways. Acquired genetic alterations and the microenvironment are supposed to critically support the establishment and expansion of the malignant PC clone in the BM.3,4 In particular, the interaction between MM cells and BM stromal cells (BMSCs) has been shown to protect the former from apoptosis.5 Additionally, osteoclasts (OCs) and endothelial cells (ECs) have recently been reported to support tumor growth.6-8
The interleukin-6 receptor/signal transducer and activator of transcription 3 (IL-6R/STAT3), the Ras/mitogen-activated protein kinase (Ras/MAPK), and the phosphatidylinositol 3-kinase/Akt (PI3K/Akt) pathways are well-characterized BM microenvironment (BMM)–triggered signal transduction cascades that sustain the viability of MM cells.9,10 It has therefore been suggested that these pathways might serve as therapeutic targets. However, we have previously demonstrated that MM cells cultured in the presence of cells from the BMM are protected from apoptosis induced by a single pathway blockade.5 Specifically, in the presence of BMSCs, disruption of either the MAPK/extracellular signal-regulated kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) module or of the IL-6R/STAT3 pathway is not sufficient to induce apoptosis of MM cells, whereas combined targeting of both pathways induces MM-cell death.11 This indicates that both pathways cooperate to maintain MM-cell growth and survival. To investigate the mechanism that might distinguish the fatal effects of combined pathway blockade from the benign consequences of any single pathway disruption, we performed gene chip analysis and found genes encoding for heat shock proteins 90 (Hsp90s) to feature prominently among down-regulated genes. We therefore analyzed the role of Hsp90s in MM in greater detail.
The ATP-dependent chaperone Hsp90 comprises 2 homologous proteins (Hsp90α and Hsp90β) that are encoded by separate genes. Hsp90 accounts for the maturation and functional stability of a plethora of polypeptides termed Hsp90 client proteins.12,13 Hsp90s are overexpressed in many cancers, and it is presumed that they are required to sustain aberrant signaling in malignant cells.14 Several components of tumor-cell–associated growth and survival pathways have been found to be Hsp90 clients, and Hsp90s are thus thought to sustain functional expression of oncoproteins while enabling the transformed cell to tolerate the imbalanced signaling this might create. These qualities make Hsp90s potential targets for anticancer drug development.15,16 Recently, it has been reported that pharmacologic inhibition of Hsp90 with 17-allylamino-17-demethoxygeldanamycin (17-AAG) suppresses growth in MM cell lines and in primary MM cells.17 However, the functional consequences of Hsp90 blockade in MM in the context of the BMM are largely unclear. Furthermore, the mechanisms that regulate Hsp90 expression in MM and the contribution of the different homologous Hsp90s to growth and survival of MM cells remain essentially unexplored.
Here, we analyzed the expression of Hsp90α and Hsp90β in MM cells in situ and identified a new mechanism for Hsp90α/β regulation in MM. We also investigated the individual role of both Hsp90 proteins in greater detail through siRNA-mediated knockdown and tested the effects of the second-generation geldanamycin-derived Hsp90 inhibitor 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG) in particular in the context of the BMM.
Materials and methods
Cell culture
Culture conditions for the human IL-6–dependent MM cell lines INA-6 and ANBL-6, the IL-6–independent MM cell line MM.1s, and primary MM cells were as previously described.5,11 In the absence of cells from the BMM (BMSCs, OCs, or ECs), INA-6 and ANBL-6 cells were kept in medium supplemented with 2 ng/mL IL-6; primary MM cells were cultured in medium supplemented with 10 ng/mL IL-6. CD138+ MM cells from 24 patients and BMSCs obtained from 3 MM patients were used in this study. All samples were taken from routine diagnostic specimens after informed consent was obtained from the patients in accordance with the Declaration of Helsinki, and after permission was granted by the ethics committees of the Charité, Berlin, and of the Medical Faculty of the University of Würzburg. Primary human OCs were generated in vitro as described.18 Briefly, mononuclear cells from buffy coats derived from healthy donors were separated by Ficoll density gradient centrifugation, and 1 × 106 cells were seeded per well of a 96-well plate in α-MEM supplemented with 10% FCS (both from Biochrom, Berlin, Germany), 100 U/mL penicillin, 100 μg/mL streptomycin (both from PAN Biotech, Aidenbach, Germany), 2 mM glutamine, 1 mM Na-pyruvate (both from Gibco, Karlsruhe, Germany), 25 ng/mL macrophage colony-stimulating factor, and 30 ng/mL receptor activator of nuclear factor–κB ligand. The adherent-cell fraction was cultured for about 3 weeks until multinucleated OCs appeared. Maturity of the OCs was verified by tartrate-resistant acid phosphatase (TRAP) staining and their morphology. Primary human umbilical vein endothelial cells (HUVECs) were cultured according to the manufacturer's protocol (PromoCell, Heidelberg, Germany). Briefly, de novo HUVECs were cultured in EC growth medium supplemented with 0.4% EC growth supplement with heparin from bovine hypothalamic tissue, 2% FCS, 0.1 ng/mL epidermal growth factor, 1 μg/mL hydrocortisone, and 1 ng/mL basic fibroblast growth factor (all from PromoCell). HUVECs were used between their third and eighth passage. All cells were grown at 37°C and 5% CO2.
Cocultures of MM cells with BMSCs, OCs, or HUVECs
Cocultures of MM cells with BMSCs, OCs, or HUVECs were performed as described before.5-7,19 Briefly, 1 × 104 INA-6, ANBL-6, or primary MM cells were cocultured with either 1 × 103 BMSCs, 2 × 103 HUVECs, or 1 × 102 OCs in 96-well plates and harvested through careful flushing that left the BMSC, OC, or HUVEC layer intact. MM cells were assayed for viability using annexin V–FITC/propidium iodide (PI) staining. For Western blot analysis, 1 × 105 MM cells were cocultured with 1 × 104 BMSCs.
Apoptosis assay
To assess the percentage of apoptotic and viable cell fractions, a human annexin V–FITC/PI staining kit (Bender MedSystems, Vienna, Austria) was used as previously described.5 Briefly, cells were washed in PBS, incubated for 15 minutes in 100 μL binding buffer (10 mM HEPES/NaOH [pH 7.4], 140 mM NaCl, 2.5 mM CaCl2) containing 2.5 μL annexin V–FITC mix and 1 μg/mL PI, and analyzed by flow cytometry (FACSCalibur/CELLQuest; Becton Dickinson, Heidelberg, Germany).
Microarray analysis
The GeneChip Human Genome U133A 2.0 oligonucleotide microarray (Affymetrix, Santa Clara, CA) comprises more than 22 000 probe sets, which analyze the expression level of more than 18 400 transcripts from more than 14 500 well-characterized human genes. For gene-expression analysis, 7.5 × 105 INA-6 cells were cultured with or without 1 × 105 primary BMSCs. After overnight adhesion of INA-6 cells to the BMSC layer, 50 μg/mL of the IL-6R antagonist Sant7 or 50 μM of MEK inhibitor PD98059 (Calbiochem, Bad Soden, Germany) or both were added and cells incubated for 30 hours. INA-6 cells were detached by short-exposure to trypsin/EDTA, pelleted, and stored at −80°C. Total RNA was prepared using the RNeasy Mini Kit according to the manufacturer's protocol (Qiagen, Hilden, Germany), and 3 μg of total RNA was applied for further proceedings. Labeling of RNA targets, hybridization, and posthybridization procedures were performed according to protocols provided by Affymetrix. After hybridization and image analysis, we excluded probe sets with absent calls in all 5 samples or with nonsignificant hybridization intensities. Following washing and staining, probe arrays were scanned twice at 3 μm resolution using a confocal argon laser scanner (Hewlett-Packard, Santa Clara, CA) controlled by Microarray Suite 5.0 software (Affymetrix). Photoemission was detected using a photomultiplier tube through a 570 nm long-pass filter. Computer-generated array images were overlaid with a virtual grid controlled by Microarray Suite 5.0 software. This step allowed definition of each feature and alignment within known array dimensions. About 40 pixels within each feature were averaged after discarding outliers and pixels close to feature boundaries. Gene-expression levels were calculated according to the average hybridization intensities of perfectly matched versus mismatched oligonucleotide probes. Arrays were scaled by Microarray Suite 5.0 software to an average hybridization intensity of 500 per gene and analyzed independently.
The Data Mining Tool 3.0 (Affymetrix) and GeneSpring software package 7.2 (Silicon Genetics, Redwood City, CA) were used for comparison between combined treated versus single treated versus nontreated INA-6 cells. The data were normalized to compensate for variability in hybridizations and hybridization artifacts. The normalization consisted of 3 steps: (1) The data transformation set measurements less than 300 to 300. (2) Each chip was normalized to the 50th percentile of the measurements taken from that chip. (3) Each gene was normalized to the median of the measurements for that gene.
Transient transfection of INA-6 and MM.1s cells with siRNA-expression constructs
Transfection studies exploiting the INA-6 transfection model were performed as previously described.11 For electroporation, 5 × 106 INA-6 cells were electroporated with 15 μg/mL pCD4Δ, and 10 μg/mL of each pSUPER construct was used. Transfection of MM.1s cells followed the same experimental procedures as described for INA-6 cells.
Construction of siRNA-expression vectors
The pSUPER-derived siRNA-expression constructs against Hsp90α and Hsp90β were designed according to the guidelines described before.20 INA-6 or MM.1s cells were transiently transfected with these siRNA-expression vectors, and Western blot analysis of the respective target in purified cells was performed after 72 hours to verify their efficiency and specificity. The sequences of the sense oligonucleotides used for construction of the most effective siRNA-expression plasmids (sequence derived from the actual gene in bold) were 5′-dGATCCCCGGAAAGAGCTGCATATTAATTCAAGAGATTAATATGCAGCTCTTTCCTTTTTG-GAAA-3′ (based on positions 218 to 236 of human HSP90α) and 5′-dGATCCCCGGCTGAGGCCGACAAGAATTTCAAGAGAATTCT-TGTCGGCCTCAGCCTTTTTGGAAA-3′ (based on positions 1923 to 1941 of human HSP90β).
Western blot analysis
All procedures followed those that previously have been published.5 The cocultured INA-6 or ANBL-6 MM cells were carefully washed to avoid contamination with BMSCs. Following separation by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), proteins were transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany) and stained with antibodies against pan-Hsp90, Hsp90α, Hsp90β, phosphorylated STAT3, total STAT3, phosphorylated ERK, or total ERK. An anti–β-actin antibody was used to assess equal loading.
Immunohistochemical analyses
Biopsies from 2 normal human tonsils with germinal center reaction, 4 lymph node biopsies with prominent extrafollicular activation of B cells, and 3 normal (non-MM) BM biopsies were used to analyze Hsp90α and Hsp90β expression in different normal PCs. To investigate Hsp90α and Hsp90β expression in PC neoplasms, 3 BM biopsies with monoclonal gammopathy of undetermined significance (MGUS) and 45 biopsies with MM (41 from BM and 4 from extramedullary localizations) were selected from the lymph node registry at the Institute of Pathology in Würzburg, Germany. The diagnosis was established according to the criteria of the World Health Organization (WHO) classification by morphologic and immunophenotypic analyses of paraffin-embedded tissue sections. Thirty-seven patients had been newly diagnosed for MM, and 8 MM patients had been pretreated.
Paraffin-embedded tissue sections were subjected to heat-induced epitope retrieval and used for immunohistochemical/immunofluorescence staining as described.21 Polyclonal antibodies against Hsp90α or Hsp90β together with an LSAB2 Kit (DakoCytomation, Hamburg, Germany) were used to detect Hsp90 proteins. An anti-CD138 antibody together with either anti-Hsp90α or anti-Hsp90β combined with species-specific Cy-3– and Cy-5–conjugated secondary antibodies (Jackson ImmunoResearch Labs, West Grove, PA) were used for double staining. The nuclei were stained with hematoxylin (Merck, Darmstadt, Germany) or DAPI (4,6 diamidino-2-phenylindole; Sigma). The images were collected either with a BX51 light microscope equipped with a DP50-CCD camera (both from Olympus, Hamburg, Germany) or acquired with a laser scanning TCS SP2 Confocal System equipped with a DMRE microscope and an HCX PL APO 40×/1.25 NA oil-immersion objective lens, and were processed with Leica Confocal Software version 2.61 (all from Leica Microsystems, Mannheim, Germany).
Reagents
Primary antibodies for Western blot analysis were anti–pan-Hsp90 (33755; Santa Cruz Biotechnology, Heidelberg, Germany), anti-Hsp90α (SPA-840), anti-Hsp90β (SPA-843) (both from Stressgen Bioreagents, Ann Arbor, MI), and anti–β-actin (A5316; Sigma). Secondary antibodies were rabbit antirat (SAB-200; Stressgen), goat antimouse (SAB-110; Stressgen), and donkey antirabbit (NA9340; Amersham, Freiburg, Germany). Primary antibodies for immunohistochemical analyses were anti-Hsp90α (AB3466) or anti-Hsp90β (AB3468) (both from Chemicon, Temecula, CA) and anti-CD138 (M7228; DakoCytomation). Construction of the expression plasmid for human truncated CD4 was previously described.11 The pSUPER vector was kindly provided by Dr Agami (The Netherlands Cancer Institute, Amsterdam). The 17-DMAG was purchased from InvivoGen (ant-dgl-1; San Diego, CA) and PD98059 from Calbiochem (513000). Human ΔN40 IL-6 and Sant7, both modified to contain a myc/His-tag at the C-terminus, were produced in our laboratory as previously described.11
Results
Down-regulation of Hsp90α and Hsp90β by combined disruption of IL-6R/STAT3 and MAPK signaling in INA-6 and ANBL-6 cells
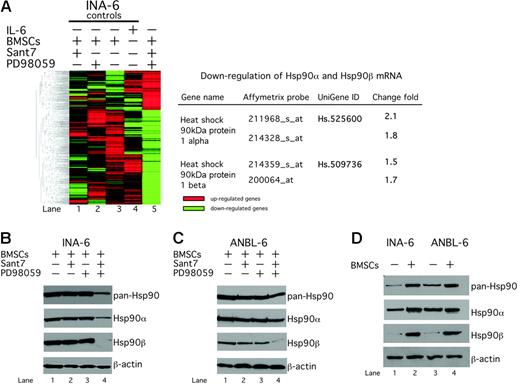
Previously, we have shown that in the presence of BMSCs concomitant inhibition of the IL-6R/STAT3 and the Ras/MEK/ERK pathways is required to induce apoptosis in IL-6–dependent MM cells.11 To gain insight into the downstream molecular mechanisms of this effect, we performed gene-expression analysis of INA-6 cells (Figure 1A). INA-6 cells were either kept in medium supplemented with IL-6 or cultured in the presence of primary BMSCs. Cocultured INA-6 cells were then treated with the IL-6R antagonist Sant7 (which specifically abrogates signaling through the IL-6R/STAT3 pathway), the MEK inhibitor PD98059 (which selectively impairs signaling via the MEK/ERK module), or with a combination of both for 30 hours. The Affymetrix GeneChip U133A platform was used to examine gene-expression profiles, and hierarchic gene cluster and Venn analyses were performed to determine molecular targets that were specifically regulated through combined treatment with Sant7 and PD98059 but remained unaffected in the untreated or the respective single treatment controls. The expression level of 18 400 different transcripts from 14 500 well-characterized human genes was analyzed. A total of 953 genes were found to be down-regulated, and 698 genes were found to be up-regulated after combined Sant7/PD98059 treatment. Heat shock proteins were strongly represented among the down-regulated genes, and we focused on the 2 HSP90 genes, HSP90α and HSP90β, for further analysis (Figure 1A).
Hsp90α and β are down-regulated by combined disruption of the IL-6R/STAT3 and the Ras/MAPK pathways in INA-6 and ANBL-6 cells. (A) Hierarchic clustering analysis of gene-expression profiles of INA-6 cells using the Affymetrix GeneChip U133A platform. Combined blockade of the IL-6R/STAT3 and the Ras/MAPK pathways in INA-6 cells caused a pattern of differently expressed genes (lane 5). HSP90α and HSP90β were found among the down-regulated genes. INA-6 cells were cultured in the presence of primary BMSCs and either treated with 50 μg/mL Sant7 (lane 1) or 50 μM PD98059 (lane 2) or a combination of both for 30 hours (lane 5). As control, INA-6 cells were kept either in the presence of BMSCs (lane 3) or only in medium supplemented with 2 ng/mL IL-6 (lane 4) and treated with DMSO. (B) Western blot analysis of Hsp90α, Hsp90β, or total Hsp90 protein expression in INA-6 and ANBL-6 cells after different drug-exposure regimens. INA-6 or ANBL-6 cells were cultured in the presence of primary BMSCs and treated with 50 μg/mL Sant7 (lane 2) or 50 μM PD98059 (lane 3) or a combination of both for 20 hours (lane 4). (C) Western blot analysis of Hsp90α, Hsp90β, or total Hsp90 protein expression in INA-6 cells cultured either without IL-6 or in the presence of BMSCs. Equal loading was assessed through immunostaining of β-actin.
Hsp90α and β are down-regulated by combined disruption of the IL-6R/STAT3 and the Ras/MAPK pathways in INA-6 and ANBL-6 cells. (A) Hierarchic clustering analysis of gene-expression profiles of INA-6 cells using the Affymetrix GeneChip U133A platform. Combined blockade of the IL-6R/STAT3 and the Ras/MAPK pathways in INA-6 cells caused a pattern of differently expressed genes (lane 5). HSP90α and HSP90β were found among the down-regulated genes. INA-6 cells were cultured in the presence of primary BMSCs and either treated with 50 μg/mL Sant7 (lane 1) or 50 μM PD98059 (lane 2) or a combination of both for 30 hours (lane 5). As control, INA-6 cells were kept either in the presence of BMSCs (lane 3) or only in medium supplemented with 2 ng/mL IL-6 (lane 4) and treated with DMSO. (B) Western blot analysis of Hsp90α, Hsp90β, or total Hsp90 protein expression in INA-6 and ANBL-6 cells after different drug-exposure regimens. INA-6 or ANBL-6 cells were cultured in the presence of primary BMSCs and treated with 50 μg/mL Sant7 (lane 2) or 50 μM PD98059 (lane 3) or a combination of both for 20 hours (lane 4). (C) Western blot analysis of Hsp90α, Hsp90β, or total Hsp90 protein expression in INA-6 cells cultured either without IL-6 or in the presence of BMSCs. Equal loading was assessed through immunostaining of β-actin.
Western blot analysis confirmed that Hsp90α was reduced and Hsp90β nearly completely lost at the protein level. Accordingly, Western blot analysis with a pan-Hsp90 antibody revealed reduced total Hsp90 protein levels (Figure 1B). We also analyzed the effects of combined pathway inhibition with Sant7 and PD98059 on the Hsp90α and β protein expression in ANBL-6, another IL-6–dependent MM cell line. Similar to the situation in INA-6, the combined pathway blockade led to a partial reduction of Hsp90α and to a strong reduction of Hsp90β protein levels even in the presence of BMSCs (Figure 1B). The IL-6R/STAT3 and the Ras/MAPK pathways in MM cells are strongly stimulated by BMSCs.11 We therefore investigated whether expression of Hsp90α and Hsp90β is induced by BMSCs. INA-6 and ANBL-6 cells were either kept in culture medium without IL-6 or cultured in the presence of BMSCs. Western blotting showed that the level of Hsp90α was moderately and the level of Hsp90β strongly increased by coculture with BMSCs (Figure 1C).
Hsp90α and Hsp90β proteins are strongly expressed in MM cells but not in MGUS or in PCs
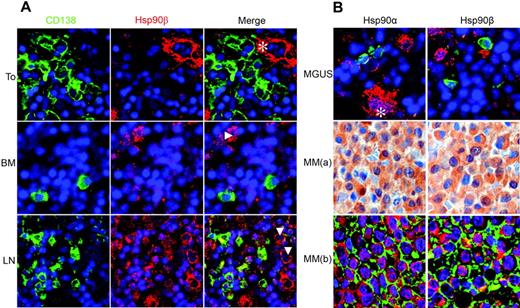
Next, we asked whether Hsp90α and Hsp90β proteins are expressed in different types of normal human PCs, thus including PCs generated during primary or recall immune responses, post–germinal center PCs of tonsils, and long-lived BM PCs. Double immunofluorescence staining was performed with antibodies specific for PC marker CD138 and for Hsp90α or Hsp90β. Normal PCs from the BM or the tonsils did not express Hsp90α (data not shown) or Hsp90β (Figure 2A). Similarly, MGUS PCs exhibited no Hsp90α or Hsp90β expression. In contrast, we observed strong expression of Hsp90α and Hsp90β in 69% (31 of 45) of investigated MM cases (Figure 2B). Of note, among these MM samples all 4 extramedullary and all 6 morphologically anaplastic MMs were strongly positive for Hsp90α and Hsp90β. Eight samples were obtained from pretreated and 37 from newly diagnosed patients. In this relatively small series of myeloma cases, we did not observe any significant differences regarding age, sex, or myeloma subtype (IgM, IgA, IgG, κ, λ) between patients that either do or do not express Hsp90. Together, these data provide evidence that Hsp90α and Hsp90β are aberrantly overexpressed in most malignant MM cases.
Hsp90α and Hsp90β proteins are strongly expressed in MM but not in MGUS or normal plasma cells. Immunohistochemical analysis of Hsp90α and Hsp90β protein expression in normal PCs (A) versus PCs from patients with MGUS or MM (B). Immunofluorescence images of sections of human tonsil (To), bone marrow (BM), and lymph node (LN) stained for CD138 (Cy-2, green), Hsp90β (Cy-3, red), and nuclei (DAPI, blue). CD138+ PCs of human tonsil and bone marrow express no Hsp90β, which is, however, expressed in ECs (*) and myeloblasts (Δ). Staining of lymph nodes shows strong Hsp90β expression in extrafollicular activated B cells (Δ) and weak expression in CD138+ PCs. The expression of Hsp90α was identical to that of Hsp90β (not shown). (B) Representative sections of BM biopsies from patients with MGUS (top panel) and MM (bottom panels 1 and 2). MGUS and MM(b) show staining for Hsp90α or Hsp90β (both with Cy-3, red) and CD138+ (Cy-2, green). Row MM(a) shows Hsp90α and Hsp90β expression staining using a secondary biotinylated antibody, a streptavidin/horseradish peroxidase complex, and 3,3 diaminobenzidine/H2O2 as the chromogen together with hematoxylin as the counterstain. PCs of MGUS express no Hsp90α and Hsp90β, which are, however, expressed in megakaryocytes (*). In contrast, Hsp90α and Hsp90β are strongly expressed in the cytoplasm of MM cells.
Hsp90α and Hsp90β proteins are strongly expressed in MM but not in MGUS or normal plasma cells. Immunohistochemical analysis of Hsp90α and Hsp90β protein expression in normal PCs (A) versus PCs from patients with MGUS or MM (B). Immunofluorescence images of sections of human tonsil (To), bone marrow (BM), and lymph node (LN) stained for CD138 (Cy-2, green), Hsp90β (Cy-3, red), and nuclei (DAPI, blue). CD138+ PCs of human tonsil and bone marrow express no Hsp90β, which is, however, expressed in ECs (*) and myeloblasts (Δ). Staining of lymph nodes shows strong Hsp90β expression in extrafollicular activated B cells (Δ) and weak expression in CD138+ PCs. The expression of Hsp90α was identical to that of Hsp90β (not shown). (B) Representative sections of BM biopsies from patients with MGUS (top panel) and MM (bottom panels 1 and 2). MGUS and MM(b) show staining for Hsp90α or Hsp90β (both with Cy-3, red) and CD138+ (Cy-2, green). Row MM(a) shows Hsp90α and Hsp90β expression staining using a secondary biotinylated antibody, a streptavidin/horseradish peroxidase complex, and 3,3 diaminobenzidine/H2O2 as the chromogen together with hematoxylin as the counterstain. PCs of MGUS express no Hsp90α and Hsp90β, which are, however, expressed in megakaryocytes (*). In contrast, Hsp90α and Hsp90β are strongly expressed in the cytoplasm of MM cells.
Combined knockdown of Hsp90α and Hsp90β proteins is required to induce apoptosis in INA-6
To investigate the respective role of the homologous Hsp90α and Hsp90β proteins in MM biology, we constructed several pSUPER-derived siRNA-expression plasmids. INA-6 cells were transiently transfected with siRNA-expression constructs against Hsp90α, Hsp90β, or both. Western blot analysis identified one highly effective and specific knockdown construct for each Hsp90 target (Figure 3A-B). Of note, the siRNA against Hsp90α caused a strong down-regulation of Hsp90α protein without reduction of Hsp90β and vice versa, verifying that both siRNAs were highly specific for their respective targets (Figure 3A-B). Interestingly, the level of Hsp90β protein increased after siRNA-mediated down-regulation of Hsp90α and vice versa, indicating compensatory up-regulation (Figure 3C-D). To study the effects of Hsp90α and Hsp90β ablation on cell survival, INA-6 cells were assayed for apoptosis 4 days after transfection (Figure 3D). Knockdown of Hsp90α had no observable effect on MM-cell viability. In contrast, Hsp90β knockdown increased the apoptotic-cell fraction up to 40% compared with the mock control. However, only the concomitant down-regulation of both Hsp90α and Hsp90β proteins led to large-scale (up to 80%) induction of apoptosis compared with the mock control (Figure 3D). Next, we investigated whether cells from the BMM can protect INA-6 cells from apoptosis induced by knockdown of Hsp90α and Hsp90β. INA-6 cells transfected with siRNA-expression constructs against Hsp90α, Hsp90β, or both were cocultured with BMSCs. The siRNA-mediated down-regulation of Hsp90α and Hsp90β proteins was effective even in the presence of BMSCs (Figure 3C). Coculture with BMSCs could not protect INA-6 cells from the deleterious consequences of simultaneous knockdown of both Hsp90s (Figure 3D).
Combined knockdown of Hsp90α and Hsp90β is required to induce apoptosis in INA-6 cells. (A-C) Western blot analysis of Hsp90α, Hsp90β, or pan-Hsp90 protein expression in INA-6 cells that were transiently transfected either with siRNAs against Hsp90α, Hsp90β, or both and cultured in the presence of BMSCs. Staining of β-actin served as a loading control. (D) Viability of the transfected INA-6 cells measured by annexin V–FITC/PI staining. INA-6 cells were transiently transfected either with siRNAs against Hsp90α, Hsp90β, or both and cultured either in the absence or presence of BMSCs for 96 hours. Error bars denote the range of values derived from at least 3 independent experiments.
Combined knockdown of Hsp90α and Hsp90β is required to induce apoptosis in INA-6 cells. (A-C) Western blot analysis of Hsp90α, Hsp90β, or pan-Hsp90 protein expression in INA-6 cells that were transiently transfected either with siRNAs against Hsp90α, Hsp90β, or both and cultured in the presence of BMSCs. Staining of β-actin served as a loading control. (D) Viability of the transfected INA-6 cells measured by annexin V–FITC/PI staining. INA-6 cells were transiently transfected either with siRNAs against Hsp90α, Hsp90β, or both and cultured either in the absence or presence of BMSCs for 96 hours. Error bars denote the range of values derived from at least 3 independent experiments.
Inhibition of Hsp90 activity by 17-DMAG attenuates levels of phosphorylated and total STAT3 and of phosphorylated ERK in INA-6 and ANBL-6 cells
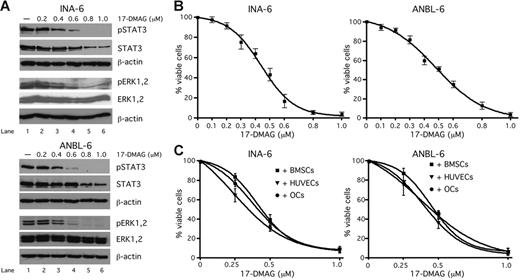
It has been reported that inhibition of Hsp90 activity affects the expression levels of several client proteins involved in signal transduction, such as Raf-1 or Akt.15 To identify Hsp90 clients in our in vitro model, we treated INA-6 or ANBL-6 cells with different concentrations of 17-DMAG (0.2, 0.4, 0.6, 0.8, or 1 μM) (Figure 4A). Western blot analysis revealed a strong concentration-dependent suppression of STAT3 phosphorylation (at Y705), whereas the levels of total STAT3 were only partially decreased. This indicates that in addition to STAT3 itself, the activity of upstream STAT3 kinases, such as the Janus kinases, might be dependent on Hsp90. In contrast, whereas phosphorylation of ERK1,2 was down-regulated through treatment with 17-DMAG, the level of total ERK1,2 protein was unaffected, suggesting that these proteins are not Hsp90 clients themselves.
Inhibition of Hsp90 activity in INA-6 and ANBL-6 cells by 17-DMAG attenuates levels of phosphorylated and total STAT3 and phosphorylated ERK1,2 and induces apoptosis also in the presence of cells from the BMM. (A) Western blot analysis of phosphorylated (Y705) and total STAT3 and of phosphorylated and total ERK1,2 in INA-6 or ANBL-6 cells that were treated with different concentrations of 17-DMAG for 20 hours. Staining of β-actin served as a loading control. (B-C) Viability analysis of 17-DMAG–treated INA-6 or ANBL-6 cells. INA-6 or ANBL-6 cells were kept either in medium with 2 ng/mL IL-6 (B) or cultured in the presence of BMSCs, OCs or HUVECs (C) and treated with different concentrations of 17-DMAG for 3 days. The viable fractions of the treated cells were determined by annexin V–FITC/PI staining.
Inhibition of Hsp90 activity in INA-6 and ANBL-6 cells by 17-DMAG attenuates levels of phosphorylated and total STAT3 and phosphorylated ERK1,2 and induces apoptosis also in the presence of cells from the BMM. (A) Western blot analysis of phosphorylated (Y705) and total STAT3 and of phosphorylated and total ERK1,2 in INA-6 or ANBL-6 cells that were treated with different concentrations of 17-DMAG for 20 hours. Staining of β-actin served as a loading control. (B-C) Viability analysis of 17-DMAG–treated INA-6 or ANBL-6 cells. INA-6 or ANBL-6 cells were kept either in medium with 2 ng/mL IL-6 (B) or cultured in the presence of BMSCs, OCs or HUVECs (C) and treated with different concentrations of 17-DMAG for 3 days. The viable fractions of the treated cells were determined by annexin V–FITC/PI staining.
Pharmacologic inhibition of Hsp90 activity by 17-DMAG induces apoptosis in INA-6 and ANBL-6 cells even in the presence of cells from the BMM
A recently published study has shown that pharmacologic inhibition of Hsp90 activity with the geldanamycin derivative 17-AAG could present a rational therapeutic strategy for MM.17 However, the role of the BMM for this therapeutic concept is poorly understood. Furthermore, a clinical application of 17-AAG is hampered by its low solubility in aqueous media resulting in formulation difficulties that may limit biologic availability.22 In this study we tested the efficacy of the novel, water-soluble, and orally bioavailable Hsp90 modulator 17-DMAG against MM cells cultured in the absence (Figure 4B) or presence of BMSCs, OCs, or HUVECs (Figure 4C). The IL-6–dependent MM cell lines INA-6 and ANBL-6 were either kept in medium supplemented with 2 ng/mL IL-6 or were cocultured with BMSCs, OCs or HUVECs and treated with different concentrations of 17-DMAG for 3 days. This treatment dramatically decreased the viability of both INA-6 and ANBL-6 cells, and the effect could not be redeemed through coculture with BMSCs, OCs, or HUVECs.
Inhibition of Hsp90 activity in MM.1s cells
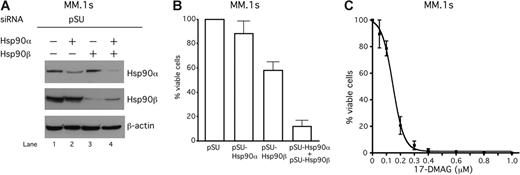
We next inhibited Hsp90 activity in MM.1s cells, another transfectable MM cell line. Similar to INA-6, either Hsp90α or Hsp90β protein was specifically down-regulated by the respective siRNA (Figure 5A). Whereas Hsp90α was almost dispensable for the viability of MM.1s cells, the loss of Hsp90β caused apoptosis in 40% of the population. Only a combined knockdown of both Hsp90 proteins strongly provoked apoptosis (Figure 5B). Blocking the Hsp90 activity in MM.1s cells by treatment with the pharmacologic Hsp90 inhibitor 17-DMAG strongly reduced their viability in a concentration-dependent manner (Figure 5C).
The siRNA-mediated knockdown of Hsp90 or treatment with 17-DMAG induces apoptosis also in IL-6–independent MM.1s cells. (A) Western blot analysis of Hsp90α and Hsp90β protein expression in MM.1s cells that were transiently transfected either with siRNAs against Hsp90α, Hsp90β, or both. Staining of β-actin served as a loading control. (B-C) Viability of MM.1s cells was assayed with annexin V–FITC/PI staining either 96 hours after siRNA-mediated knockdown of Hsp90 (B) or 72 hours after treatment with different concentrations of 17-DMAG. Error bars denote the range of values derived from at least 3 independent experiments.
The siRNA-mediated knockdown of Hsp90 or treatment with 17-DMAG induces apoptosis also in IL-6–independent MM.1s cells. (A) Western blot analysis of Hsp90α and Hsp90β protein expression in MM.1s cells that were transiently transfected either with siRNAs against Hsp90α, Hsp90β, or both. Staining of β-actin served as a loading control. (B-C) Viability of MM.1s cells was assayed with annexin V–FITC/PI staining either 96 hours after siRNA-mediated knockdown of Hsp90 (B) or 72 hours after treatment with different concentrations of 17-DMAG. Error bars denote the range of values derived from at least 3 independent experiments.
Pharmacologic inhibition of Hsp90 activity by 17-DMAG induces apoptosis in primary MM cells cocultured with cells from the BMM
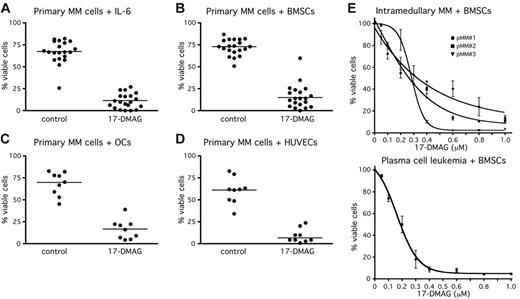
Because pharmacologic inhibition of Hsp90 activity in primary MM cells cultured in the presence of cells from the BMM has so far not been investigated, we analyzed the survival of primary MM cells after treatment with 17-DMAG (Figure 6). Primary MM cells of 24 patients were freshly isolated from BM aspirates (n = 23) or from peripheral blood (n = 1). Twenty-one samples were either kept in medium with 10 ng/mL IL-6 or cultured in the presence of cells from the BMM. Due to limited material, 11 samples were cocultured only with BMSCs, whereas 9 samples were cocultured with BMSCs, OCs, or HUVECs. The primary MM cells were exposed for up to 7 days to 1 μM 17-DMAG (Figure 5A-D). Treatment with 1 μM 17-DMAG drastically decreased the number of viable cells to median values of 12% (medium supplemented with IL-6) (Figure 5A) and to 20% (BMSCs) (Figure 5B), 20% (OCs) (Figure 5C), and 8% (HUVECs) (Figure 5D) of controls. With an additional 4 primary myeloma samples (3 of medullary origin and 1 PC leukemia) 17-DMAG titration curves were performed. EC50 values for 17-DMAG–induced cell death ranged from 0.2 to 0.3 μM (Figure 6E). Thus, in primary MM cells, too, pharmacologic inhibition of Hsp90 activity efficiently overcomes the resistance to apoptosis imparted by BMSCs, OCs, and HUVECs.
Treatment of primary MM cells with 17-DMAG induces apoptosis also in the presence of cells from the BMM. Primary MM cells were freshly isolated from 24 patients. (A-D) Primary MM cells were kept in medium with 10 ng/mL IL-6 (n = 20) or cultured either in the presence of BMSCs (n = 20) or OCs (n = 9) or HUVECs (n = 9). The primary MM cells were exposed for up to 7 days to 1 mM 17-DMAG. (E) 17-DMAG titration experiments with 3 BM-derived primary MM samples and with cells from a patient with PC leukemia (PCL). Primary MM cells were cocultured with BMSCs and exposed to different concentrations of 17-DMAG for 1 week. Viable cell fractions were assessed by annexin V–FITC/PI staining. Horizontal lines (A-D) indicate the median of each treatment cohort.
Treatment of primary MM cells with 17-DMAG induces apoptosis also in the presence of cells from the BMM. Primary MM cells were freshly isolated from 24 patients. (A-D) Primary MM cells were kept in medium with 10 ng/mL IL-6 (n = 20) or cultured either in the presence of BMSCs (n = 20) or OCs (n = 9) or HUVECs (n = 9). The primary MM cells were exposed for up to 7 days to 1 mM 17-DMAG. (E) 17-DMAG titration experiments with 3 BM-derived primary MM samples and with cells from a patient with PC leukemia (PCL). Primary MM cells were cocultured with BMSCs and exposed to different concentrations of 17-DMAG for 1 week. Viable cell fractions were assessed by annexin V–FITC/PI staining. Horizontal lines (A-D) indicate the median of each treatment cohort.
Discussion
Recently, we have shown that signaling through both the IL-6R/STAT3 and the Ras/MAPK pathways is triggered by BMSCs and contributes to the survival of MM cells. Consequently, combined disruption of both pathways is required to effectively induce MM-cell death, especially if the cells grow in the presence of BMSCs.11 To elucidate how these 2 pathways mediate their prosurvival effect, we compared the gene-expression profiles of combined pathway blockade versus single pathway disruption in INA-6 cells. Among the genes specifically down-regulated after combined pathway blockade we found HSP90α and HSP90β. This treatment also reduced the respective protein levels in INA-6 cells as well as in ANBL-6, a second IL-6–dependent MM cell line. Conversely, we have demonstrated that protein expression of Hsp90α and Hsp90β in MM cells is up-regulated by coculture with BMSCs, which are known to activate STAT3 and MAPK signaling in MM cells. Our data are in good accordance with observations from other experimental models suggesting that in addition to stress-induced regulation, Hsp90 expression might also be regulated by signal transduction pathways. Thus, increased Hsp90 protein levels were observed in IL-6–transgenic mice that exhibit elevated IL-6 levels.23 Likewise, it was demonstrated that IL-6 can induce increased levels of Hsp90 in a variety of cell types.24 Furthermore, it has been shown that the transcription factors STAT3 and CCAAT/enhancer-binding protein β (C/EBPβ) cooperatively bind and activate the HSP90β promoter and increase Hsp90 levels.25 Whereas STAT3 is exclusively regulated by signaling through the IL-6R, transcriptional activity of C/EBPβ can be regulated by both the IL-6R and the MAPK pathway.26 Hsp90 acts as a molecular chaperone that stabilizes components of survival pathways.15 This is confirmed by our own observations concerning the inhibition of Hsp90 activity in MM cells. Here we show for the first time that targeting Hsp90 activity in MM cells through treatment with 17-DMAG leads to a strong down-regulation of phosphorylated STAT3 and activated MAPK (phosphorylated ERK1,2) and a partial decrease of total STAT3. The suppression of STAT3 phosphorylation suggests that activator kinases upstream of STAT3 count among the major Hsp90 clients. Candidates would be the Janus kinases 1 and 2, as has recently been reported.27 Furthermore, STAT3 has previously been described as an Hsp90 client.28 Raf and its substrate, MEK, which are signaling components upstream of ERK, are both Hsp90 clients, and inhibition of Hsp90 has been shown to deplete them from a variety of tumor cells.29,30 Raf-1 has also recently been shown to be an Hsp90 client in the MM.1s MM cell line.17 These results might explain the decreased levels of ERK phosphorylation that we have observed. Thus, both the IL-6R/STAT3 and Ras/MAPK pathways maintain the expression of Hsp90α and β, which then supports the functionality of these very pathways. These observations suggest that a positive feedback loop consisting of STAT3, MAPK, and Hsp90α/β drives the survival of MM cells.
The PI3K/Akt pathway has also been reported to mediate oncogenic signaling in MM.31 Akt, too, is a client of Hsp90 in MM cells.17 Thus, Hsp90 seems to be central to the maintenance of a number of important oncogenic signaling pathways in MM. This might explain the strong proapoptotic effect of Hsp90 blockade in MM cells, even if they reside in their protective microenvironment.
In a recent study no significant differences in Hsp90 mRNA levels were found between in vitro purified MM cells and normal PCs.17 In contrast, our in situ immunohistochemical Hsp90-expression analysis of BM biopsies revealed strong expression of both Hsp90α and Hsp90β proteins in 69% of primary MM samples analyzed but no expression in PCs from patients with MGUS. These observations suggest that Hsp90α and Hsp90β might play an important role for malignant growth and disease progression of MM, as has recently been reported for other hematologic malignancies.32,33 Because of the relatively small number of MM patients in our study, statistical correlation of the Hsp90-expression status with clinical features has not been attempted.
The 2 isoforms of Hsp90 (Hsp90α and Hsp90β) are encoded by different but highly conserved genes that arose through duplication during evolution.12 To investigate the respective role of Hsp90α and Hsp90β proteins for MM pathogenesis we used siRNAs to specifically knock down each isoform in INA-6 as well as in MM.1s cells. Selective knockdown of one Hsp90 isoform was always accompanied by compensatory up-regulation of the other isoform. Interestingly, knockdown of Hsp90α alone had no observable effect on MM-cell viability, whereas knockdown of Hsp90β led to moderate induction of apoptosis. We thus provide the first comparative knockdown analysis of Hsp90α and Hsp90β in tumor cells, which has been lacking before. Our data are in a good accordance with very recent data implying that in mast cells an interaction of the Hsp90β isoform with the protein Bcl-2 is required for its antiapoptotic function.34 Unlike selective knockdown of either Hsp90α or Hsp90β, a concomitant down-regulation of both proteins efficiently drove INA-6 cells into apoptosis. This effect was observed even in the presence of cells from the BMM. This indicates that both Hsp90 isoforms might cooperate to maintain the survival of MM cells.
Because of the potential therapeutic value of pharmacologic inhibition of Hsp90, development and investigation of suitable Hsp90 inhibitors are needed. 17-DMAG is the first water-soluble analog of 17-AAG. It shows promising antitumor activity in preclinical models, has an excellent bioavailability, is widely distributed to tissues, and is quantitatively metabolized much less than is 17-AAG.35,36 Here, we show that treatment with this novel Hsp90 inhibitor strongly induced apoptosis in MM cell lines and in primary MM cells, which is in accordance with recent studies on MM and other tumor cells.17 However, evaluation of the effects of Hsp90 blockade in the context of the tumor microenvironment has so far been scant. MM cells become more resistant to various antitumor treatments, including selective pathway inhibitors and conventional cytotoxic drugs, if they grow in the presence of BMSCs.5,37 Here, we show that neither for MM cell lines nor for primary MM cells are the proapoptotic effects of Hsp90 blockade mitigated by BMSCs. Importantly, the biologic effects of pharmacologic Hsp90 blockade matched those obtained through siRNA-mediated knockdown of Hsp90 in INA-6 and in MM.1s cells, supporting the notion that they are indeed caused by loss of Hsp90 activity.
Recently, it has been shown that in addition to BMSCs other cellular components of the BMM such as OCs and ECs contribute to the malignant growth and drug resistance of MM cells as well.6-8,38 We therefore extended our analysis of Hsp90 blockade to MM cocultures with OCs and ECs. Neither coculture with OCs nor with ECs prevented the induction of apoptosis in either MM cell lines or primary MM cells through Hsp90 blockade.
Drug titration experiments revealed that in vitro the apoptosis-inducing concentration of 17-DMAG ranged between 0.1 and 1.0 μM—levels that are known to be achievable in vivo.39
Taken together, these observations indicate that activity of Hsp90α and Hsp90β critically contributes to tumor-cell survival in the context of their microenvironment and therefore strengthens the potential value of Hsp90 as a therapeutic target in MM.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: M.C. conceived and designed the research, performed viability assays and Western blot analyses, and wrote the manuscript; S.J. performed the GeneChip experiment, constructed analyses, and wrote the manuscript; T.S. contributed to the construction of siRNA expression vectors, performed transfection experiments, and wrote the manuscript; M.A., A.G., and H.K.M.-H. performed and evaluated the immunohistochemical analyses; R.J.-K. statistically analyzed the raw data generated from the GeneChip experiment; U.U. performed the hybridization of the GeneChip; H.L. kept the cell culture and performed the experiments with MM.1s cells; K.B. performed the experiments with the OCs; M.T., D.K., and H.E. provided primary patient samples; and R.C.B. established the research plan, supervised the project, wrote the manuscript, and approved the data and final version of the manuscript.
M.C. and S.J. contributed equally to this work.
Acknowledgments
This work was supported by the International PhD Program of the Max Delbrück Center for Molecular Medicine and the Humboldt University of Berlin (S.J.) and by grants fom the Deutsche Forschungsgemeinschaft and the Wilhelm-Sander-Stiftung.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal